Introduction
Reintroduction refers to the purposeful movement of endangered organisms into the wildness of their native distribution from which they had been extirpated in historic times (Armstrong and Seddon Reference Armstrong and Seddon2008; Seddon et al. Reference Seddon, Armstrong and Maloney2007). To evaluate reintroduction success for decision-making around threatened or endangered species conservation, trends in reintroduction biology have been analysed in various research studies and practices, including captive-veterinary management, population dynamics, genetics, habitat use, social-economic effects, and behaviour (Sarrazin and Barbault Reference Sarrazin and Barbault1996; Seddon et al. Reference Seddon, Armstrong and Maloney2007). Founder populations of a small number established in a recovery site must cope with a novel environment that is often different from their historical context, which can cause a weak or even a negative population growth (i.e. Allee effect; Deredec and Courchamp Reference Deredec and Courchamp2007). To perform long-term population projection with stochastic uncertainties, post-release demographics should account for the survival and fecundity of founders, which can also be affected by sex ratio and age structure (Converse et al. Reference Converse, Moore and Armstrong2013). In addition, understanding detailed behavioural characteristics of released animals can enrich reintroduction success in a given environment, including habitat selection (e.g. foraging, breeding, and roosting), seasonal movement (e.g. habitat use–availability, habitat requirements, and release-site fidelity), migration routes (e.g. breeding, stopover, and wintering sites), predation risk, and/or social interaction (Berger-Tal and Saltz Reference Berger-Tal and Saltz2014; Reading et al. Reference Reading, Miller and Shepherdson2013). Including these components in the process of management decision-making could improve reintroduction success for threatened species that are extinct in the wild globally or locally (Converse et al. Reference Converse, Moore and Armstrong2013; Sarrazin and Barbault Reference Sarrazin and Barbault1996).
The Oriental Stork Ciconia boyciana is globally listed as “Endangered” on the International Union for Conservation of Nature (IUCN) Red List of Threatened Species due to habitat loss and overhunting in the past (BirdLife International 2018), with distribution in Russia, China, Korea, and Japan. Its northern population breeds in south-east Siberia and parts of north-east China and winters mainly in south-east China, with some in Japan and the Korean Peninsula (Elliott et al. Reference Elliott, Kirwan, Garcia, del Hoyo, Elliott, Sargatal, Christie and de Juana2020). Its global population has been recently estimated at 1,000–2,499 mature individuals (BirdLife International 2018), including approximately 700 pairs of the migratory population in the Russian Far East (see also Elliott et al. Reference Elliott, Kirwan, Garcia, del Hoyo, Elliott, Sargatal, Christie and de Juana2020). This species was previously a locally common resident in the Korean Peninsula (Austin Reference Austin1948; Austin and Kuroda Reference Austin and Kuroda1953), which formerly bred in Japan (until 1960s) and Korea (until 1977 or later in North Korea; 1971 in South Korea). Its resident populations were extirpated from Japan and the Korean Peninsula although they were formerly common. According to the process of heteropatry (Winker Reference Winker2010), migratory and resident populations of the Oriental Stork were likely to breed allopatric (i.e. Russia versus the Korean Peninsula) and winter sympatric (i.e. southern parts of the Korean Peninsula) along the Russian Far East flyways between south-east Siberia and the Korean Peninsula.
Currently, reintroduction programmes (e.g. foreign imports, captive propagation, and release together with habitat management) for the Oriental Stork have been underway in South Korea and Japan to restore their exterminated resident populations (Ezaki and Sagara Reference Ezaki and Sagara2014; Park et al. Reference Park, Yoon and Kim2011). Only a limited number of studies have documented the adaptiveness and life-history perspectives of founder Oriental Storks in comparison to many studies of northern, migratory populations. Russian populations seasonally leave their breeding ground in September–October (i.e. fall or autumn migration) and return in March–April (i.e. vernal migration) of the next year. According to satellite tracking data (Higuchi Reference Higuchi2010), their migration patterns covered short distances each day (e.g. a total distance 2,760 km for 103 days on average). The migratory behaviour of this species seems to indicate they travel distances of approximately 20–30 km/day during the migration period of 3–4 months. Their reproductive schedule has been reported to vary with different latitudes with the on-set of breeding being one or two months delayed in Russia compared with Japan and South Korea (Elliott et al. Reference Elliott, Kirwan, Garcia, del Hoyo, Elliott, Sargatal, Christie and de Juana2020). However, the factors directly affecting such variation remain unknown. Neither variation in reproduction (e.g. clutch size and length of incubation–nestling periods) nor seasonal variation in moult (e.g. rate and intensity) has been reported for Oriental Stork in the wild.
The purpose of the present study was to examine the characteristics of founder Oriental Storks with respect to population demography and behavioural ecology (i.e. habitat use, home range, and seasonal movements) at an early stage of reintroduction in South Korea. Prior to the extirpation of the resident population in South Korea in 1971, a few fundamental studies and observations existed which described some ecological aspects (e.g. demography and habitat use) and could have aided the current reintroduction biology for Oriental Storks. Unfortunately, we also failed to propagate the last wild birds for ex situ conservation. Instead, captive individuals (i.e. 38 wild or captive migratory birds from Russia, and Japanese and German zoos in 1996–2001) were imported and propagated. Since 2015, some have been released into the wild to restore the extirpated resident population. However, little scientific information is available to evaluate the founder population and compare current and past situations in the Korean Peninsula. Using the first two-year ecological data of reintroduced Oriental Storks, this study aimed to evaluate the ecological status of the founder population (i.e. demography, habitat use, and movement) and discuss its potential adaptiveness as a resident population to the current environment in South Korea.
Methods
Study subject
The Oriental Stork exhibited monogamous mating with a long-term pair bond in which both sexes incubated and provided the chicks with post-hatching care throughout the spring–summer breeding season (Cheong Reference Cheong2005; Elliot et al. Reference Elliott, Kirwan, Garcia, del Hoyo, Elliott, Sargatal, Christie and de Juana2020; Yoon, Ha et al. Reference Yoon, Ha, Jung and Park2015). They started to display courtship and nest-building behaviours in December (Yoon, Ha et al. Reference Yoon, Ha, Jung and Park2015; Yoon, Yoon et al. Reference Yoon, Yoon and Park2015). To date, 118 captive storks have been managed in breeding facilities in South Korea. Since 2015 some have been released into recovery sites in Yesan County (36°36′32″N, 126°48′05″E). Prior to reintroduction, captive storks were bred and managed in two breeding facilities: the Ecological Institute for Oriental Stork, Korea National University of Education (KNUE) 36°36′15″N, 127°21′33″E and the Yesan Oriental Stork Park (YOSP) 36°32′32″N, 126°48′42″E. Since 1996 these facilities have been responsible for veterinary and quarantine management in the processes of introduction, captive propagation, and reintroduction, including habitat management (e.g. ecological rice paddy fields with artificial nest platforms) (Park and Cheong Reference Park and Cheong2002; Park et al. Reference Park, Yoon and Kim2011). Starting in September 2015 with the first reintroduction, our preliminary observations showed that the large-scale home range (i.e. kernel density estimate 50%) of reintroduced Oriental Storks included increasing rice paddy fields with decreasing forested areas in habitat use (Ha et al. Reference Ha, Kim, Shin and Yoon2021). In captivity, wing feather moult occurs over a long period from spring to fall, with adults seeming to moult during the breeding season in terms of moult-breeding overlap (Yoon et al. Reference Yoon, Yoon, Nam and Choi2021). However, few data were available on the ecology of founder Oriental Storks, including ecological characteristics of population demography and seasonal habitat use (e.g. habitat characteristics, home range, and movement behaviour) at the early stage of reintroduction.
Population demography
To conduct demographic analyses of released and wild-born Oriental Storks (i.e. 28 individuals) from September 2015 to September 2017 (Supplementary material), two population management software packages, Poplink 2.5.2. (Faust et al. Reference Faust, Bier, Schowe and Gazlay2019) and PMx version 1.6.5. (Ballou et al. Reference Ballou, Lacy, Pollak and Callicrate2022), were utilised. Studbook information (i.e. hatch data, sex, parentage, and all events) of the 28 individuals for a two-year reintroduction period were fed into Poplink. This information was also linked to studbook management at KNUE and YOSP. Detailed demographic trends were produced using the PMx software (Ballou et al. Reference Ballou, Lacy, Pollak and Callicrate2022). Demographic, genetic relations, and census data were exported from Poplink to PMx. Demographic analyses were performed using known life history-related traits such as survival and reproduction (e.g. age class = one-year, early mortality = 30 days, and sex ratio at birth maturity = 0.5 in captive and wild situations). We accounted for a future population trend based on population growth rate (λ) through deterministic and stochastic models.
Seasonal habitat use and movement
To inspect habitat characteristics within their home range and their seasonal movement behaviour, spatial data of 17 Oriental Storks were collected during the two tracking years (i.e. September 2015 to September 2017 (Table 1). The tracker (WT-300, KoEco, South Korea) with a solar charging system was attached to the back of each individual prior to release. Trackers had features of Code Division Multiple Access, positioning rate (12 locations per day), communication rate (once per day), size (66 mm × 38 mm × 33 mm), and weight (62 g; less than 3% of body weight). Our trackers used Cellular Tracking Technology (CTT), which transmitted geographical locations with a range error of less than 50 m using cellular networks (see also Lee et al. Reference Lee, Yu, Martínez-López, Yoon, Kang and Hong2020). Regarding the data points used for each individual, an average of 4,391 data points were used, ranging from 768 to 9,104, depending on release time, death, and/or tracker conditions. We also excluded one-month tracking data right after release because it might take time for released storks to acclimate to the wild environment.
Table 1. Tracking list of 17 Oriental Storks Ciconia boyciana recently reintroduced into South Korea. N = non-breeding; B = breeding; N→B = the transition from non-breeding to breeding status during the tracking period

To produce polygons of home range, a Brownian Bridge Movement Model (BBMM) dealing with temporal spatial data, position-related estimated errors, and assigned grid-cell size (Horne et al. Reference Horne, Garton, Krone and Lewis2007) was used first. Our spatial data satisfied the two assumptions (i.e. bivariate normal distribution of spatial errors followed by random conditions between start and end positions in continuous spatial movements) of the BBMM (Horne et al. Reference Horne, Garton, Krone and Lewis2007). Errors of normal distribution are likely to occur in spatial data where hourly continuous point-movement data could be inconsistent and less realistic with increasing time intervals (Horne et al. Reference Horne, Garton, Krone and Lewis2007). We used the BBMM package in the program R version 4.13 for computing home range polygons (i.e. in 50% scale). Although a core range should not be strictly determined by home range area (Powell Reference Powell, Boitani and Fuller2000), we here chose 50% kernel volume contour to examine spatial variations as a function of breeding status and/or season, which was based on observations (e.g. nest proximity in c.2 km; see also Ezaki and Sagara Reference Ezaki and Sagara2014) under the core isopleths ranging 90–50% kernel volume contour (Börger et al. Reference Börger, Franconi, de Michele, Gantz, Meschi and Manica2006). Considering breeding phenology and agricultural schedule (i.e. rice paddies) in South Korea, individual tracking data (September 2015 to December 2017) were split into winter (December–February for pairing and nest-building), spring (March–May for egg-laying, incubating, and provisioning), summer (June–August for post-fledging), and fall (September–November for post-breeding) according to the four bio-seasons for further home range analyses. Polygons of BBMM 50% scaled home ranges were used to extract seven types of land covers (i.e. paddy, forest, river, coast, village, meadow, and bare land) following the large-scale classification method provided by the Korean Ministry of Environment. We also calculated individual-based movement distance using Track Intervals to Line in Tracking Analyst Tools of ArcGIS (version 10.1, ESRI). A generalised mixed model was then used to analyse variations in habitat characteristics, home range size, and daily movement distance as a function of season, breeding status, and interaction terms, controlling for each individual as a random effect. All statistical analyses were performed with SPSS ver. 16 (SPSS Inc., Chicago, IL, USA). We did not need to transform any variables to meet model assumptions.
Results
Population trends of founders
For the first two years after initiating reintroduction (17 releases), the founder population of Oriental Storks consisted of 28 individuals (11 males and 17 females), including dead, rescued, and disappeared individuals in the wild. Among them, we counted 15 survivors, six missing individuals, five known deaths (one collision with an airplane, two electrocutions, one trapped in fish farm lines, and one potential poisoning), and two rescues (leg fracture and starvation) in breeding facilities in 2015–2017. There were four breeding events with 11 fledglings (two from a pair in 2016 and nine from three pairs in 2017). Based on analytical results using PMx software, the population trend of founder Oriental Storks at the early stage of reintroduction after two years was then determined (i.e. average λ = 1.075 with 1.102 for males and 1.048 for females). The future population size was projected to be 81 individuals in the deterministic model and 39 individuals in the stochastic model over 20 years (i.e. 2015–2035) (Figure 1).

Figure 1. Deterministic and stochastic population projections of reintroduced Oriental Storks Ciconia boyciana in South Korea from 2015 to 2035. The founder population consisted of 17 released and 11 fledged individuals, including six missing individuals, five known deaths, and two rescues in breeding facilities during 2015–2017.
Seasonal change in habitat use
Patterns of habitat use for 11 Oriental Storks with five breeding, four non-breeding to breeding, and 10 non-breeding cases with 385 home range polygons (BBMM 50%; average 152 ha) could be significantly explained by a mixed effect of land cover type and breeding status (Table 2). Lacking seasonal variations, their home ranges included rice paddies (63%), which had the highest rate, followed by forested areas (22%), river–reservoirs (9%), coastal areas (3%), and others (3%) (Figure 2a). Interestingly, breeding individuals seemed to use relatively more paddy–forest areas whereas non-breeding individuals utilised areas with diverse land cover types (e.g. river–reservoirs) (Figure 2b).
Table 2. Results of a mixed model for land coverage (%) as a function of breeding season (i.e. December–February for winter, March–May for spring, June–August for summer, and September–November for fall as bio-seasons), breeding status (i.e. breeding versus non-breeding), and land cover type (i.e. paddy, forest, river–reservoir, coastal area, village, meadow, bare land), including two-way and three-way interaction terms

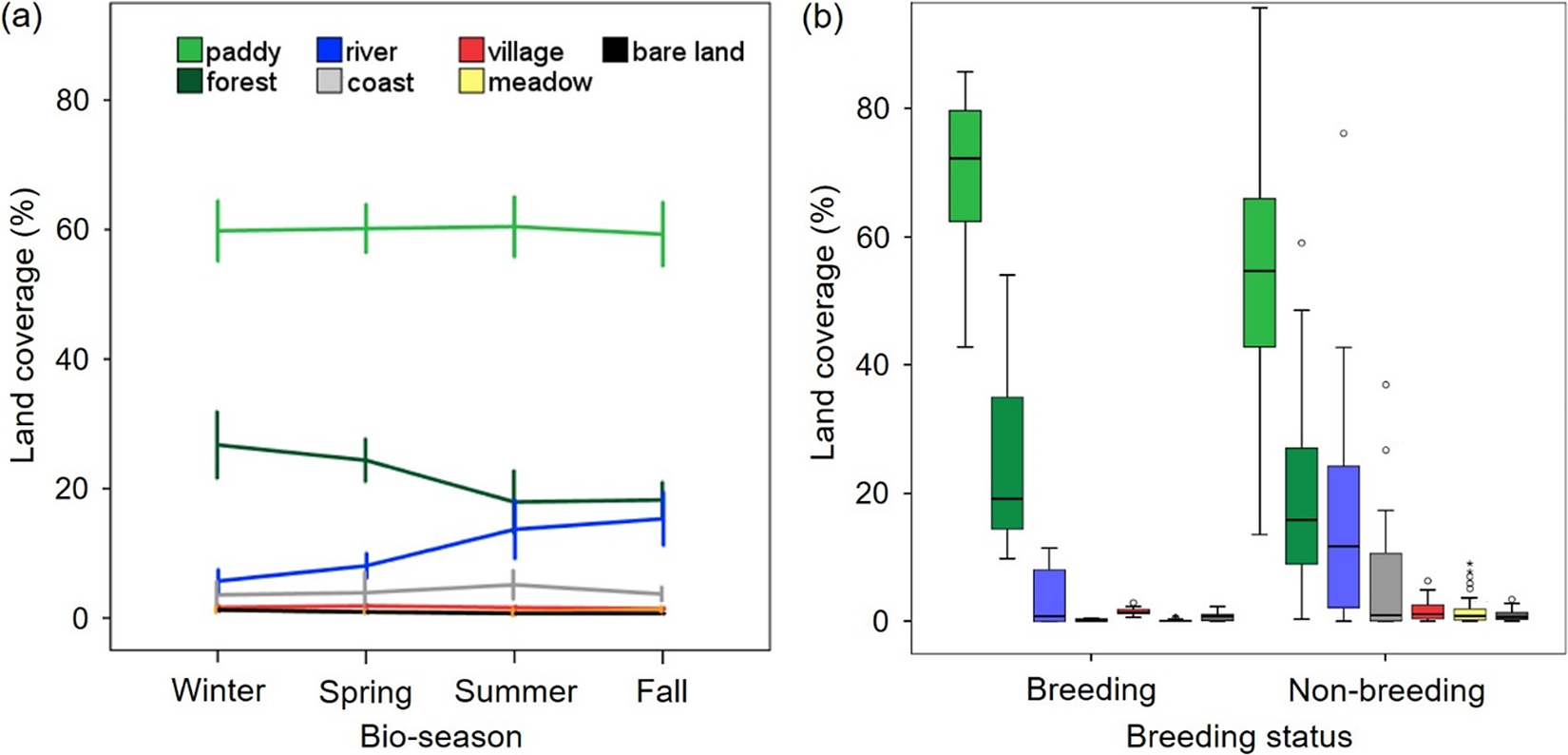
Figure 2. Seasonal variation in land coverage (%) within home ranges of reintroduced Oriental Storks Ciconia boyciana (n = 11 individuals) categorised by (a) land cover type (i.e. paddy, forest, river, coastal area, village, meadow, and bare ground) and (b) breeding status. Bio-season includes winter (December–February), spring (March–May), summer (June–August), and fall (September–November), considering Oriental Stork’s breeding season and agricultural schedule in paddy fields. Error bar denotes mean ± 1·SE.
Seasonal change in home range size
Variation in home range size of Oriental Storks was weakly explained by season and breeding status whereas their two-way interactive term was marginally significant (mixed model: SEASON F 3, 370 = 1.50, P = 0.21; BREEDING F 1, 201 = 0.73, P = 0.39; SEASON × BREEDING F 3, 370 = 2.29, P = 0.08) (Figure 3a). The home ranges of non-breeding individuals (184 ± 64 ha on average) seasonally varied more than those of breeding ones (129 ± 72 ha). The home ranges of non-breeding individuals were smaller in winter (90 ± 83 ha) and summer (68 ± 83 ha) than in spring (306 ± 87 ha) and fall (272 ± 645 ha), while breeding birds consistently maintained their home range area.

Figure 3. Seasonal variations in home range size (ha) and daily movement distance (km) of reintroduced Oriental Storks Ciconia boyciana according to breeding status during the tracking period (see Table 1).
Seasonal movement distance with breeding status
Reintroduced Oriental Storks tended to exhibit seasonal variations in movement behaviour, partially depending on breeding status (mixed model: MONTH F 11, 168 = 3.78, P <0.001; BREEDING F 1, 178.1 = 1.58, P = 0.21; MONTH × BREEDING F 11, 168 = 1.69, P = 0.08) (Figure 3b). That is, Oriental Storks tended to move most in spring and fall (e.g. low in June) and breeders moved less than non-breeders, a pattern which was marginally significant.
Discussion
The present study documented the behavioural ecology of 28 founder Oriental Storks with respect to population projection and seasonal changes in habitat use, home range size, and movement together with breeding status. The population trend seemed to be stable or steadily increasing when it was projected at the first two-year reintroduction (λ = 1.075). Here, factors affecting population growth (e.g. breeding density or availability of food and nesting sites) should be tested in target recovery sites from the early stage of reintroduction over a long-term period (see van Horne Reference Van Horne1983). In home range analysis, their core areas (BBMM 50%; average 152 ha in 385 home ranges) included rice paddies (63%), the highest rate, followed by forested areas (22%), river–reservoirs (9%), coastal areas (3%), and others (3%). Breeding status seemed to be a better predictor in explaining variations in habitat characteristics within the home range and its size than seasons. Breeders utilised relatively less variation in land cover types and maintained smaller home ranges than non-breeders. Oriental Storks moved in spring and fall at a high rate while non-breeders moved more and maintained larger home ranges in spring and fall than breeders in smaller home ranges with less movement. Overall, founder Oriental Storks inhabited rice paddy fields for breeding and foraging, where habitat use, home range, and movement varied with the mixed effects of season and breeding status.
In Japan and South Korea, Oriental Storks forage extensively in cultivated areas, especially rice paddy fields. They also breed in nearby woods (Kim et al. Reference Kim, Kim, Cheong, Kim, Sung and Park2008; Naito and Ikeda Reference Naito and Ikeda2007; Yoon et al. Reference Yoon, Na, Kim and Park2012; Yamada et al. Reference Yamada, Itagawa, Yoshida, Fukushima, Ishii and Nishigaki2019). Our results indicated that the habitat use and breeding phenology of Oriental Storks might be adaptive to the anthropogenic management of rice paddy fields. These artificial wetlands were historically man-managed for cultivation. They might have provided Oriental Storks with suitable foraging habitats as alternative wetlands for nutritional sources in South Korea and Japan. In general, rice farming involves a cycle of rice cultivation irrigation (February–March), rice planting (April–May), and drainage-harvesting (September–October) with some temporal variations in regions and farming techniques in South Korea. The breeding schedule of Oriental Storks has been observed in terms of egg-laying (February–March), incubation (March), post-hatching care (April–May), and fledging (June) with some temporal variations in captivity and wildness (Jongmin Yoon, personal observation; see also Yoon et al. Reference Yoon, Na, Kim and Park2012, Yoon, Ha et al. Reference Yoon, Ha, Jung and Park2015). The breeding phenology of Oriental Storks for chick-rearing appears to match rice farming, which can provide food resources (e.g. fish, amphibians, and aquatic invertebrates) during the breeding period more than in other seasons. According to the evolution of migratory behaviour and empirical studies (Hall and Tullberg Reference Hall and Tullberg2004; see also Zhou et al. Reference Zhou, Xue, Zhu, Shan and Chen2013), some long-distant migratory storks from Russia might have established resident populations in South Korea and Japan in association with river–rice farming environments.
Rice farming was very common in terraced fields and flood plains until the Goryeo Dynasty (918–1392) in the Korean Peninsula. It might have provided unstable foraging and breeding environments for Oriental Storks that lacked anthropogenic irrigation management. Oriental Storks were also influenced by stochastic events of droughts and floods. Farm irrigation structures for rice cultivation have become systematic in flood plains since the Joseon Dynasty (1392–1897) (Dong-Jin Kim, personal communication), which might have provided stable and spacious foraging habitats for breeding Oriental Storks on their migration route and/or wintering grounds. In the present study, their reproductive status (i.e. breeding versus non-breeding) appeared to be related to degrees of home range and seasonal movement where breeding individuals might be more localised than non-breeding ones. This pattern is potentially linked to the fact that breeding storks need to remain in fixed localities to incubate eggs and provide for post-hatching chicks until fledging, resulting in smaller home ranges and a lower level of movements of breeders with some territoriality than non-breeders (Newton Reference Newton2011). Seasonal variation in space use appeared to match the schedule of rice farming, where reintroduced Oriental Storks showed reduced movements during the rice cultivation season.
In general, three main energy-demanding stages (i.e. breeding, moult, and migration) occur in the annual cycle of migratory birds (Ketterson Reference Ketterson2020; Wingfield Reference Wingfield2008), which tend to take place at different times and in different spaces with minimal overlaps (Foster Reference Foster1974). The present study and previous studies showed that the degrees of movement and moult of Oriental Storks seemed to be seasonally partitioned with a schedule of moult–breeding overlap (see also Yoon et al. Reference Yoon, Yoon, Nam and Choi2021). Moult is anticipated to be more flexible in timing than other events under time constraints among species and/or populations (Kiat et al. Reference Kiat, Izhaki and Sapir2019; reviewed in Newton Reference Newton2011). This is because moult could reduce flight efficiency for foraging and travelling and increase thermoregulation costs, especially during the moult period of large feathers (Kiat et al. Reference Kiat, Izhaki and Sapir2019). Breeding also needs fixed localities during egg incubation and post-hatching care. Thus, the timing and locality of the moult stage could be more flexible than those of breeding and migration stages. The moult season of Oriental Storks is likely to correspond to their nesting period with less movement, presumably in fixed localities for territories and/or nestling areas. Overall, seasonal variations in spatial patterns (i.e. habitat use, home range size, and movement) might be influenced by a mixed effect of food availability in artificial and natural wetlands as well as breeding status, lacking highly seasonal long-distance movements.
Many researchers have questioned whether reintroduced Oriental Storks in Japan and South Korea exhibit characteristics of migrants or residents because all incipient captive storks have originated from Russian migratory populations (e.g. total migration distance 2,760 km for 103 days on average in spring and fall; movement distance 20–30 km/day; see Higuchi Reference Higuchi2010). For Oriental Storks, the breeding habitat of migrants is also known to differ from that of residents. Our results indicated that reintroduced Oriental Storks exhibited patterns of resident or short-distance movement with fewer than 5 km of daily movements. Considering ecological and life history-related perspectives (i.e. habitat use, moult, and breeding phenology), it could be judged that recently reintroduced Oriental Storks could adapt to the environment of South Korea as a novel wildness. Based on collected evidence, founder storks seemed to have some resident-related characteristics according to their habitat use and life stages of their annual cycle. Their reproductive status (i.e. breeding versus non-breeding) appeared to be related to the degree of seasonal movement where breeding individuals might be more localised than non-breeding ones. The availability of foraging habitats in cultivated fields (i.e. low in winter versus high in summer) could play a crucial role in providing breeding storks with food (e.g. fish and amphibians), affecting their phenology along with changes in home range and distance of movement. To our knowledge, the storks may seasonally utilise small areas with less movement for different reasons, such as the high food availability in rice paddies in summer, but with the limitation of unfrozen wetlands in winter, compared with the availability and concentration of foraging habitats in spring and fall. Their variations in seasonal movement and moult might be also seasonally partitioned to cope with changing environments in South Korea.
The Oriental Stork is monotypic and only distributed in Russia, China, Japan, and the Korean Peninsula (Elliott et al. Reference Elliott, Kirwan, Garcia, del Hoyo, Elliott, Sargatal, Christie and de Juana2020). The past and present breeding populations, including recently reintroduced populations, exhibited differences in migratory behaviour. Few studies have examined its life-history perspectives. Thus, empirical data with respect to its breeding, moult, and migration are lacking. For endangered species, the study of habitat use towards management and/or migration behaviour towards flyways has been focused on more than other perspectives (Yang et al. Reference Yang, Chen, Jia, Xu, Wang and Wei2023). Recently, Japan and South Korea have propagated and reintroduced captive Oriental Storks which originated in northern, migratory populations (i.e. Russia) to restore extirpated resident populations. These reintroduced individuals in South Korea were likely to possess resident-related characteristics that showed overlapping moult and breeding phases in a rice cultivation season with high food availability and low movements. Their breeding phenology also seemed to depend on environmental conditions such as food availability in rice paddies and surrounding wetlands. Our study had some uncertainties (e.g. habitat condition, parent age with experience, and releasing method) while performing the population projections because we used empirical data from founders with a small number to evaluate their ecological and behavioural adaptiveness. The first two-year demographic and tracking data which were used in this study might be insufficient to predict long-term population dynamics and/or to reveal habitat use with movement. Our limited information should be taken with caution for generalisation. Gender effect influencing home range size and movement pattern was not tested due to the limitation of pair-based tracking data. Instead, we focused on breeding status in the present study at the population level. Future studies should investigate variations in life histories (e.g. sex-related survival–reproduction, parental care, and habitat use) for Oriental Storks along their global distribution at different latitudes through international cooperation under on-going climatic changes and anthropogenic land uses. In the future, man-managed areas (e.g. cultivation fields), upon which Oriental Storks depend, should be managed in an eco-friendly manner, with minimal or no pesticide use, and high connectivity between paddies and river systems for their conservation.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0959270924000194.
Acknowledgements
We are grateful to the anonymous reviewers whose comments led to the finalisation of this manuscript. This study was part of a conservation project that included captive propagation and reintroduction of Oriental Storks in the Ecological Institute for Oriental Stork, Korea National University of Education with Yesan Oriental Stork Park. This research was funded as an ex situ Conservation Project for Endangered Species by the Ministry of Environment, Korea Heritage Service, and local governments of South Korea. The writing of the manuscript was supported by a research project funded by the National Institute of Ecology [grant number NIE-BaseStudy-2024-46].







