Introduction
Over long time scales, the distribution of wildlife species is subject to changes related to climatic variation and human activities (Lemoine et al. Reference Lemoine, Bauer, Peintinger and Boehning-Gaese2007, Sodhi and Ehrlich Reference Sodhi and Ehrlich2011). The geography of recent changes in distribution - mostly contractions and extinctions - is largely related to human settlement and subsequent habitat destruction (Channell and Lomolino Reference Channell and Lomolino2000a,Reference Channell and Lomolinob). In recent times, humans try to retain biodiversity by designing nature reserves that encompass the full suite of species, communities and ecosystems in a region (Soulé Reference Soulé1987). However, reserves are often established in remote areas because of the relative lack of value of the land for commercial use or human habitation, for scenic or recreational value, or for the protection of the central range of endangered species. This may result in an inefficient reserve network that does not necessarily accomplish the original goal of preserving biodiversity (Margules and Pressey Reference Margules and Pressey2000, Araujo and Williams Reference Araujo and Williams2001).
The low latitude margins of a species’ distribution (i.e. southern areas in the northern hemisphere) have frequently acted as glacial refugia (Hampe and Petit Reference Hampe and Petit2005, Rodríguez-Muñoz et al. Reference Rodríguez-Muñoz, Mirol, Segelbacher, Fernandez and Tregenza2007, Bajc et al. Reference Bajc, Čas, Ballian, Kunovac, Zubić, Grubešić, Zhelev, Paule, Grebenc and Kraigher2011). Populations residing in these low latitude margins may contribute to the long-term survival and evolution of the species (Channell and Lomolino Reference Channell and Lomolino2000a,Reference Channell and Lomolinob). Environmental factors in low latitude margins often differ from those in the core of the species’ range. For instance, it is now generally accepted that the planet is warming at the highest rate ever recorded, and warmer climates are expected in low latitude distribution margins (IPCC 2007). In turn, low latitude populations are expected to cope better with the predicted vegetation changes in the current global change scenario (Huntley et al. Reference Huntley, Collingham, Green, Hilton, Rahbek and Willis2006, Ohlemueller et al. Reference Ohlemueller, Anderson, Araujo, Butchart, Kudrna, Ridgely and Thomas2008, Pearce-Higgins et al. Reference Pearce-Higgins, Stephen, Langston, Bainbridge and Bullman2009, Renwick et al. Reference Renwick, Massimino, Newson, Chamberlain, Pearce-Higgins and Johnston2012).
Most endangered species, including many indicator species, currently persist in habitats in the periphery of their historic distribution - often remote areas that are less disturbed by humans (e.g. mountain ranges; Naves et al. Reference Naves, Wiegand, Revilla and Delibes2003). In particular, forest species that require large areas of suitable habitat suffer generalised declines due to habitat fragmentation, leaving peripheral populations isolated from the core of the remaining population (Harrison and Bruna Reference Harrison and Bruna1999, Donald et al. Reference Donald, Collar, Marsden and Pain2010). These peripheral areas are particularly important for maintaining biological diversity; however, they usually remain unprotected, which allows continued habitat destruction and jeopardizes conservation of the species (Furlow and Amrijo-Prewitt Reference Furlow and Amrijo-Prewitt1995, Channell and Lomolino Reference Channell and Lomolino2000a,Reference Channell and Lomolinob). In this paper, we document the anthropogenic activities that affect an endangered grouse in peripheral unprotected habitat (Mediterranean deciduous forests) in the south-west of their European distribution.
We studied the Cantabrian Capercaillie Tetrao urogallus cantabricus, a subspecies of the Western Capercaillie T. urogallus, inhabiting montane forests of the Cantabrian range (north-west Spain). The population has been declining dramatically in the last decades and is currently restricted to an area of less than 1,700 km2 with a very low population size, of great concern in terms of population viability (Storch et al. Reference Storch, Jose Banuelos, Fernandez-Gil, Ramon Obeso, Quevedo and Rodriguez-Munoz2006, Moran-Luis et al. Reference Moran-Luis, Fameli, Blanco-Fontao, Fernandez-Gil, Rodriguez-Munoz, Quevedo, Mirol and Banuelos2014). Hence, further habitat destruction or landscape fragmentation will likely be very detrimental to this population (Quevedo et al. Reference Quevedo, Banuelos and Obeso2006a,Reference Quevedo, Banuelos, Saez and Obesob).
Previous studies have localised a peripheral population at the southern edge of the Cantabrian Capercaillie distribution (González et al. Reference González, Olea, Robles and Ena2010, Alda et al. Reference Alda, González, Olea, Ena, Godinho and Drovetski2013). This population inhabits Pyrenean oak Quercus pyrenaica forests within the Mediterranean biogeographic region (González et al. Reference González, Olea, Robles and Ena2010). A better understanding of the quality of the habitat occupied by this population may improve recommendations for its preservation. Here, we use a simple descriptive approach to document which human activities (i.e. forest fragmentation, risk fire and human direct disturbance) potentially affect Cantabrian Capercaillie at the margin of the species’ distribution (sensu Hampe and Petit Reference Hampe and Petit2005) in the Cantabrian Mountains of northern Spain.
Methods
Study area
The study area covers approximately 1,500 km2 and is located in the Mediterranean region of the Cantabrian range (Figure 1). The landscape is mountainous, with moderate slopes and elevations ranging from 800 to 1,700 m asl. Climate is temperate (winter mean temperature is -4°C, and summer mean temperature is 9°C) with 866–1,100 mm annual precipitation. The forested landscape is covered by native Pyrenean oak forest (30,500 ha; 20% of the total study area) and Scots pine Pinus sylvestris plantations (12,500 ha; 8%). The remaining areas covered by a fine-grained mixture of heather Erica australis, brooms Genista spp., meadows, and riparian lowland forest mostly composed by Populus sp., Fraxinus excelsior and Alnus glutinosa. Forest understorey is mainly dominated by heath Erica arborea and broom Cytisus scoparius, while bilberry Vaccinium myrtillus is uncommon (< 0.5% of the forest ground cover) (see González et al. Reference González, Olea, Robles and Ena2010).
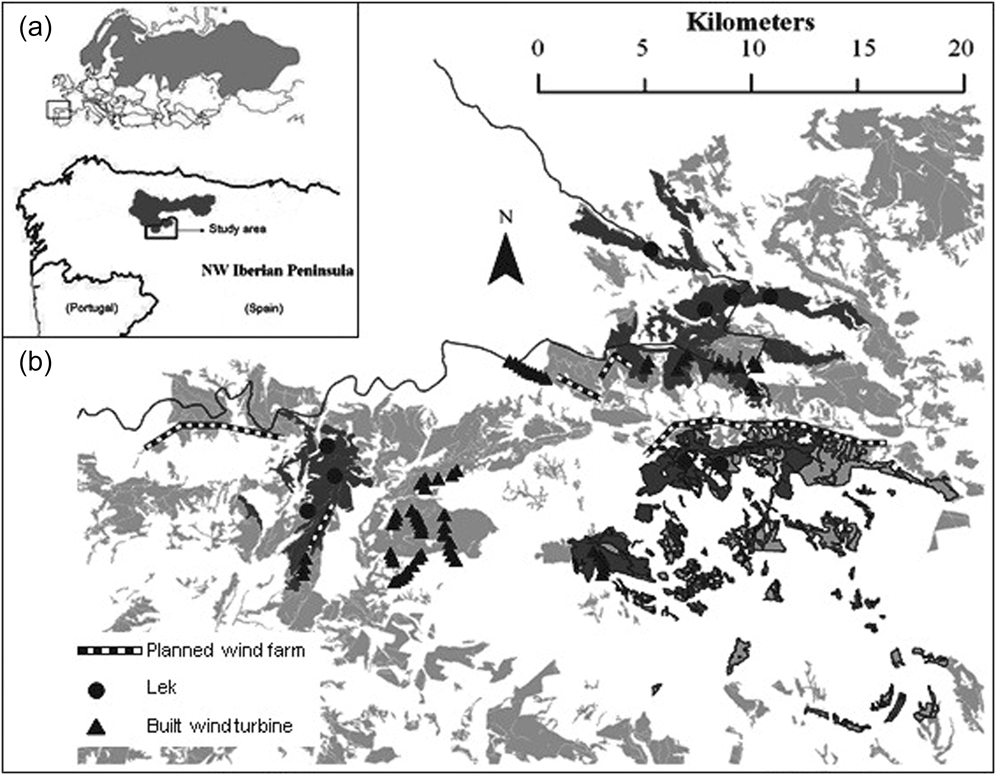
Figure 1. (a) Inset shows the global Capercaillie distribution (light grey) (Storch Reference Storch, Jose Banuelos, Fernandez-Gil, Ramon Obeso, Quevedo and Rodriguez-Munoz2006) and Cantabrian Capercaillie distribution (dark grey). (b) Distribution of i) fragmented forests with Capercaillie presence (dark grey) and without Capercaillie presence (light grey) within the study area; ii) Cantabrian Capercaillie leks, iii) wind turbines operating up to date, and iv) planned wind farms. Forests with a narrow black line indicate forests at high risk of fire; the grey line shows the border of an Important Bird Area (IBA).
Much of the study area is not protected and open to hunting (Pollo Reference Pollo2001, Pollo et al. Reference Pollo, Robles and García-Miranda2004). Only the northern section (c.7,500 ha) lies within the Natura 2000 network (i.e. Important Bird Area of Sierras de Gistreo y Coto, SEO-Birdlife 2011; Figure 1). Human population density is very low (0.6 people/km2) and has declined by 85% since the 1950s (INE 2008). The peripheral population of Cantabrian Capercaillie has been previously estimated using presence/absence signs at approximately 40 adults (González unpubl. data).
Sampling design
Presence-absence data of Cantabrian Capercaillie were obtained from a digital map of the distribution generated in previous studies (González et al. Reference González, Olea, Robles and Ena2010, Reference González, Olea, Mateo-tomás, García-tejero, De Frutos, Robles, Purroy and Ena2012) using: 1) species data from observations; we gathered information on the distribution of Cantabrian Capercaillie in the study area between 2002 and 2009 from questionnaires and reports sent by forest wardens, hunters and local people to the regional environmental agency (Consejería de Medio Ambiente of the Junta de Castilla y León). An experienced observer then validated these data through field surveys which consisted of 3–4 hour systematic zig-zag transects in the fragment looking for signs of Cantabrian Capercaillie presence (direct sightings, display grounds, footprints, droppings or feathers), every 1–2 months up to six times throughout the year, or until signs were found. Some biases may exist in this non-systematic sampling, mainly due to different accessibility of observers to forest fragments and differences between habitats in detectability of Cantabrian Capercaillie. To minimise bias from this non-systematic sampling, we pooled the data on presence signs over all the study years (2002–2009). We considered a fragment to be occupied by Cantabrian Capercaillie when at least one presence sign was registered during 2002–2009 and unoccupied when no sign was observed in that period (González et al. Reference González, Olea, Robles and Ena2010, Reference González, Olea, Mateo-tomás, García-tejero, De Frutos, Robles, Purroy and Ena2012). 2) Species data from radio-tracking: we trapped four Cantabrian Capercaillie (two females and two males) using funnel trap boxes in the study area in May 2000, November 2006 and November 2007 (Robles Reference Robles and Robles2007). Trapped birds were one adult male (> 2 years old), one sub-adult male (< 2 years old) and two sub-adult females. All birds were radio-collared with adjustable necklace transmitters (Biotrack-TW 3, 21 g weight with mortality sensor). The total weight of transmitter plus harness did not exceed the recommended limit of 3–5% of body weight (Kenward Reference Kenward2001). Tracking equipment comprised an Icom ICR-20 receiver, a directional three-element Yagi antenna (150–152 MHz; Biotrack Ltd, Wareham, UK) and a hand-held GPS (Garmin e-Legend HCx). We determined the location of the radio-tagged Cantabrian Capercaillie using the standard triangulation technique (Millspaugh and Marzluff Reference Millspaugh and Marzluff2001). We tracked each bird over more than 18 months (range: 72–96 weeks) and at least twice a week 2 days apart in order to reduce autocorrelation (Harris et al. Reference Harris, Cresswell, Forde, Trewhella, Woollard and Wray1990). For each triangulation point we recorded x and y coordinates, habitat type (i.e. Pyrenean Oak, Scots Pine or non-forested) and date. Following Kenward (Reference Kenward2001), we estimated by an experimental trial our inherent error in determining the real position by triangulation of the transmitter, which resulted in an error estimate of 35.56 ± 3.75 m (mean ± SE). We therefore buffered each location with a 35-m radius. A habitat patch (fragment) with at least one radio location of a tagged Cantabrian Capercaillie was considered occupied (González et al. Reference González, Olea, Robles and Ena2010, Reference González, Olea, Mateo-tomás, García-tejero, De Frutos, Robles, Purroy and Ena2012). 3) Species data from leks: selected sites were locations where survey after survey we found Cantabrian Capercaillie signs. The visits were performed throughout April and May (i.e. display season) after 12h00 to avoid disturbing birds. A lek is defined as a site where one or more cocks consistently display for hens, plus the adjacent surrounding forest habitat. It has traditionally been used as Cantabrian Capercaillie occurrence measure in the Cantabrian range (Obeso and Bañuelos Reference Obeso and Bañuelos2003). During April–May where fresh Cantabrian Capercaillie signs were found, 2–3 observers (forest wardens and L. Robles) visited the site at night as many as four times until the display finished, or until well past dawn. A site was considered as an occupied lek when at least one cock was heard calling or seen displaying. Every detected lek was surveyed each year to label it as occupied or unoccupied by looking for presence signs after 12h00 (see above). In April–May 2009 every occupied lek was surveyed at night to get the number of displaying cocks (González et al. Reference González, Olea, Robles and Ena2010, Reference González, Olea, Mateo-tomás, García-tejero, De Frutos, Robles, Purroy and Ena2012). The map reveals the forest fragments with and without detected Cantabrian Capercaillie presence using surveys (questionnaires and reports), field observations, radio-tracking and lek locations (González et al. Reference González, Olea, Robles and Ena2010, Reference González, Olea, Mateo-tomás, García-tejero, De Frutos, Robles, Purroy and Ena2012). Two types of habitat were considered as available for Cantabrian Capercaillie, namely Pyrenean oak forest and Scots pine plantation, according to the dominant tree species.
Threats
Habitat fragmentation
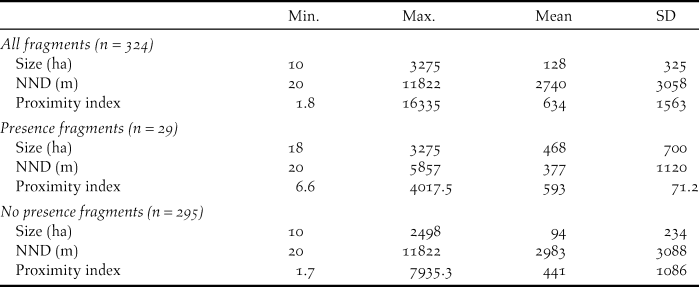
In order to assess habitat fragmentation, we used three variables: i) fragment size, ii) nearest neighbour distance (NND), and iii) a proximity index. All variables were estimated using the ArcGIS 9.3 software from ESRI and V-LATE extension for ArcGIS, a vector tool for quantitative analyses of landscape structure. The fragment size was the area (ha) of a single forest fragment. We considered as a forest fragment a patch of uniform species composition (i.e. Pyrenean oak forest or pine plantation) surrounded by a different habitat type (García et al. Reference García, Quevedo, Obeso and Abajo2005). To create a forest fragment digital layer, we first digitized aerial photographs of the study area (PNOA 2008). NDD was the minimum distance of a focal fragment to the next nearest fragment, representing a simple measure of connectivity. The proximity index calculates proximity as implemented in the FRAGSTATS program (McGarigal et al. Reference McGarigal, Cushman, Neel and Ene2002), which was a fragment-level measure of neighbourhood isolation that considers the size and proximity of all fragments whose edges are within a specified search radius of the focal fragment. This proximity index characterises the tendency for fragments to be relatively isolated from other forest fragments within a neighbourhood (Moilanen and Nieminen Reference Moilanen and Nieminen2002). For this metric, we specified a search radius of 5 km according to the mean documented dispersal distances of Capercaillie in fragmented habitats (Bollmann et al. Reference Bollmann, Graf and Suter2011). The proximity index was computed as the sum of all fragments of the corresponding fragment type whose edges are within the specified radius of the focal fragment, divided by the square of its distance from the focal fragment. The proximity index is dimensionless, and therefore, the absolute value of the index has little interpretive value; instead it is used as a comparative index whose value is inversely related to fragmentation such that a relatively high proximity index indicates relatively low levels of fragmentation (McGarigal et al. Reference McGarigal, Cushman, Neel and Ene2002). To compare fragment size, nearest neighbour distance, and the proximity index among fragments with presence and with no presence of Cantabrian Capercaillie, we performed Wilcoxon tests using R software (R Development Core Team 2015).
Fires
The fire risk in forests fragments with Cantabrian Capercaillie presence was analysed using data from the regional environmental agency on the location of forest fires between 2000 and 2009. We built a vector layer of the study area to create an index of fire history at the municipality scale. This index accounted for fire frequency and time since fire for each forest fragment, c, with i = 1, …n c fires since January 2000. The value of the index of fire history (F c ) is calculated as:
T i is the time in years from the month of fire i until January 2009 and the sum is all fires that took place in that time period in a forest fragment c. If there was no fire in a given forest fragment since January 2000, then F c = 0. The index accounts for fire frequency and time since the last fire while assuming that the impact of the last fire declines over time and that the effect of successive fires is additive (Rhodes et al. Reference Rhodes, Wiegand, McAlpine, Callaghan, Lunney, Bowen and Possingham2006). We grouped F c data into three categories representing relative risk of fire: low risk (1–10), medium risk (11–20), and high risk (> 20). We also categorised each forest fragment according to its inclusion or exclusion in the fire prevention programme approved by the local governmental in 2002 (namely, Plan 42; García Reference García2002). We overlaid fire layers with layers representing Cantabrian Capercaillie presence (see above).
Human infrastructure
We measured density and frequency of anthropogenic infrastructure as indicators of disturbance with the following metrics: i) distance (m) from centre of each occupied forest fragment to the nearest human infrastructure (wind farm, road, off-road track, and human settlements; ii) density of roads and tracks (km of roads or tracks per km2 of land area); and iii) frequency of human settlements (%) within 3- and 4-km buffer zones around the centre of each occupied forest fragment. Buffer zone sizes were chosen based on recommendations to protect an adequate area around lek centres according to the mean Capercaillie home range in similar fragmented landscapes like this (Storch Reference Storch1995).
We calculated distance metrics in ArcGIS 9.3. Locations and numbers of constructed and planned wind turbines and farms were obtained in the field, from the private data base of a conservation NGO (Plataforma para la Defensa de la Cordillera Cantábrica; http://www.cordilleracantabrica.org), and from reviewing the Official Bulletin of Castilla y León (http://bocyl.jcyl.es). Information on wind farm distribution was geo-referenced on a digital map and overlaid with the Cantabrian Capercaillie presence layer (see above). Between 2009 and 2010 the number of wind farms increased from 0 to 5 in the study area, and the number of wind turbines increased from 0 to 65. Six additional wind farms are authorised for construction in the near future and will increase the total number of wind turbines to 127 at 11 wind farms (Figure 1). We compared distances between forest fragments with Capercaillie presence to the nearest constructed wind farm with those to the planned wind farm using means in paired Wilcoxon tests in R (R Development Core Team 2015).
Results
Over one quarter (416 km2) of our study area was considered potential Cantabrian Capercaillie habitat on a previous study (González et al. Reference González, Olea, Robles and Ena2010). Pyrenean oak forest fragments covered 71.5% of the total forest suitable area, and the Scots pine plantations covered the remaining 28.5% (Table 1) measured in km2. Cantabrian Capercaillie presence was detected in 39% (162 km2) of the forested area (59% were Pyrenean oak forests and 41% pine plantations measured in km2). Only 18 km2 (13% of the total forested surface) is part of the Natura 2000 network (EU Habitats Directive) protected by the EU as Important Bird Area (IBA). Three of the nine leks were within the IBA, while the remaining six leks were on public hunting lands with no specific protection for Cantabrian Capercaillie or their habitat (Figure 1).
Table 1. Percentage (%) of: i) the total forest area (TFA), ii) forest area with high risk of fires (FAF), iii) forest area with Capercaillie presence (FAP), iv) forest area with high risk of fires with Capercaillie presence (FAPF) in the study area. Numbers in brackets refer to the percentage related to each forest type.

Habitat fragmentation
Cantabrian Capercaillie occurred in 9% (n = 29) of the forest fragments (Table 2) as measured in km2. Table 2 shows the average values of the fragment size, nearest neighbour distance, and proximity index. Fragments in which Cantabrian Capercaillie were detected were significantly larger, closer to the nearest occupied fragments, and had a higher proximity index than fragments in which no Cantabrian Capercaillie were detected (Wilcoxon tests: W = 1477; P < 0.001, W = 7724; P < 0.001, W = 2,382; P < 0.001 respectively).
Table 2. Metrics of forest fragments in the study area. Size of fragment (ha): area of a focal fragment (ha); NND (m): nearest neighbour distance (m); Proximity index: measure of neighbourhood isolation.
Fires
Between 2000 and 2009, 183 forest fires were recorded in the study area; only three of these occurred in forest fragments in which Cantabrian Capercaillie presence was detected (two in Pyrenean oak fragments and one in Scots pine plantations) accounting for a total of 387.2 ha of burned forest.
High risk of fire (i.e. F c >20) was observed in 23% of the Scots pine plantations and 7.2% of the Pyrenean oak fragments. Combined, these areas totalled 11.7% of the forested area measured in km2. Eighteen percent of the area of fragments in which Cantabrian Capercaillie presence was detected was placed in the high risk of fire category. This area accounted for 33.5% of the total surface covered by Scots pine plantations and 5.8% of the total area covered by Pyrenean oak fragments measured in km2 (Table 1).
Human infrastructure
The 3-km buffer zone around fragments with Cantabrian Capercaillie presence had higher density of roads and tracks, and higher percentage of human infrastructure than areas within 4-km buffer zone (Table 3). The distance between known leks and the nearest existing wind farm was 2,932 ± 1,569 m (mean ± SD, range = 350–5,330). After the additional authorised wind farms are constructed, this distance will decrease to 2,058 ± 1,694 m (mean ± SD, range = 250–5,330; Paired Wilcoxon test, Z = -1.826; P = 0.068). The mean distance from lek to nearest road was 1,205 m ± 763.31 m (mean ± SD, range = 325–2,561), to nearest track, 159 ± 157 m (mean ± SD, range = 25–435), and to nearest human settlement, 1,415 m ± 666 m (mean ± SD, range = 675–2,355). Five wind turbines were within 3 km of leks and 16 wind turbines were within 4 km of leks. Cantabrian Capercaillie leks are located outside of the cluster of the remaining 49 (n = 65) existing wind turbines (Figure 2).
Table 3. Density of roads and tracks (km of roads or tracks per km2 of land area), and area occupied by human settlements within the 3- and 4-km buffers from the centre of the leks.

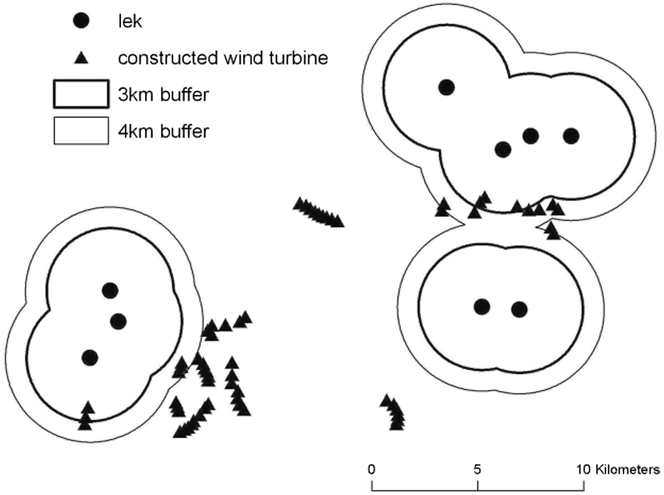
Figure 2. Distribution of the constructed wind turbines and Cantabrian Capercaillie leks with 4 km (narrow line) and 3 km (thick line) buffers around the leks in the study area.
Discussion
The peripheral population at the southern edge of the endangered Cantabrian Capercaillie distribution was affected by forest fragmentation. Cantabrian Capercaillie was detected in forest fragments significantly larger, closer to the next nearest occupied fragments, and with a lower degree of isolation compared to fragments in which no Capercaillie were detected. In our study area, the maximum distance to the nearest occupied fragment was 5.8 km which should not pose a threat to Capercaillie dispersal based on data from other populations (Segelbacher et al. Reference Segelbacher, Hoglund and Storch2003). At a regional scale in fragmented landscapes, the presence of Capercaillie mainly depends on the availability of suitable habitat where distances among fragments do not exceed average dispersal distances (Bollmann et al. Reference Bollmann, Graf and Suter2011). Our data supported previous studies in the Cantabrian range, showing that forest fragment size and connectivity are more important drivers of Capercaillie occupancy than forest type (Quevedo et al. Reference Quevedo, Banuelos and Obeso2006a,Reference Quevedo, Banuelos, Saez and Obesob). Thus, the peripheral population is vulnerable to forest fragmentation as it may affect negatively Cantabrian Capercaillie numbers within the study area. Habitat loss and fragmentation have been widely acknowledged contributing significantly to the decline of Cantabrian Capercaillie in the Euro-Siberian area (García et al. Reference García, Quevedo, Obeso and Abajo2005, Quevedo et al. Reference Quevedo, Banuelos and Obeso2006a,Reference Quevedo, Banuelos, Saez and Obesob).
The peripheral population studied is located in the Mediterranean area, which is characterised by a two-month period of drought during summer that increases the risk of fires compared to the more humid Euro-Siberian forests (Luis-Calabuig et al. Reference Luis-Calabuig, Calvo, Marcos, Valbuena and Trabaud2000, del Río et al. Reference del Río, Herrero and Penas2007). In addition, human-caused fires are required to enhance grasslands within the Mediterranean forest ecosystem. Between 2000 and 2009, prescribed fires burned 8.9% of the total forested surface area in our study area, of which 2.4% were forests where we detected Capercaillie, and the remaining 6.5% were good potential Capercaillie habitat. Consequently, prescribed fires may significantly reduce suitable habitat for Capercaillie and, thus, fire-exclusion policies should be developed in Cantabrian Capercaillie habitat. For example, the regional programme “Plan 42”, which aims to reduce fires in the area by providing environmental education pertaining to forest management, use of prescribed burns, and legal restrictions (García Reference García2002), should be applied at least in forests where we detected Cantabrian Capercaillie.
Our results showed that density and frequency of anthropogenic infrastructure (wind farm, road, off-road track, and human settlements) were high within the 4-km buffer zone around active Capercaillie leks. Roads can show negative effects in the Cantabrian Capercaillie habitat. In our study area, wind farms are rapidly being constructed (APECYL 2010) and are associated with habitat destruction and fragmentation due to accompanying infrastructure, roads, and tracks (de Lucas et al. Reference de Lucas, Guyonne and Ferrer2007, Bright et al. Reference Bright, Lanyston, Bullman, Evans, Gardner and Pearce-Higgins2008). Moreover, wind farms and associated infrastructure constitute barriers to movement in grouse and other related galliforms. Our study area is not highly humanised in comparison to other Capercaillie habitat in Western Europe except for wind farms (and particularly newly forecast ones). No well-documented evidence exists that wind farms constitute barriers to movement for grouse. It is a working hypothesis which needs further investigation. For example, Grünschachner-Berger and Kainer (Reference Grünschachner-Berger and Kainer2011) found a negative effect of wind farms on Black Grouse Tetrao tetrix in Austria. Capercaillie are considered to be particularly sensitive to wind farms due to disturbance displacement, collision, and habitat loss and damage (Langston and Pullan Reference Langston and Pullan2003). Both the other closely related grouse species as well as Capercaillie have been negatively impacted by wind energy development with effects ranging from pronounced population declines to avoidance behaviour (Pruett et al. Reference Pruett, Patten and Wolfe2009, González and Ena Reference González and Ena2011, González et al. Reference González, García-Tejero, Wengert and Fuertes2015). Therefore, wind farm development should be halted within the current and historical distribution of the Cantabrian Capercaillie in the Mediterranean area to reduce habitat loss, fragmentation, and disturbance.
Our study shows therefore that the peripheral population of the endangered Cantabrian Capercaillie in the Mediterranean area remains vulnerable to the human disturbance explained above (forest fragmentation, risk fires and human infrastructure). These human disturbances can be mitigated if the habitat of the peripheral population studied is protected. The Mediterranean area described here (∼ 10% of the entire distribution of this subspecies; Quevedo et al. Reference Quevedo, Banuelos and Obeso2006a) is mostly lacking any specific protection while the well-known Euro-Siberian habitat of the Cantabrian Capercaillie is entirely within the Natura 2000 network (Sundseth and Creed Reference Sundseth and Creed2008, BOCyL 2009). The regional Cantabrian Capercaillie Recovery Plan (BOCyL 2009) suggests including within the Natura 2000 network any area where this grouse occurs, but currently the plan is failing to achieve its basic purpose of protecting the entire distribution of the Cantabrian Capercaillie. A priority conservation measure should therefore be to include our study area within the Natura 2000 network. This would also preserve the valuable genetic resource of the peripheral population of the Cantabrian Capercaillie, which has a relevant role maintaining genetic contact with the northern Cantabrian Capercaillie (Alda et al. Reference Alda, González, Olea, Ena, Godinho and Drovetski2013). Moreover, if our study area is included within the Natura 2000 network, reforestation programs of Pyrenean oak forests could easily be developed in order to spread out southwards increasing both habitat availability and forest connectivity and reducing fire risk relative to Pine forests. Additional conservation measures should include the establishment of a permanent monitoring programme for Cantabrian Capercaillie population trends and distribution, and the control of anthropogenic activities wherever Capercaillie populations exist. Although we consider this habitat as a vast departure from its natural state and highly modified by human activities, to achieve an efficient reserve network for the Cantabrian Capercaillie the area here described should be promptly protected.
Acknowledgements
We specially thank Virginia Winder and Rolando Rodríguez for their comments. Ramón Balaguer, Luis Robles, Alvaro Ortiz, Jose Manuel Castro and Fernando Gonzalo for their field work assistance and Carlos González-Antón for adequate legal advertisements; Miguel de Gabriel, Emilio de la Calzada, and PDC (Plataforma para la Defensa de la Cordillera Cantábrica) for providing data on wind farms; Pedro García-Trapiello for working with the local media on Capercaillie and wind farm conflicts, and Isabel Muñoz and Juan Carlos González from the Junta de Castilla y León who helped us with fire data. This manuscript was improved by constructive comments from Dr Marc Montadert and an anonymous referee. This work was supported by the Universidad de León Pre-Doctoral Programme 2007–2012.







