Introduction
The Caribbean region, with over 700 islands, constitutes one of 35 currently recognised “biodiversity hotspots”, containing 2.3% of globally endemic plants and 2.8% of globally endemic vertebrates, many of them highly threatened through habitat loss and other factors (Myers et al. Reference Myers, Mittermeier, Mittermeier, Da Fonseca and Kent2000, Brooks et al. Reference Brooks, Mittermeier, Mittermeier, Da Fonseca, Rylands, Konstant, Flick, Pilgrim, Oldfield, Magin and Hilton‐Taylor2002). The Bahamas archipelago forms the northernmost component of this hotspot; its low-lying islands (highest point 63 m) are home to a total of 375 bird species, of which seven are endemic (BirdLife International 2022). Of these seven only three, Bahama Hummingbird Nesophlox evelynae, Lyre-tailed Hummingbird N. lyrura, and Bahama Yellowthroat Geothlypis rostrata, are listed as having a secure conservation status, i.e. IUCN category Least Concern (BirdLife International 2022). Three others are more exposed to extinction risk: in 2018 Bahama Warbler Setophaga flavescens was listed as Near Threatened while both Bahama Oriole Icterus northropi and Bahama Nuthatch Sitta insularis were listed as Critically Endangered (BirdLife International 2022). Moreover, Bahama Swallow Tachycineta cyaneoviridis, which breeds only in the Bahamas but has a wider range in winter, was also then listed as Endangered, while Brace’s Emerald Chlorostilbon bracei represents the country’s one documented global avian extinction to date (Hume and Walters Reference Hume and Walters2012, BirdLife International 2022). Thus more than half the endemic birds of the Bahamas are judged in danger of global extinction, although there has been little international engagement to help remedy the situation. Moreover, despite the critical importance of species-specific biological and ecological studies, particularly concerning habitat selection and breeding success, in establishing an evidence base for the effective management of populations, only two of these endemics – the oriole (seven peer-reviewed papers on Google Scholar) and the swallow (three papers) – have been the target of such studies.
Each of these endemic species has a unique range within the Bahamas, but three of them, the warbler, nuthatch, and swallow, inhabit the island of Grand Bahama at the north-west end of the archipelago. The Bahama Warbler, which otherwise only occurs on Abaco, was treated as a subspecies of the continental Yellow-throated Warbler Setophaga dominica until relatively recently, but is distinctive in both morphology and voice (McKay et al. Reference McKay, Reynolds, Hayes and Lee2010, del Hoyo and Collar Reference del Hoyo and Collar2016): with its notably elongated bill, it is adapted in part to forage for invertebrates on tree trunks in the manner of a creeper (family Certhiidae), a behaviour rarely noted in the species from which it has been split (Hayes et al. Reference Hayes, Hayes and Spencer2008, White Reference White2011, pers. obs.). Thus it has been characterised as a bark, twig, needle, and pine-twig gleaner, both feeding and breeding in Caribbean Pine forest, occurring on trunks and in the overstorey (Emlen Reference Emlen1977, McKay et al. Reference McKay, Reynolds, Hayes and Lee2010).
There is no information that its historical range was different from present, but its population is thought to have decreased considerably since the 1970s (Lloyd and Slater Reference Lloyd and Slater2011). The most recent evidence on its conservation status dates from a survey in Grand Bahama in April 2007, which produced a population estimate of 2,116 (CI: 1,239–3,614) individuals (Lloyd and Slater Reference Lloyd and Slater2011). Based on the same survey’s results McKay et al. (Reference McKay, Reynolds, Hayes and Lee2010) estimated an approximate global Bahama Warbler population of 3,150–3,500 individuals, given a maximum of a 1,000 km2 of remaining forest left in Grand Bahama and Abaco combined (Hayes et al. Reference Hayes, Hayes and Spencer2008).
The dominant vegetation type of Grand Bahama is native pine forest, which mostly comprises Caribbean Pine Pinus caribaea var. bahamensis and forms a key habitat for the endemic Bahamian avifauna (Lloyd and Slater Reference Lloyd and Slater2011). The understorey is commonly characterised by Thatch Palms Thrinax radiata, which are strikingly distinctive in shape, size, and structure, and are evidently used by a number of bird species for nesting (Lloyd and Slater Reference Lloyd and Slater2011, Price et al. Reference Price, Lee and Hayes2011). However, this forest has been under threat from urban development, human-induced fires, logging, and the increasing frequency and severity of hurricanes (Myers et al. Reference Myers, Wade and Bergh2004, Lloyd et al. Reference Lloyd, Slater and Metcalf2008). In October 2016, Hurricane Matthew (category 5) struck Grand Bahama directly, and towns in the east of the island (e.g. Eight-mile Rock and Holmes Rock) sustained severe damage, roads became impassable, and forest trees were devastated by winds up to 220 km/h (Stewart Reference Stewart2017). Prompted by this extreme event, we aimed to clarify the conservation status of the Bahama Warbler on Grand Bahama in 2018 by conducting a comprehensive survey to update its distribution and to research its habitat preferences, particularly in relation to hurricane damage to forests.
Methods
Study area
Grand Bahama island is approximately 153 km long (west to east) and 24 km at its widest covering an area of 1,373 km2; it is the fourth largest island in the Bahamas. In addition to the native Caribbean Pine species, the non-native She-oak Casuarina equisetifolia and C. glauca make up the remainder of the conifer/conifer-like tree species; they were introduced on land west of Freeport during the nineteenth century and have since spread to coastal areas across the island (Sykes and Clench Reference Sykes and Clench1998, pers. obs.). Grand Bahama has a subtropical climate and commonly experiences extreme natural events in the form of hurricanes, which occur seasonally from June to November. It has, however, suffered several particularly destructive hurricanes in recent years, notably Frances (category 4) and Jeanne (category 3) in 2004 (Lloyd and Slater Reference Lloyd and Slater2011), Matthew (category 5) in 2016 (Stewart Reference Stewart2017), and Dorian (category 5) in 2019 (Avila et al. 2019), 15 months after our fieldwork (see Discussion). After Matthew, sections of Caribbean Pine, particularly in the north of the Lucayan Estates, were badly damaged by strong winds followed by saltwater incursion (E. Weir, pers. comm.). The research was carried out on the island by DJP and MAG in April, May, and June 2018, which is the breeding season for birds on the island (Emlen Reference Emlen1977, Lloyd and Slater Reference Lloyd and Slater2011). There is no evidence that the Bahama Warbler uses different habitats outside this season.
Point-count transects with playback
Two observers, working as a pair, conducted two separate studies focusing on the Bahama Warbler (present study) and Bahama Nuthatch (Gardner et al. Reference Gardner, Pereira, Collar and Bellin prep.). Each observer surveyed his own count station alone, gathering data using the same methods. Delayed slightly by poor weather and resulting transport problems, 116 transects in total were conducted throughout the island of Grand Bahama. This resulted in 464 points surveyed once between 19 April and 26 June 2018. Transect locations were selected by laying a 1 km2 grid over a 2,000 forest cover map of Grand Bahama (Hansen et al. Reference Hansen, Potapov, Moore, Hancher, Turubanova, Tyukavina, Thau, Stehman, Goetz, Loveland and Kommareddy2013), which was the most recent available at the time. A random-point generator was used to distribute transects on the grid squares, and sites were accessed via old logging tracks.
Birds were counted using point counts spaced along an 800-m transect (Figure 1). Each transect was surveyed only once during the survey period. At the start of each transect, the observers began from “point zero” (usually the vehicle), each walking 100 m in the opposite direction to the other along the logging track, to ensure all count stations were separated by 200 m, regarded as the minimum distance required to achieve sample independence (Bibby et al. Reference Bibby, Jones and Marsden1998). Each observer then walked 100 m from the logging track into the forest to start the first transect, stopped to conduct a 10-minute point count followed by a habitat survey, and retraced steps to walk 100 m into the forest on the opposite side of the track for a second point count, beginning the second transect. Both observers then returned to the main path and walked a further 200 m in their initial directions, to reach a second pair of count stations and applied the same protocol. A previous survey protocol was followed (Hayes et al. Reference Hayes, Barry, McKenzie and Barry2004), and surveys were conducted primarily in the morning, from 06h45 to 12h00, but occasionally continuing to 14h00 if bird activity persisted under overcast skies. Surveys were not undertaken during inclement weather or when transport access problems occurred (6/48 days).

Figure 1. Diagram of point-count transects method used in this study. Top and bottom group of four points were each a separate transect.
Playback recordings of songs of Bahama Warblers obtained from xeno-canto (https://www.xeno-canto.org/) and of the “rubber ducky” call (see Boesman and Collar Reference Boesman and Collar2020) of Bahama Nuthatch kindly provided by J. Lloyd and G. Slater were spliced into a 10-minute playback sequence using “Soundtrap”, consisting of the following: two minutes silence, one minute warbler songs, one minute silence, one minute warbler songs, one minute silence, one minute nuthatch calls, one minute silence, one minute nuthatch calls, one minute silence. All bird species seen or heard were recorded during the 10-minute period, including sex and age when these were possible to identify visually.
Habitat surveys
Following each bird count, the observer remained at the point and delineated a habitat survey plot using three foliated trees (or another plant when a foliated tree was not available) ~15 m away as reference points. They faced the main path and selected a trunk at eye level, turned 90° to the right to select a second trunk, and chose a third tree at 45° between the first two, thereby creating a quadrant plot in which to record habitat characteristics. Data for calculating the heights of the three trees were obtained by rangefinder hypotenuse readings fed into a modified version of the Pythagoras theorem formula allowing for observer eye-level height:
where a = tree height, 1.5 m = observer eye-level height, c = distance from count station to tree crown (i.e. hypotenuse), and b = distance from count station to tree trunk, e.g. 1.5 + (√(20² – 15²)) = 14.7 m. Girth of the same three trees was tape-measured and averaged. The number of dead tree trunks (upright trunks without crown, needles, or branches; hereafter “snags”) in the plot was counted. All living trees present within the plot were also counted and allocated to four classifications: 1 = foliated mature: trees ≥8 m in height, with branches and needles, 2 = needleless mature: trees ≥8 m in height, with branches but without needles; 3 = foliated young: trees 1–8 m in height, with branches and needles; 4 = needleless young: trees 1–8 m in height, with branches but without needles. Understorey height was measured based on the tallest foliated understorey plant present within the plot (excluding trees and Thatch Palms, which were measured separately; see below) and allocated to two categories: 1 = <140 cm; 2 = >140 cm. Understorey characteristics were allocated to seven categories based on presence/absence and abundance of ferns or plant species such as Poisonwood Metopium toxiferum plus overall ground coverage: 1 = >50% bare ground, understorey height <1 m, Poisonwood absent; 2 = <50% bare ground, understorey height <1 m, Poisonwood absent; 3 = same as 2 but ferns present; 4 = same as 3 but small Poisonwood (<1m) present; 5 = same as 4 but large Poisonwood (>1m) present; 6 = same as 5 but understorey height >1 m; 7 = same as 6 but access to targeted count station unfeasible timewise due to dense vegetation (only two such points; data collected where approach halted). Thatch Palms, as potentially important nesting substrates for warblers, were tape-measured and allocated to the same categories as understorey height. Wind damage to trees was assigned to three categories based on Rodgers and Gamble (Reference Rodgers and Gamble2008): 0 = no damage; 1 = one uprooted tree and/or multiple trees with snapped twigs and broken limbs; 2 = multiple tree trunks snapped in the same direction and/or multiple uprooted trees. Fire disturbance was divided into five categories and determined by the presence of burn signs on trees and flora, based on fire ecology studies (Barlow et al. Reference Barlow, Lagan and Peres2003, Gleason and Nolin Reference Gleason and Nolin2016): 0 = no burn signs; 1 = charred debris on forest floor, char marks on tree trunks, vegetation height >100 cm, Poisonwood present; 2 = same as 1 but vegetation height <100 cm; 3 = same as 2 but Poisonwood absent; 4 = same as 3 but ash throughout forest floor.
Statistical analyses
All statistical analyses were conducted using R (R Core Team 2021 version 4.1.2) unless otherwise stated. Binary logistic regression analysis was used with warbler presence/absence as the product of the independent variable. Elevation, number of foliated mature trees per plot, number of foliated young trees per plot, number of needleless mature trees per plot, number of needleless young trees per plot, number of snags within plot, average tree height per plot, average girth per plot, understorey characteristics categories (1–7), understorey height categories (1–2), Thatch Palm height categories (1–2), wind damage categories (0–2), and fire disturbance categories (0–4) were used in the analysis as independent variables, whilst transects were included in the model as random factors. We used a backwards stepwise approach to select the model that best explained Bahama Warbler presence and absence. Using a correlation matrix, we detected multi-collinearity between fire disturbance and understorey density (r = −0.81, n = 464, P <0.001), fire disturbance and understorey height (r = −0.53, n = 464, P <0.001), understorey height and understorey density (r = 0.62, n = 464, P <0.001), as well as between average girth per plot and average tree heights per plot (r = 0.54, n = 464, P <0.001). Starting with all candidate variables except understorey density and understorey height (since these were explained by fire disturbance), and average tree heights per plot, we excluded all non-significant variables (using a threshold of P <0.05) and refitted the model. We repeated this process with subsequent models until all variables had a significant effect on Bahama Warbler presence. Based on Hegyi and Garamszegi (Reference Hegyi and Garamszegi2011), we reintegrated each previously removed variable to the model one by one. We repeated the process of reintegrating all non-significant variables to the final model, but none had a significant effect on warbler presence. We inspected model residuals to evaluate the model. We calculated odds ratios (OR) to make comparisons between the model’s categorical variable levels.
To compare habitat differences between plots in Lucayan Estates and East End (where warblers were recorded) with West End and Freeport (where they were not), we combined warbler presence from Lucayan Estates and East End and compared the habitat within these plots (n = 209) with those in West End and Freeport, which only contained warbler absences (n = 20). Differences in the three predictors from the stepwise model were compared using a two-sample t-test for the continuous variable and, given the disparity between both regions’ sample sizes, we used observed frequencies within each level of categorical variables to calculate the representation percentage within both regions, taking into account the total plots surveyed within each region (e.g. if 30/209 plots in Lucayan Estates + East End had shorter palms, 14% of surveyed plots had shorter palms; if 3/20 West End + Freeport surveyed plots had shorter palms, 14% of surveyed plots had shorter palms).
Results
A total of 464 points were surveyed and 209 warbler presences (i.e. each presence consisting of one or more warblers detected during a count) were recorded, 146 (70%) of them in Lucayan Estates and 63 (30%) in East End. A total of 233 warblers (71%) were recorded in Lucayan Estates and the remainder 94 warblers (29%) were recorded in East End. A total of 255 warbler absences was recorded, 174 (68%) in Lucayan Estates, 61 (24%) in East End, 8 (3%) in West End, and 12 (4.7%) in Freeport (Figure 2A, B, and C).

Figure 2. Bahama Warbler absences and presences recorded during the 2018 point-count survey in Grand Bahama. A: a full map of warbler absences and presences on Grand Bahama; B: zoomed-in locations of warbler absences and presences in Lucayan Estates; C: zoomed-in locations of warbler absences and presences in East End.
Our final model (χ2 = 30.826, df = 8, P = 0.0002; AIC = 570) (Table 1) (Figures 3, 4, and 5) used three of our variables to explain warbler presence and absence. Warblers were more likely to be present in transects with fewer needleless mature trees (Figure 3; β = −0.15 ± 0.06, P = 0.007), taller Thatch Palms (Figure 4; β = 0.75 ± 0.34, P = 0.029), and some burnt vegetation (Figure 5; category 2 [β = 2.5 ± 1.03, P = 0.015], category 3 [β = 2.25 ± 1.02, P = 0.028], category 4 [β = 2.62 ± 1.14, P = 0.022]).
Table 1. Stepwise regression model’s best predictor variables explaining the likelihood of warbler presence.


Figure 3. The relationship between the probability of Bahama Warbler presence and needleless mature trees.
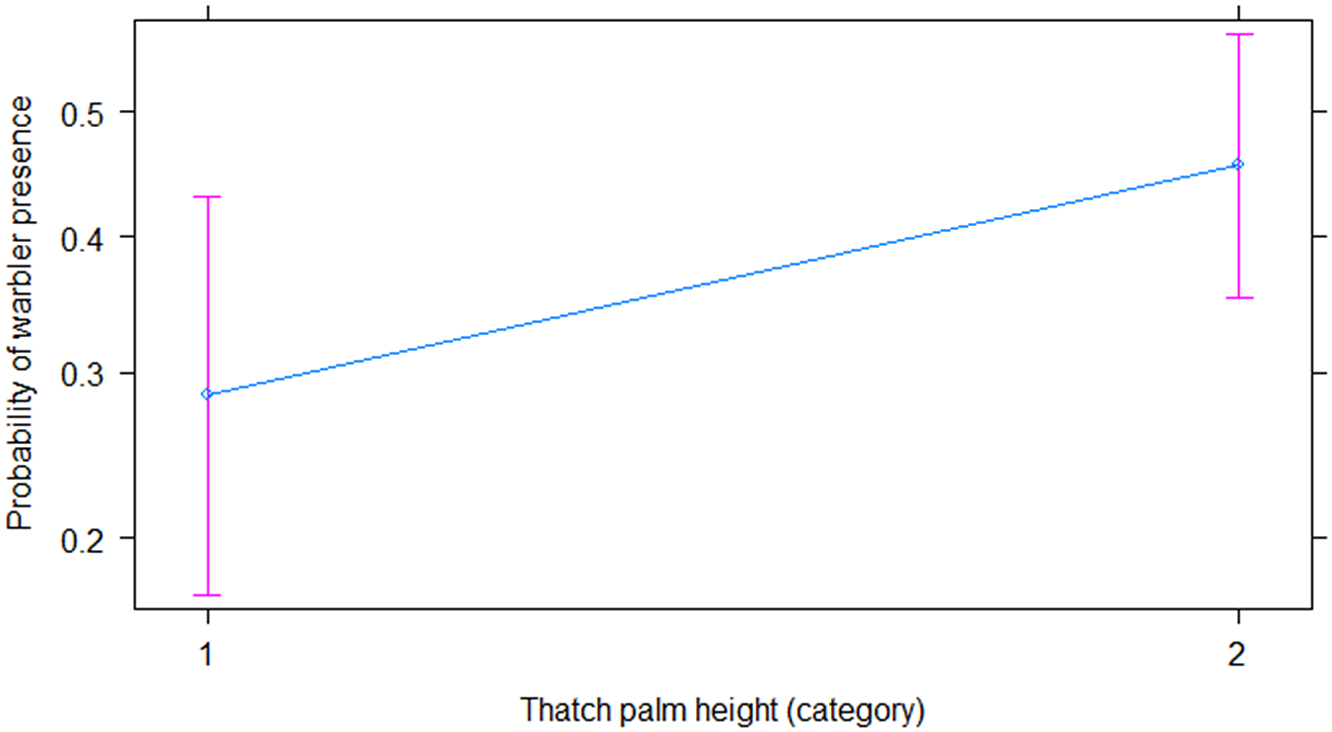
Figure 4. The relationship between the probability of Bahama Warbler presence and Thatch Palm height.

Figure 5. The relationship between the probability of Bahama Warbler presence and fire disturbance.
Warblers were more likely to be found in Thatch Palm heights category 2 (OR = 0.86, CI = [1.08, 4.18]) than in category 1. They were also more likely to be found in plots with some fire disturbance rather than none (category 1 OR = 12.18, CI = [0.61, 45.95]; category 2 OR = 9.50, CI = [1.82, 118.18]; category 3 OR = 13.67, CI = [1.45, 91.49]; category 4 OR = 0.05, CI = [1.62, 160.20]).
The number of needleless mature trees was not significantly different in the Lucayan Estates and East End (t = −0.23, df = 24.775, P = 0.8) than in West End and Freeport. However, Lucayan Estates and East End held a higher proportion of taller Thatch Palms than West End and Freeport (81% vs. 50%). Moreover, Lucayan Estates and East End held a lower proportion of points in the “no fire disturbance category” (1% vs. 15%) and a lower proportion in the “severe fire disturbance” category (7% vs. 10%) than West End and Freeport. Additionally, Lucayan Estates and East End held a higher proportion of points within two of the three middle fire disturbance categories than West End and Freeport (category 1 [5% vs. 5%]; category 2 [38% vs. 30%]; category 3 [48% vs. 40%]).
Discussion
The distribution of the Bahama Warbler on Grand Bahama in 2018 – restricted to the Lucayan Estates and East End – is a clear reflection of its preference for relatively intact, mature pine forest, a condition indicated by the presence of mature Thatch Palms in the understorey, no or few needleless trees, and some disturbance from fire. Such a preference is predictable, given that mature Caribbean Pine forest was the dominant vegetation across Grand Bahama in previous centuries (March Reference March1949, Bounds Reference Bounds1968), and that the pine’s fire regime involves frequent (every 1–10 years) but low-intensity (“cool”) surface (not tree crown) fires occurring either naturally or by prescription, as in Abaco, where according to Myers et al. (Reference Myers, Wade and Bergh2004) it is also needed to maintain the island’s biodiversity. Nevertheless, it indicates a degree of adaptation by the warbler which should be factored into assessments of its conservation status and management requirements.
As our primary fieldwork concern was to find the Bahama Nuthatch, we reduced our sampling effort to just 4% combined in the visibly poor-quality forests in West End and Freeport. By contrast, we increased effort to 27% in East End and 69% in the Lucayan Estates, where the nuthatch had last been seen. Consequently, it is impossible to demonstrate a statistically robust difference in the warbler’s choice of area. Nevertheless, the narrower girths and lower heights of pine trees and greater preponderance of small Thatch Palms and wind-damaged trees in West End and Freeport, where Emlen (Reference Emlen1977) found both nuthatch and warbler, indicate a degradation of habitat in the past half-century that explains the absence of records of both species in those areas in 2018. Perhaps the single most compelling reason for warblers to use areas of mature forest is that it provides food that is less energetically expensive to obtain, given that wider and taller trees offer a larger foraging substrate per unit area (Airola and Barrett Reference Airola and Barrett1985). Moreover, such trees have more fissured bark, which presumably provides habitat for greater numbers and varieties of invertebrates. They are also likely to withstand and survive fire events due to their higher moisture content and thicker bark (Hare Reference Hare1965, VanderWeide and Hartnett Reference VanderWeide and Hartnett2011).
Despite their name, “cool” fires can still damage the bark of old trees, thereby increasing their susceptibility to insect infestation (Dajoz Reference Dajoz2000, Parker et al. Reference Parker, Clancy and Mathiasen2006, Santolamazza-Carbone et al. Reference Santolamazza-Carbone, Pestana and Vega2011), hence the attractiveness of burnt areas to the warblers. In 2018 Bahama Warblers were more likely to be present in plots with some fire disturbance (categories 1–4, none more preferred than others) rather than none (category 0); observers (DJP, MAG) repeatedly noticed that warblers foraging on trunks often probed under burnt peeling bark. These pieces of evidence suggest a good fit between the warbler’s foraging ecology and the standard fire regime in its climax pine forest habitat.
This fit extends to Thatch Palms, which also have strong fire-resistant properties (Bergh and Wisby Reference Bergh and Wisby1996). During the 2018 surveys, aside from Caribbean Pine, Thatch Palms were the only surviving flora in severely burnt areas, even though they too showed burn signs. The presence of warblers was significantly predicted by the presence of taller Thatch Palms, while comparisons between occupied and unoccupied regions showed that shorter palms were dominant in unoccupied regions. It therefore seems likely that taller palms are more capable of surviving intense fires than shorter ones, and that taller (i.e. mature) palms provide a larger substrate on which warblers can forage and which presumably also sustain a higher abundance of arthropods. In 2018, Bahama Warblers were observed both foraging for invertebrates on the leaves and removing nest material from the trunks of Thatch Palms.
A powerful predictor of warbler absence is, unsurprisingly, related to hurricane impacts. Sites with a higher proportion of needleless mature trees failed to hold the species, presumably because they provide poor foraging opportunities and cover. This latter circumstance is produced not by the direct impact of the wind but by the wind-driven incursion of saltwater (“storm surge”) on to and below the forest floor (Walker Reference Walker1991). The salt increases osmotic tension in soil water, preventing water uptake by trees and in severe situations even drawing water from them, thus rapidly desiccating them. Consequently, pine trees lose their needles, resulting in a loss of food and cover for invertebrates, which in turn diminishes the presence of insectivores such as warblers.
A major impact of hurricanes on any bird species in their path will, inevitably, involve direct mortality. It can reasonably be assumed that Hurricane Matthew, which struck Grand Bahama only 18 months before our 2018 survey began, killed a significant proportion of the Bahama Warblers on the island, and it is possible that our findings on the bird’s preferences largely reflect the habitat that provided the best shelter. However, the subsequent failure of the species to recolonise habitats from which it may have been swept away by Matthew indicates that such preferences are not necessarily unrepresentative of its overall requirements. Even so, it is difficult to apply such speculation to the earlier circumstance in which surveyors in April 2007 found 21 warblers in 46 transects and considered the species “far less abundant than reported by Emlen (Reference Emlen1977)” with a small population and a patchy distribution (Lloyd and Slater Reference Lloyd and Slater2011): Hurricane Wilma struck Grand Bahama 18 months before the Lloyd and Slater (Reference Lloyd and Slater2011) survey, but unlike Matthew it left the centre and east of the island intact (World Meteorological Organization 2006). The apparent great decline in abundance since Emlen’s work requires further research to find an explanation, but we would very tentatively judge that our 2018 findings, which produced proportionately higher numbers of birds per point count than the 2007 transects, presumably owing to the use of playback, reasonably match and possibly surpass the abundance level found by Lloyd and Slater (Reference Lloyd and Slater2011).
Whatever the circumstance, on 2018 evidence the central focus of any programme or plan for the long-term survival of the Bahama Warbler must be the preservation of the oldest, tallest pine stands and their full complement of mature Thatch Palms on both Grand Bahama and Abaco, and the adaptive management of their fire regimes (Myers et al. Reference Myers, Wade and Bergh2004). Just as important will be the restoration over several decades of many currently degraded pine stands in order to create a broad geographical spread of habitat refugia, which will improve the chances of populations surviving in the event of further hurricane strikes.
These simple prescriptions might seem relatively straightforward to implement, but there are inevitably serious obstacles. The first is the increasing anthropogenic degradation of forests, mainly through commercial development, which Hayes et al. (Reference Hayes, Barry, McKenzie and Barry2004) and Lloyd and Slater (Reference Lloyd and Slater2011) long ago cited as a threat to the integrity, size, and ecological function of the pine forests in Grand Bahama. Another issue however is the fly-tipping of old household goods in the forest, creating potential sources of fire, as well as shelter for alien invasive animals. The second is the presence on Grand Bahama of precisely such introduced animals, among which feral cats would predate adult warblers, while Raccoons Procyon lotor and Corn Snakes Elaphe guttata would predate their nests; the Corn Snake in particular could easily represent a largely unrecognised but highly significant threat (wider discussion in Gardner et al. in prep.). The third is hurricanes, particularly when working in combination with the two preceding sources of concern (fragmented habitat is more exposed, and alien invasive species can multiply rapidly in the wake of serious habitat disruption), and particularly given the already fulfilled prediction of their increasing frequency and strength (Wiley and Wunderle Reference Wiley and Wunderle1993, Bender et al. Reference Bender, Knutson, Tuleya, Sirutis, Vecchi, Garner and Held2010).
Fifteen months after our fieldwork ended, Hurricane Dorian (category 5) devastated Grand Bahama with winds of 295 km/h for over 24 hours, creating such human misery and economic damage that three years later the situation of the island’s wildlife remains unclear. It is possible that Grand Bahama’s entire population of Bahama Warblers was wiped out, but the only other population of the species, on Abaco, has survived in the south of the island, where much forest remained standing (G. Wallace pers. comm. 2021); the status of the species has since been updated to Endangered (BirdLife International 2022). Further surveys are required to determine both the current status of Bahama Warbler on both islands and the extent to which hurricane damage has reduced habitat availability. A survey of the warbler on Abaco should aim to assess numbers, confirm habitat preferences similar to those on Grand Bahama in 2018, and review the dangers of anthropogenic factors such as disturbance, rubbish, roads, and fire that might need addressing by management. Analysis of satellite imagery (see methods used on Andros by Antalffy et al. Reference Antalffy, Rowley, Johnson, Cant-Woodside, Freid, Omland and Fagan2021) and ground surveys are needed as a matter of great urgency in order to identify the management options for restoring and preserving forests in pine-dominated islands across the Bahamas as economic, recreational, and natural heritage resources and as part of the overall resilience of the islands to the future effects of hurricanes like Dorian.
Acknowledgements
We are most grateful to Thrigby Hall Wildlife Gardens and the Sir Philip Reckitt Educational Trust for financially supporting this research, the Bahamas National Trust (BNT) for their assistance and encouragement, Ellsworth Weir, David Clare, Jinnel Sturridge, Lisa Wildgoose, and the other staff and volunteers of the Rand Nature Centre for their invaluable help, knowledge, and friendship, David Wege and BirdLife International for various forms of assistance, Zeko Mackenzie, John Lloyd, and Gary Slater for sharing their nuthatch survey data (via David Wege), and the people of Grand Bahama for their assistance, advice, and warm hospitality. Two referees significantly improved the paper with their comments.








