Introduction
The Little Bustard Tetrax tetrax is a Palearctic, ground-nesting, medium-sized grassland bird (Cramp and Simmons Reference Cramp and Simmons1980). It is listed as ‘Near Threatened’ globally and as a vulnerable species in Europe (BirdLife International 2016) due to a dramatic population decline in recent years. Agricultural intensification, habitat loss and degradation across the species’ geographical range are thought to be the main factors depleting its populations (e.g. Silva et al. Reference Silva, Pinto and Palmeirim2004, García de la Morena et al. Reference García de la Morena, Morales, De Juana and Suárez2007, Suárez-Seoane et al. Reference Suárez-Seoane, García de la Morena, Morales Prieto, Osborne and De Juana2008, De Juana Reference De Juana2009, Delgado et al. Reference Delgado, Morales, Traba and Garcia De La Morena2009, Iñigo and Borov Reference Iñigo and Borov2010).
Two widely separated breeding populations are recognized: the western population is found across Morocco, Spain, Portugal, France and Italy and the eastern population from China to Ukraine and Iran (Iñigo and Borov Reference Iñigo and Borov2010). In contrast to the western European populations which are mostly short-distance migrant birds (García de la Morena et al. Reference García de la Morena, Morales, Bota, Silva, Ponjoan, Suárez, Mañosa and Juana2015), except for the French Atlantic populations, the eastern population is fully migratory (Cramp and Simmons Reference Cramp and Simmons1980), wintering in the Caucasus, Azerbaijan and Iran (Gauger Reference Gauger2007). Recent Little Bustard winter counts in these wintering sites highlight the importance of the Iran/Azerbaijan region as a wintering site (Gauger Reference Gauger2007, Sehhatisabet et al. Reference Sehhatisabet, Abdi, Ashoori, Khaleghizadeh, Khani, Rabiei and Shakiba2012), possibly comprising the large majority of the eastern population (Iñigo and Borov Reference Iñigo and Borov2010). Azerbaijan holds by far the highest known winter concentrations of Little Bustards in the world, with an estimated 150,000 birds found wintering in the region (Gauger Reference Gauger2007). This is a remarkable figure considering the world population of the Little Bustard is estimated at 260,000 individuals (Iñigo and Borov Reference Iñigo and Borov2010). While the western population is declining across most of its range (e.g. Inchausty and Bretagnolle Reference Inchausti and Bretagnolle2005, De Juana Reference De Juana2009), the eastern population until 2000 was described as recovering, significantly increasing its population size (Gauger Reference Gauger2007, Iñigo and Borov Reference Iñigo and Borov2010) and gaining international importance.
The population of Little Bustard that winters in Iran probably breeds in Kazakhstan and Russia (Gauger Reference Gauger2007, Aghayari-Samian et al. Reference Aghayari-Samian, Mousavi, Mahdizadeh and Khodaparast2014) as the species is also a wintering migrant in the areas immediately north of Iran (Sehhatisabet et al. Reference Sehhatisabet, Abdi, Ashoori, Khaleghizadeh, Khani, Rabiei and Shakiba2012). Every year between November and February, Iran harbours an important wintering population with an estimated size of 5,000–10,000 individuals (Sehhatisbet et al. Reference Sehhatisabet, Abdi, Ashoori, Khaleghizadeh, Khani, Rabiei and Shakiba2012). While alfalfa and other favourable agricultural crops for the species are expanding rapidly across northern parts of Iran and providing attractive winter habitat, hunting has been identified as a major threat (Sehhatisbet et al. Reference Sehhatisabet, Abdi, Ashoori, Khaleghizadeh, Khani, Rabiei and Shakiba2012).
The winter ecology of the Little Bustard has been extensively studied for the western populations. Here they were found mostly associated with cereal stubbles within the extensive cereal agricultural system (Silva et al. Reference Silva, Pinto and Palmeirim2004) and alfalfa in more intensified agricultural sites (García de la Morena et al. Reference García de la Morena, Morales, De Juana and Suárez2007). However, its habitat preferences in its eastern range are largely unknown, and the environmental factors driving wintering area selection are likely to differ. Since the Little Bustard is now greatly dependent on landscapes shaped by man (Iñigo and Borov Reference Iñigo and Borov2010), outlining adequate management and conservation actions is essential to ensure the preservation of the species. Hence, the objectives of this work are (1) to update wintering counts and assess population trends and range modification of the Little Bustard in northern Iran and (2) to understand the main environmental factors influencing its occurrence and predict areas with greater habitat suitability for the species during winter.
Material and methods
Data collection
Winter surveys were carried out during October–February in 2010–2015 in northern Iran. A first assessment of the species’ distribution and population size was made by Sehhatisabet et al. (Reference Sehhatisabet, Abdi, Ashoori, Khaleghizadeh, Khani, Rabiei and Shakiba2012). This earlier study, carried out between 2003 and 2010, identified the main wintering habitats used by the species in five northern Iranian provinces including the plains of Ardebil, Gilan, Mazandaran, Golestan and Khorasan-e-Razavi. In the present study we covered all Little Bustard areas previously identified by Sehhatisabet et al. Reference Sehhatisabet, Abdi, Ashoori, Khaleghizadeh, Khani, Rabiei and Shakiba2012 plus another 38 sites dominated by open agricultural landscapes that had not been surveyed in northern Iran. These sites were of variable size (mean = 2,136 ha, SD = ± 4,463) and surveyed by covering all available roads and tracks by car at slow speed and with regular stops to scan for Little Bustard flocks using binoculars. Overall we surveyed 49 sites covering the agricultural lands in the six northern provinces of Iran (Figure 1). We surveyed those sites annually that were occupied by Little Bustards. Each flock when counted in flight was carefully watched and we noted where it landed to avoid double counts.
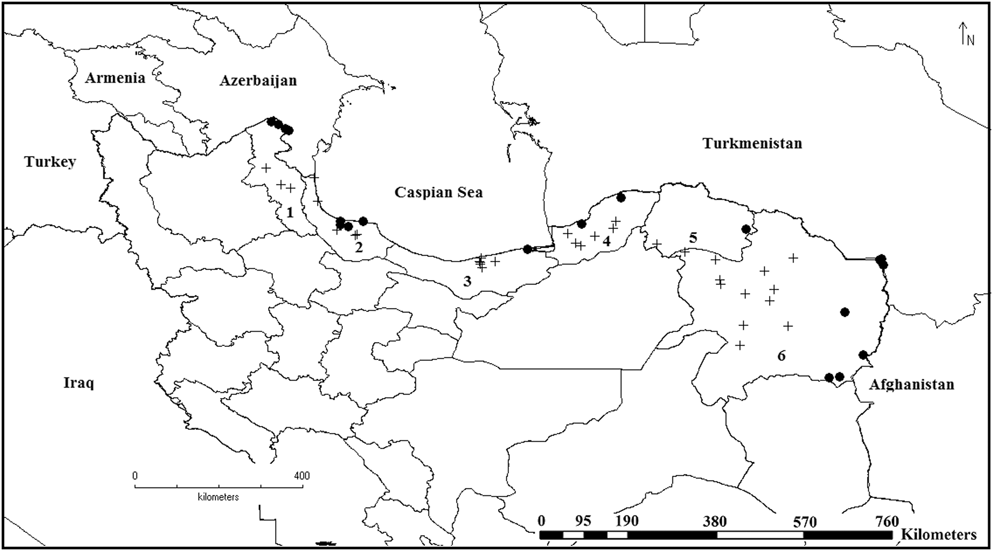
Figure 1. Presence records (dots) and unoccupied habitats (crosses) of the Little Bustard in northern Iran in winter. Ardebil province (1), Gilan province (2), Mazandaran province (3), Golestan province (4), Khorasan-e-Shomali province (Northern Khorasan) (5) and Khorasan-e Razavi province (6).
Predictor variables
To predict the suitable ranges for the Little Bustard across the northern parts of Iran, we used three categories of eco-geographic factors, including climate, topography and land-cover variables (Table 1) previously known to influence the species’ occurrence (Martínez Reference Martınez1994, Silva et al. Reference Silva, Pinto and Palmeirim2004, Reference Silva, Faria and Catry2007, Sehhatisabet et al. Reference Sehhatisabet, Abdi, Ashoori, Khaleghizadeh, Khani, Rabiei and Shakiba2012). All environmental variables were mapp at with 1 km2 (30 × 30 arcseconds) grid size.
Table 1. List of predictors variables used for modelling Little Bustard distribution in the north of Iran.
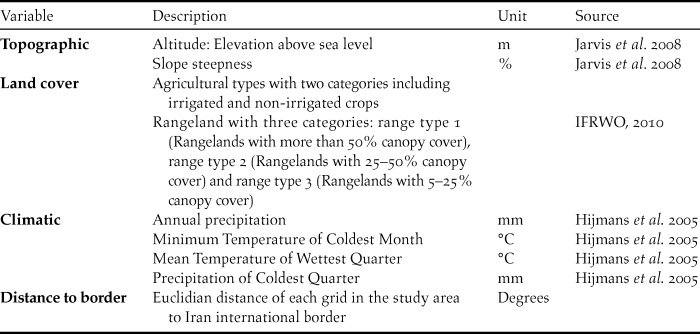
Climatic variables were obtained from the WorldClim database (Hijmans et al. Reference Hijmans, Cameron, Parra, Jones and Jarvis2005). This database consists of 19 climatic variables which result from the interpolation of data derived from climatic stations. Land cover data were obtained from the National Land Cover map (IFRWO 2016) based on the Landsat Enhanced Thematic Mapper Plus - ETM+ which consists of imagery for conterminous Iran in the year 2010. Using the Shuttle Radar Topography Mission (SRTM) elevation model, two topographic explanatory variables were compiled: altitude and slope.
We also used distance to the border in order to test our hypothesis that vicinity to an international border influences the distribution pattern of Little Bustard in Iran, as this relates to areas where hunting is forbidden and disturbance is possibly reduced. For this purpose, we calculated the Euclidian distance of each cell of the grid in the study area to the international border using ArcMap Spatial Analyst tools. The climatic variables were found highly correlated (Pearson > 0.70) and therefore to prevent multicollinearity we chose annual precipitation to run in the model (Tabachnick and Fidell Reference Tabachnick and Fidell1996) because this had most support from the literature (e.g. Delgado and Moreira Reference Delgado and Moreira2010). The remaining environmental variables (Table 1) showed lower correlation values. The pairwise correlations were calculated using ENMtools (Warren et al. Reference Warren, Glor and Turelli2010).
Data analysis
We used the maximum entropy algorithm (Phillips et al. Reference Phillips, Anderson and Schapire2006) to build a habitat suitability map for Little Bustard in an area of about 249,166 km2 in northern Iran. This algorithm can be used to predict the suitability of the study area for the species under a specified set of environmental constraints (Phillips et al. Reference Phillips, Anderson and Schapire2006) and is known to perform well even with limited presence data (Pearson et al. Reference Pearson, Raxworthy, Nakamura and Townsend Peterson2007). MaxEnt was set to run with a maximum of 1,000 iterations, convergence threshold of 0.0001 and 10,000 background points. Overall, 20 Little Bustard occurrences were recorded within the 1 km2 grid within 16 sites. We used these 20 occurrence points in MaxEnt analysis, 16 points for training and four points for test.
The performance of the model was assessed using the Area under the Curve (AUC) metric of a Receiving Operator Characteristic (ROC) (Phillips et al. Reference Phillips, Anderson and Schapire2006), which is considered adequate to assess the accuracy of SDMs (Fourcade et al. Reference Fourcade, Engler, Rodder and Second2014). MaxEnt plots all sensitivity values (true positives) against specificity (false positive) values and calculates the AUC to provide a threshold-independent metric of overall accuracy, ranging between 0.5 (no predictive ability or randomness) and 1.0 (perfect predictive ability). Variables contributing less than 1% to the model were sequentially removed until all variables entered in the model contributed over 1%. Models with AUC > 0.75 are considered adequate and > 0.90 are considered excellent (Swets Reference Swets1988, Elith Reference Elith, Ferson and Burgman2002). We used the 10-percentile training presence logistic threshold (Young et al. Reference Young, Carter and Evangelista2011), defined at 0.27, to convert the continuous suitability predictions into binary suitable/unsuitable maps.
To confirm MaxEnt outcomes, we additionally carried out a binomial Generalized Linear Model (GLM) with a logit link function. The 49 surveyed sites resulted in 16 presences and 33 absences. To reduce autocorrelation, one location was selected per site: for presence sites that recorded more than one flock the location was randomly selected; and for absence sites the location was chosen taking into account its habitat suitability for the species according to Sehhatisabet et al. (Reference Sehhatisabet, Abdi, Ashoori, Khaleghizadeh, Khani, Rabiei and Shakiba2012), principally the proportion of open area and presence of irrigation. To reduce collinearity, we eliminated all variables showing correlations over 0.7 (Tabachnick and Fidell Reference Tabachnick and Fidell1996) and selected the one of greater biological importance based on the literature (Silva et al. Reference Silva, Pinto and Palmeirim2004, Delgado and Moreira 2009). This reduced the number of variables to three: distance to border; land cover and annual precipitation. In the analysis, for the categorical variable land cover, we used irrigated crop as the reference category. We computed GLM models with all possible variable combinations, resulting in a total of seven models. Akaike’s information criterion adjusted to small data sets (AICc) was used for model selection (Burnham and Anderson Reference Burnham and Anderson2002). We defined our top concurrent models as those that fell within five AICc (Δ AICc < 5). We then used model averaging (Burnham and Anderson Reference Burnham and Anderson2002) of this best set of models to determine the relative importance of each parameter for explaining the variance. Analyses were performed in R (R Development Team 2016), using the MuMIn package (Bartón Reference Bartón2016).
Results
Winter counts
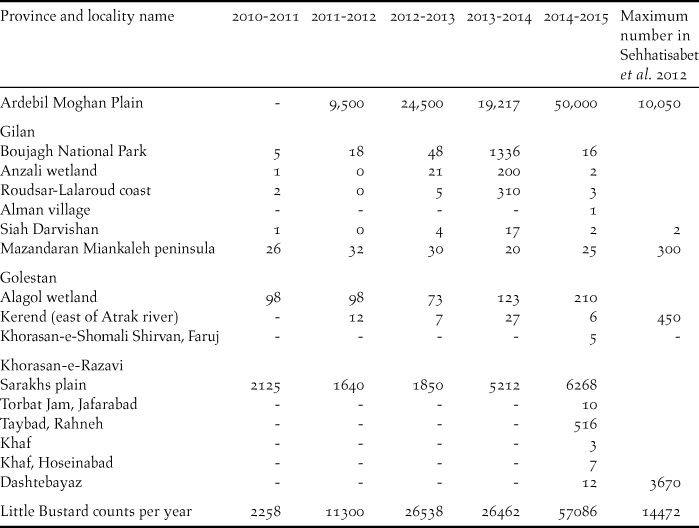
Over a five-year period, populations appeared to be increasing, with a count of 57,086 individuals recorded in the last survey year of 2014–2015 (Table 2). We obtained 20 Little Bustard records within 16 sites in Northern Iran (Figure 1), five of which correspond to new areas, where the species was not previously recorded: four new localities in Khorasan-e-Razavi province and one new locality in Khorasan-e-Shomali.
Table 2. Number of Little Bustard counted in each province along with maximum number of Little Bustards in each province counted by Sehhatisabet et al. (Reference Sehhatisabet, Abdi, Ashoori, Khaleghizadeh, Khani, Rabiei and Shakiba2012). “-“ stands for no data available and “0” means that no birds were counted at that giving site.
Habitat suitability
Results of habitat suitability modelling indicated that irrigated agricultural land in low-elevation plains in north, north-east and north-west Iran that are close to the international border provided the most suitable wintering habitats for Little Bustard (Figure 2). Unsuitable habitats were mostly located in the southern parts of study area as well as in areas far away from Iran’s international border with high elevation, as in Alborz and Kopet-Dagh Mountains, stretching to northern and eastern Iran.

Figure 2. Predicted suitable and unsuitable habitats for Little Bustard in Iran along with presence records (white dots).
Variable importance
The variables that most influenced the habitat suitability model were distance to border (44.6%) followed by land cover (25.8%), irrigated land and altitude (15.2%) (Appendix S1 in the online supplementary material). Response curves (Appendix S1) indicate that an increase in distance from the border reduces the habitat suitability for the Little Bustard. A similar pattern was also obtained for altitude, indicating that the Little Bustard prefers low elevation plains with predominantly agricultural landscapes. In contrast, there was a positive relationship between the species, probability of occurrence and minimum temperature of coldest month. This may suggest that areas with higher temperatures would be of greater suitability for the species during their wintering period in Iran. The modelling procedure also indicates that there is a clear preference for irrigated agricultural land. The overall predictive ability of the model (AUC = 0.971 for training and 0.971 for test data) showed high discriminatory capacity in determining suitable and unsuitable habitats.
As for the GLM models, four concurrent models had a ΔAICc < 5 and were retained (Table 3). Model averaging of these models then showed that there was a higher probability of occurrence of Little Bustard flocks next to the border, coinciding with areas with lower annual precipitation, selecting irrigated crops and avoiding areas with greater canopy area (Table 4). Distance to border was the most important variable explaining the variance (with the maximum importance of 1) followed by annual precipitation (0.74) and land cover (0.07). The GLM and model averaging procedure therefore corroborated the MaxEnt analysis, that the distance to the international border was the most important predictor in the variables we considered.
Table 3. AICc results for the combination of all models, also indicating ΔAICc.
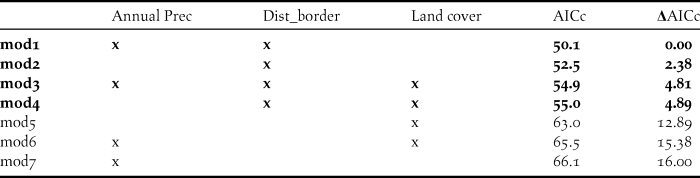
Table 4. Coefficients of the model averaging procedure, indicating the relative importance of the variables.

Discussion
Winter population and range increase in Iran
Sehhatisabet et al. (Reference Sehhatisabet, Abdi, Ashoori, Khaleghizadeh, Khani, Rabiei and Shakiba2012) surveyed probable Little Bustard habitats in northern Iran from 2003 to 2010 and confirmed its presence in 15 localities. Maximum counts in 2010 were of approximately 14,000 birds (Sehhatisabet et al. Reference Sehhatisabet, Abdi, Ashoori, Khaleghizadeh, Khani, Rabiei and Shakiba2012). Since then yearly counts have shown a steep increase (Table 2). Now maximum counts are of 57,086 which represents more than four times what was counted in 2010. Ardebil province alone registered in 2015 a total count of approximately 50,000 individuals which is five times more than the maximum wintering population estimated for the province in 2010 (Sehhatisabet et al. Reference Sehhatisabet, Abdi, Ashoori, Khaleghizadeh, Khani, Rabiei and Shakiba2012). The Little Bustard was found in five new locations, including two new regions: Khorasan-e-Razavi and Khorasan-e-Shomali, indicating that its winter range may also be expanding. Our work now updates the Little Bustard winter range and maximum counts in Iran.
Even though we cannot fully exclude the possibility of birds being double counted at different sites at different times, or that there might have been an increase in survey effort, our repeated counts at the different sites show a trend of increasing numbers.
Within the eastern population, the most important breeding Little Bustard populations coincide with the former Soviet Union (Iñigo & Borov Reference Iñigo and Borov2010). In the late 20th century, with the break-up of the former Soviet Union, vast areas of arable fields were abandoned and pristine steppe left ungrazed, resulting in a period of significant population increase for many steppe species (Kamp et al. Reference Kamp, Urazaliev, Donald and Hölzel2011), including the Little Bustard (Gauger Reference Gauger2007). This possibly also led to a population increase in their wintering range. However, since 2000 there has been an intensification of agricultural and pastoral systems, which will tend to increase with the recent reclamation of abandoned land and consequently lead now to the decline of steppe birds (Kamp et al. Reference Kamp, Urazaliev, Donald and Hölzel2011). Therefore, the population and range increase of wintering Little Bustards in Iran is more likely related to a higher level of concentration of the wintering population within the Caucasus region, than by an actual population increase, due to the safe heaven provided by the non-hunting zone combined with the increase of attractive agricultural crops.
Factors influencing Little Bustard winter distribution in Northern Iran
Both MaxEnt and the GLM and model averaging approaches showed that distance to a border was the most important predictor of Little Bustard occurrence in Iran. Iran’s international borders are likely to strongly influence the species’ distribution pattern, due to the severe military restriction as a non-hunting area, and therefore providing safe habitats for Little Bustards. Hunting has been identified as a major threat for the species leading to high rates of non-natural mortality and considerable disturbance (Gauger Reference Gauger2007, Sehhatisabet et al. Reference Sehhatisabet, Abdi, Ashoori, Khaleghizadeh, Khani, Rabiei and Shakiba2012). A vast area next to the border, including next to the Caspian Sea, which can cover a belt of more than 10 km with strict non-hunting policy may therefore play a crucial role in providing refuge areas for the species.
Land cover is another important predictor of Little Bustard distribution in Iran. Little Bustard distribution was shown to be associated with irrigated crops, likely including crops such as alfalfa, which is a known preference for the species during winter (García de la Morena et al. Reference García de la Morena, Morales, De Juana and Suárez2007). Even though a land cover map discriminating all land uses was not available for our study area, alfalfa is a crop that is expanding in Iran and is well represented in irrigated areas. A previous study in Iran also found a significant proportion of the wintering population in alfalfa crops (Sehhatisabet et al. Reference Sehhatisabet, Abdi, Ashoori, Khaleghizadeh, Khani, Rabiei and Shakiba2012). These legume crops offer a suitable vegetation structure for the species and are of high nutritional value, providing a suitable food resource (Bretagnolle et al. Reference Bretagnolle, Villers, Denonfoux, Cornulier, Inchausti and Badenhausser2011) that can support large wintering flocks (Iñigo and Borov Reference Iñigo and Borov2010).
As for elevation, its importance in the model probably relates to the preference for lowland agricultural sites with milder temperatures. Altogether, the Little Bustard preferred sites closer to the border within lowland irrigated agricultural landscapes. For the GLM models, because elevation was found to be highly correlated with distance to border, it was removed from analysis, however areas of lower annual precipitation are mostly located in plains closer to the border.
Our study is the first to address winter habitat selection of the eastern Little Bustard population, although previous studies have identified some patterns of habitat use (Gauger Reference Gauger2007, Sehhatisabet et al. Reference Sehhatisabet, Abdi, Ashoori, Khaleghizadeh, Khani, Rabiei and Shakiba2012). The predicted distribution map produced in this study largely matched the distribution outlined by Sehhatisabet et al. (Reference Sehhatisabet, Abdi, Ashoori, Khaleghizadeh, Khani, Rabiei and Shakiba2012) based on extensive fieldwork, which further confirms the ability of MaxEnt to accurately predict the suitable areas for the species even with limited data availability. MaxEnt and GLM analysis agree that distance to border is the most important variable explaining the pattern of Little Bustard occurrence in Northern Iran. Although land cover also enters in both modelling procedures, they disagree on its relative importance. This discrepancy is likely related to the difference in land cover variation captured by the absences (with the GLM analysis) and background points (with the MaxEnt). Species distribution models are useful tools to identify potential new areas for target species (Williams et al. Reference Williams, Seo, Thorne, Nelson, Erwin, O’Brien and Schwartz2009, Yousefi et al. Reference Yousefi, Ahmadi, Nourani, Behrooz, Rajabizadeh, Geniez and Kaboli2015). In this study, the MaxEnt model highlighted some new patches that seem to connect known areas. Further monitoring is needed to confirm the importance of these areas.
Iran now holds an important wintering population of the eastern Little Bustard population. Because these birds can aggregate in large numbers in relatively small areas, depending on highly anthropogenic habitats, it is essential that conservation action takes place. The non-hunting area next to the border represents a rare opportunity for the conservation of this species. Here the priority should be given to maintaining suitable habitats for the species. Additionally, in suitable agricultural areas, further from the border, poaching could be prevented by promoting awareness campaigns next to farmers and hunters and add greater protection to agricultural sites next to areas of greater habitat suitability.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270917000181
Acknowledgements
We would like to thank Ricardo Correia for reviewing a previous version of the manuscript. J.P.S. was funded by Grant SFRH/BPD/72311/2010 and SFRH/BDP/111084/2015 from Fundação para a Ciência e Tecnologia.








