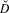Introduction
The White-naped Crane Antigone vipio (WNC) is classed as ‘Vulnerable’ by the IUCN, based on a declining population and a reduction in habitat occupancy (BirdLife International 2012). Direct threats to the WNC population in Mongolia, identified by national stakeholders and species experts, included “fire” and “habitat loss” resulting from livestock-based agricultural practices (Fine et al. Reference Fine, Ochirkhuyag, Didier, Redford and Grippo2008, Wildlife Conservation Society 2011, Harris and Mirande Reference Harris and Mirande2013). The species has a disjunct breeding range, with a western population breeding in north-eastern Mongolia and north-eastern China, encroaching into Russian territory in Chita, and an eastern population along sections of the Amur and Ussuri Rivers (Archibald et al. Reference Archibald, Meine, Garcia, del Hoyo, Elliott, Sargatal, Christie and de Juana2013). Populations migrate south to winter in the Yangtze River Valley in China, the Korean Peninsula and the Japanese Island of Kyushu (Higuchi Reference Higuchi1997, Higuchi et al. Reference Higuchi, Pierre, Krever, Andronov and Fujita2004, Archibald et al. Reference Archibald, Meine, Garcia, del Hoyo, Elliott, Sargatal, Christie and de Juana2013). Obtaining reliable population estimates across such a widely dispersed and often remote breeding range is challenging, and movement of birds between wintering areas complicates population assessment during the non-breeding season (BirdLife International 2014). The Chinese wintering population is largely confined to the vast wetland at Poyang Lake, where annual counts vary, but have shown a negative trend from a mean maximum count of 2,278 between 1996 and 2004 (n = 8), to a mean of 1,167 from 2005 to 2012 (n = 6) (Li et al. Reference Li, Wu, Harris and Burnham2012). The wintering population in the Korean Peninsula and Japan appears to have been more stable, with numbers exceeding 3,000 birds at the artificial feeding site at Izumi, Japan since the late 1990s (BirdLife International 2001, 2012, Li et al. Reference Li, Bloem, Delany, Martakis and Quintero2009). The majority of WNCs breeding in Mongolia are thought to winter in China, although some cranes marked in Mongolia have alternated between wintering areas in China and Japan in successive years (B. Nyambayar in litt. 2014).
In Mongolia, WNCs nest along river valleys in open steppe country, particularly the watersheds of the Kherlen and Ulz Rivers, and extending north into the edges of the taiga zone in the Onon River watershed (Bradter et al. Reference Bradter, Gombobaatar, Uuganbayar, Grazia and Exo2005). The cranes require wetlands, characterised by wet meadows or reeds, favouring shallow pools in areas with low grazing intensity for nesting (Bradter et al. Reference Bradter, Gombobaatar, Uuganbayar, Grazia and Exo2005). In 2000 and 2001 Bradter et al. (Reference Bradter, Gombobaatar, Uuganbayar, Grazia and Exo2005, Reference Bradter, Gombobaatar, Uuganbayar, Grazia and Exo2007) surveyed breeding WNCs along a 270 km stretch of the Ulz River, collecting data on reproductive performance and nest site selection. The authors noted high densities of territorial WNCs (mean encounter rate of 1.5 pairs per 10 km of river basin, to a maximum of 8.8 pairs per 10 km), and a moderately high breeding productivity (with fledging success defined as the percentage of pairs raising at least one chick up to 52%, and a brood size up to 0.7 per breeding pair). This was despite the survey coinciding with what was noted as a particularly dry period. Thus, it was hypothesized that these productivity levels may fall below what might be achieved with higher precipitation and ground saturation. Since 1955 the climate in eastern Mongolia has undergone two cycles characterised by periods of above average precipitation followed by periods of drought (Simonov et al. Reference Simonov, Goroshko, Egidarev, Kiriliuk, Kochneva, Obyazov, Tkachuk and Somonov2013). The drought noted by Bradter et al. in 2001 has continued through at least 2012 (Simonov et al. Reference Simonov, Goroshko, Egidarev, Kiriliuk, Kochneva, Obyazov, Tkachuk and Somonov2013), resulting in a considerable drop in water levels. Considering the apparent decline in the wintering population in Poyang Lake, assessing the impact of a decade of drought on the numbers and productivity of Mongolian WNCs is seen as a priority.
The objective of this study was to revisit the 270 km transect along the Ulz River that was surveyed by Bradter et al. in 2000 and 2001 to assess the distribution, numbers and breeding performance of WNCs after a decade of drought. Surveys were extended to cover a more generalised survey area including sections of the Kherlen River, Onon River catchment (including Onon Balj National Park) and Khurkh Khuiten Ramsar site where WNCs have been recorded previously. Finally, we employed distance sampling and occupancy methods to assess the detectability of cranes within survey areas. Although cranes can be relatively visible in the open landscape of the Mongolian steppe, detection is less than certain, with terrain, vegetation and crane posture reducing detectability. Any assessment of species density or distribution is likely to produce inaccurate results, unless variation in detectability is taken into account. Distance sampling has the potential to provide unbiased estimates of density, if assumptions of the method can be met. Occupancy accounts for detectability during an assessment of distribution and was also used to investigate the factors influencing both detectability and distribution.
Methods
Study area
The Mongolian Eastern Steppe is the largest intact temperate grassland ecosystem in the world. The landscape is predominantly open grassland steppe, transitioning to taiga forest in the north, and is crossed by three large river drainages, the Ulz, Kherlen and Onon Rivers, which form part of the Amur River catchment (Figure 1). Surveys took place in the aimags (provinces) of Dornod and Khentii, and the municipality of Ulaanbaatar. Habitat suitable for WNCs was found intermittently along the river drainages and consisted of seasonal and permanently flooded meadows, shallow pools, lake margins, as well as the river channels themselves. These areas were predominantly open, although stands of willow Salix sp. grew along some sections, particularly along the Onon drainage. Human population density is generally low, with traditional nomadic pastoralists and their livestock found throughout much of the survey areas, and several permanent settlements serving as administrative centres (soums). Select areas of critical habitat for WNCs are protected within two Ramsar Sites, Mongol Daguur (Ramsar # 924) and the Lakes in the Khurkh-Khuiten River Valley (Ramsar # 1380), and by Onon Balj National Park. Further details of the Ulz River study area are provided in Bradter et al. (Reference Bradter, Gombobaatar, Uuganbayar, Grazia and Exo2005).
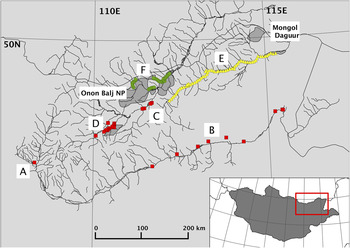
Figure 1. A map of study areas, including locations covered during the generalized surveys (squares): Gun Galuut Nature Reserve (A), the Kherlen River (B), Onon River (C) and the Khurkh Khuiten Valley (D); Ulz River transect (triangles, E); and Onon Balj surveys (circles, F). Shaded polygons indicate protected areas, including the Mongol Daguur Ramsar site and Onon Balj National Park.
Ulz River surveys
In 2010 and 2011, surveys were conducted along the 270 km section of the Ulz River Valley visited by Bradter et al. (Reference Bradter, Gombobaatar, Uuganbayar, Grazia and Exo2005) a decade earlier (hereafter referred to as the Ulz River transect). Surveys took place on a monthly basis between 22 May and 10 July 2010, and between 19 May and 18 August 2011. The search effort to locate pairs of WNC differed in each of the survey years. In 2010, searches were targeted on areas of wet meadow, pond, and marsh along the Ulz River. Observations were made using binoculars (8x32 mm) and telescope (80 mm, 20-60x zoom) from elevated positions along the river valley. All sites where cranes were observed were revisited during subsequent months. In 2011, observations were made from 63 fixed points randomly generated along the entire length of the Ulz River transect, located at 5 km intervals along navigable tracks running parallel to the river basin. As visibility of the river basin could not be assured from randomly generated points, observers were given flexibility to select elevated positions within 200 m of the random points. Additional observations were made from 20 non-random points where cranes had been sighted in 2010. Two or more observers used binoculars and telescopes to scan for cranes at each observation point, and recorded the bearing and estimated distance of each crane observed. All observation points were visited on a monthly basis in 2011.
All cranes were identified to species and age based on plumage characters. We defined territorial pairs (TPs) and breeding pairs (BPs) in the same manner as Bradter et al. (Reference Bradter, Gombobaatar, Uuganbayar, Grazia and Exo2005). Territorial pairs met one or more of the following criteria: 1) signs of nest building, 2) evidence of breeding activity (incubation or the presence of one or more chicks), and/or 3) were recorded in the same location after a minimum interval of 10 days. Breeding pairs were a subset of TPs, consisting of those cranes showing signs of breeding activity (evidence of an egg and/or a chick). Bradter et al. noted that non-breeding pairs departed their territories as early as late May, thus raising a dilemma when classifying pairs that were not observed until June or later. In cases where these pairs showed no signs of breeding activity, they were assumed to be non-breeding pairs that may have been recorded as TPs at another location earlier in the study, and so were not considered to be TPs, to prevent double-counting. In several instances we recorded pairs where breeding activity was not observed until June or later. These birds could either have been late to establish their territories, or could have been overlooked during May surveys. To facilitate comparison with Bradter et al., we defined these as late breeding pairs (LBPs). All other pairs that did not meet these criteria, but showed signs of consorting together were defined as presumed pairs (PPs).
Bradter et al. used two methods to define breeding productivity. The first of these, termed ‘fledging success’ focused on BPs only, and was defined as the percentage of BPs that successfully fledged at least one chick. The second, termed ‘recruitment’ accounted for the observation that some pairs did not attempt to nest, despite holding a territory. This was expressed using the following formula:
where R denotes recruitment and FJ denotes the number of fledged juveniles.
We attempted to estimate the conservative minimum number of individual WNCs in the transect by summing the number of individuals represented by observed TPs, PPs and non-paired cranes in each survey month. This estimate assumes that birds were not moving between observation points while the survey was taking place.
Generalized survey
Additional surveys were performed in 2010 and 2011 away from the Ulz River, to provide further data on crane numbers and breeding activity on the Eastern Steppe. These consisted of historic breeding sites provided by N. Tseveenmyadag (Mongolian Academy of Sciences) for the period 1981–2003. These included sites at Gun Galuut Nature Reserve, and locations along the Kherlen River Valley, Onon River and the Khurkh Khuiten Valley (Figure 1). Monthly visits were made to each of these locations between 16 May and 16 July 2010, and between 15 May and 19 August 2011. In 2011, the Onon River and Khurkh Khuiten Valley were only surveyed on 1 June and 3 June respectively, and so it was not possible to estimate the total number of TPs present due to the departure of birds whose breeding attempts failed early in the season. However, all breeding birds observed were likely to have initiated breeding activity before the end of May and so were counted as BPs not LBPs (and therefore also qualified as TPs). A record was made of all cranes observed and their territorial and breeding status was noted using the same system as along the Ulz River transect.
Intensive Onon Balj surveys
Observations were made between 30 May and 2 June 2011, in Onon Balj National Park and neighbouring areas along the Onon River watershed by four independent teams. A total of 60 sampling points were randomly generated along navigable tracks at intervals of 3 km, which followed watercourses (streams and rivers), constituting potential WNC habitat. Independent observations were made by four teams of experienced observers, who visited each of the points and spent ten minutes scanning for the presence of WNCs. Details of all cranes were recorded as described above. In addition, observers made a record of habitat (as the percentage ground cover corresponding to wet meadow, willow, reed or steppe in a circle of 500 m radius, extending from the point), ground moisture (categorized as wet or dry), human settlements (numbers of individual structures, or gers [yurts used by nomadic herders]), sign of fire (the proportion of circular area with evidence of recent fire), and grazing intensity (categorized as none, low, medium, or high). Visibility during the visit to the sampling point was coded as poor, moderate, good, and very good. The proportion of the circular area with an unobstructed view was also recorded and integrated into subsequent analyses as a sampling fraction (distance analysis) or sampling covariate (occupancy analysis).
Crane detectability
Despite the open landscape of the Mongolian steppe, WNCs could be surprisingly difficult to observe at times, particularly when they retreated into long grass or reed. In order to consider the impact of this, we tested two different approaches to estimate the detection probability of WNCs in the eastern steppe, namely distance sampling and occupancy methods. The former approach also has the potential to provide unbiased estimates of density. The latter approach has the potential to provide unbiased estimates of the probability of occupancy and was also used to assess the factors influencing WNC distribution and detectability.
Distance sampling techniques have been successfully used to obtain density and abundance estimates for many birds and other species (Buckland et al. Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001). However, there was some uncertainty as to whether point transect distance sampling could be successfully applied to WNCs due to the need to position transects at vantage points that might potentially invalidate the assumption that birds are uniformly distributed with respect to the observer. Nonetheless, distance sampling methods were tested by applying them to the 2011 Ulz River surveys using both data from the random and non-random sampling points to increase sample size to estimate detectability accurately. Data were analysed based on WNC groups (usually pairs, but occasionally single birds or larger aggregations) comprising both adults and subadults with temporal stratification by monthly visit for encounter rate and group size to obtain density estimates stratified by the four survey months. Distance sampling methods were also applied to the data collected at the 60 points during the 2011 Onon Balj surveys with the same potential issue of non-uniform distribution of the cranes relative to the observation points. The data were pooled across observers with survey effort accounting for the observations by multiple independent observers at each point. A sampling fraction was associated with each of the point transects, as the view was not always unobstructed in all directions, neither during the Ulz River nor Onon Balj surveys.
Radial distance data were analysed using Distance 6 (Thomas et al. Reference Thomas, Buckland, Rexstad, Laake and Strindberg2010). Variance was estimated empirically using the replicate transect points as samples. Exploratory analyses were first conducted to examine options for truncation and grouping intervals to improve model fit for the detection function. Following Buckland et al. (Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001), a variety of key functions and adjustment term combinations were considered to model the detection function (e.g. uniform + cosine or simple polynomial, half-normal + cosine or hermite polynomial, hazard rate + cosine or simple polynomial). Goodness of fit tests were used to identify violations of assumptions. Akaike’s Information Criterion for small sample sizes (AICc) was used in model selection (Burnham and Anderson Reference Burnham and Anderson2002), with particular attention paid to model fit at distances near zero since the fit of the shoulder near zero is most important for robust estimation (Buckland et al. Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001). Maximum likelihood methods were used to estimate the variance of the effective detection radius (EDR). To test for bias in the estimate of group size we applied a statistical hypothesis test at the 15% a-level to the regression of natural logarithm of group size against the probability of detection at distance x from the line, using Distance. If the regression was statistically significant, the expected group size was used, otherwise average group size was used to estimate population density. Estimates of density were obtained by survey month for the Ulz River surveys and overall for the Onon Balj surveys.
Occupancy methods that estimate the proportion of sample sites occupied when detectability is less than 100% have also been successfully applied to birds and other species (Mackenzie et al. Reference Mackenzie, Nichols, Lachman, Droege, Royle and Langtimm2002, Reference Mackenzie, Nichols, Royle, Pollock, Bailey and Hines2006). The methods were used to analyse the 2011 Onon Balj survey data to provide estimates of the probability that a WNC is detected, the probability that a sampling unit is occupied by WNC, and to investigate the factors that might influence these. Site covariates associated with each sampling point that had the potential to influence occupancy included habitat, ground moisture, human settlements, sign of fire, and grazing intensity. The sampling covariates thought to influence detectability that were recorded for each of the four visits to each point included observer team, proportion of unobstructed circular area available for observation (referred to as circular area), and visibility. Aside from WNCs, other crane species (Common Crane Grus grus, and Siberian Crane Leucogeranus leucogeranus) were also present in the areas sampled by the observers, and the assumption is that there were no errors in identifying species. Observations that were not identified to species were omitted, so estimates of occupancy may be underestimated for WNCs. Data were analysed using the single-season option in the Presence software (Hines Reference Hines2006). Model selection was done using the Akaike’s Information Criterion (AIC) values produced by Presence (Burnham and Anderson Reference Burnham and Anderson2002). The MacKenzie and Bailey (Reference Mackenzie and Bailey2004) goodness-of-fit test was run using 1,000 bootstrap iterations within Presence to assess model fit.
Factors influencing distribution of WNC along the Ulz River
Our spatial modeling efforts focused on the Ulz River data as this area was surveyed the most intensely (both by Bradter et al. in 2001, and in 2010 and 2011 during the present study). To develop a habitat model, we divided the 270 km Ulz River transect into 5 × 5 km blocks. We then assessed the presence or absence of WNCs in each block to derive a binary response variable. To estimate habitat quality, we used Normalized Difference Vegetation Index (NDVI) data acquired by the Moderate Resolution Imaging Spectro-radiometer (MODIS) on board the TERRA satellite. We obtained three 16-day (May 25–Jun 09) NDVI composites (for 2001, 2010 and 2011) in 250-m resolution from NASA’s Earth Observing System Gateway (http://reverb.echo.nasa.gov) and re-projected the data to Transverse Mercator (UTM zone 49 N). The mean NDVI raster cell value for the each 5 × 5 km block was calculated using Focal Statistics Tool in ArcMap 10.0. To examine the effects of human disturbance on WNC distribution, we calculated Euclidean distances to the nearest soum (towns/villages) from the centre of each block using the Near function of Analysis Tool in ArcMap 10.0.
We used a t-test to compare differences in habitat quality or wetness (indexed as NDVI) between 2001 and 2011. Generalized Linear Mixed Models (GLMM) with a binomial error distribution were fit to the data using the “lme4” library in R (R Development Core Team 2008), to investigate WNC distribution in relation to environmental and human associated variables. WNC distribution was defined based on the location of TPs, using data from 2001 (provided by U. Bradter in litt. 2014), as well as data collected during the present study. We excluded 2010 data from the analysis because sample size was small. Model selection was performed using AIC values (Burnham and Anderson Reference Burnham and Anderson2002). We incorporated year (2001 vs. 2011) as a random factor in the analysis to account for potential differences in vegetation quality Moran’s I correlograms of model residuals were used to investigate potential spatial autocorrelation. We used the area under the receiver operating characteristic curve (AUC) to measure the discrimination ability of the final models, with 0.5 showing no discrimination ability and 1.0 showing perfect discrimination ability of a model (Pearce and Ferrier Reference Pearce and Ferrier2000).
Results
Ulz River transect
Along the Ulz River transect we recorded 10 TPs in 2010, of which seven (64%) were confirmed to be BPs (Table 1). The more intensive surveys in 2011 detected 17 TPs but only three (18%) BPs. A single LBP was recorded in 2010, but no LBPs were observed along the Ulz River transect in 2011. Recruitment was found to be 37.5% in 2010 and 10.5% in 2011. All BPs along the Ulz were observed to fledge at least one chick in both 2010 and 2011, and so fledging success was 100% in both years as defined by Bradter et al. (Reference Bradter, Gombobaatar, Uuganbayar, Grazia and Exo2005). The LBP recorded in 2010 was also successful, thus maintaining the 100% fledging success for all nests observed during the survey. Mean densities of TPs were 0.37 per 10 km in 2010 and 0.60 per 10 km in 2011. Highest density of TPs was 7 per 10 km in the western extent of the survey area in 2011. The conservative minimum number of individual WNCs present within the transect was estimated as 35 in May 2010 and 71 in May 2011.
Table 1. Summary of Ulz River transect results.
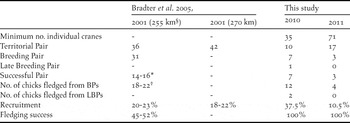
§ In 2001 Bradter et al. (Reference Bradter, Gombobaatar, Uuganbayar, Grazia and Exo2005) was unable to exclude the possibility of failed breeding attempts prior to first surveys in late May in 15 km of the transect.
* Two pairs still had dependent (unfledged) chicks at the end of the study period.
† 18 chicks had fledged at the end of the study period, and four chicks were still dependent.
Generalized survey
A total of 27 TPs were recorded during the generalized surveys (covering sites in Gun Galuut, the Kherlen River, Onon River and Khurkh Khuiten) in 2010 of which 11 (41%) were found to be BPs (Table 2). The behaviour of one pair on the Onon River was consistent with the presence of a chick, but vegetation cover prevented confirmation. These sites were revisited in 2011 and yielded 34 TPs, of which 15 were BPs (44%). However, in 2011 the Onon River and Khurkh Khuiten were only visited in early June, and so numbers of TPs and BPs should be considered an underestimate, as pairs that failed early will not be captured in the total. Overall fledging success was found to be 55% in 2010, and 50% in 2011 for BPs where outcome was known (i.e. excluding Khurkh Khuiten BPs).
Table 2. Summary of results from generalized surveys, and Onon Balj National Park.

§ One pair on the Onon River behaved as if tending a chick, but confirmation was not possible.
* One Late Breeding Pair in Khurkh Khuiten in 2010 was observed to be incubating in July, and so outcome of this nesting attempt could not be determined.
† Surveys along the Onon River, Khurkh Khuiten and Onon Balj National Park were only performed in early June in 2011, therefore outcome could not be determined.
Intensive Onon Balj surveys
Surveys within Onon Balj National Park by the four independent teams resulted in 144 sightings of WNC, 67 sightings of Common Crane, six sightings of Siberian Crane and five sightings that could not be assigned to species. As survey teams were independently visiting the same 60 locations many of these will constitute repeat sightings (for instance only two Siberian Cranes were observed independently by three teams). Most of the cranes showed no signs of breeding activity, with many observed in loose mixed species congregations of up to 36 individuals). However, taking account of duplicate observations, a minimum of 52 WNCs were observed, of which five were thought to be TPs, and two to be BPs.
Crane detectability
For the Ulz River survey of 2011, the final model – a half-normal with no adjustment terms – included all 130 observations of WNC groups to a maximum distance of 3,200 m, and the detection probability was estimated as 0.27 (95% CI = 0.22–0.34) with an effective detection radius (EDR) of 1,672.7 m (Figure 2a). Data were grouped into six equal-sized intervals for the final analysis due to severe rounding in the radial distance values. Although the majority of observations were pairs of WNC, the average group size was larger in July and August, and there was also occasionally size bias in the estimate of group size with expected group size used in those instances. The encounter rates and densities for each survey month are shown in Table 3. The histograms of observation frequency relative to distance from the sampling point indicated that the assumption of uniform distribution of the birds relative to the observers was likely violated, so these densities should be treated as relative rather than absolute measures. The density estimates corresponding to the random sampling points seem to indicate a peak in July, whereas non-random points have higher densities overall (as they focused on areas where WNCs had been recorded previously) and a later peak in August.
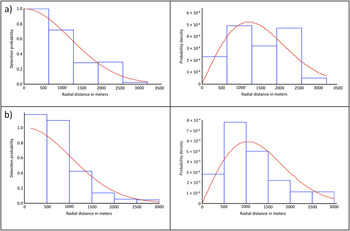
Figure 2. Detection probability functions (left) and probability density functions (right) for (a) Ulz River, and (b) Onon Balj surveys in 2011. The probability density function reflects the true distribution of observations as a function of distance from the observer, and permits the estimation of detectability at zero radial distance.
Table 3. Distance sampling estimates for the Ulz River (also stratified by month visited) and Onon Balj surveys. Encounter rates (n / K), estimates of density (
![]() $\hat D$
) in number/km2 with their 95% confidence intervals (95% CI) and overall percent coefficient of variation (%CV) are shown.
$\hat D$
) in number/km2 with their 95% confidence intervals (95% CI) and overall percent coefficient of variation (%CV) are shown.
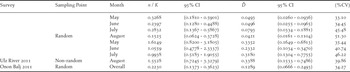
For the Onon Balj survey of 2011, the final model - a half-normal with no adjustment terms - included all 36 observations to a maximum distance of 3,000 m, and the detection probability was estimated as 0.24 (95% CI = 0.18–0.32) with an effective detection radius (EDR) of 1,453 m (Figure 2b). Exact distances rather than distance intervals were used by this model. Unlike the Ulz River survey, the majority of observations constituted larger aggregations rather than singletons or pairs of WNC, thus the average group size was larger overall (3.83). There was no indication of size bias in the estimate of group size. The encounter rate and density estimate are shown in Table 3. Again there were indications that the assumption of uniform distribution of the birds relative to the observers was likely violated, so these densities should again be treated as relative rather than absolute measures.
For the 60 Onon Balj points covered during 2011, the naive estimate of occupancy was 0.2667. The model without covariates that assumed constant occupancy across the habitat patches and constant detectability provided an estimate for probability of occupancy of 0.3093 (SE = 0.0704) and detection probability of 0.3907 (SE = 0.0737). These estimates are likely biased as not all covariates causing heterogeneity in detectability are included. Reductions in AIC value were greatest when both visibility and circular area were included as covariates for detectability with increases in estimated detectability for increasing area, as expected, but with a decrease in detectability for improved visibility (perhaps due to an increase in crane activity in conditions of poor visibility, such as the early morning when mist may hamper observation, or due to heightened concentration of observers in conditions of poor visibility). The inclusion of observer team created numerical problems, probably due to the limited number of replicate observations for some observer teams, and was thus omitted. Estimated probability of occupancy increased when ground was wet and was positively associated with the wet meadow habitat. Particularly as the proportion of wet meadow habitat increased, the estimated probability of occupancy also increased significantly. Estimated probability of occupancy decreased as grazing intensity increased and was negatively associated with reed habitat. There was no clear positive or negative association with willow or steppe habitats. There was no statistically significant relationship found to the number of gers or other structures, nor to the proportion of area with signs of recent fire. Table S1 in the online supplementary material lists the model set with model likelihood values. The top-ranked model that had considerable weight in the data included grazing intensity and the wet meadow habitat as covariates for occupancy, and visibility and circular area as covariates for detectability. The goodness-of-fit test indicates no problems with model fit and no overdispersion. For this top-ranked model the estimated probability of occupancy and detectability was on average 0.5439 (SE = 0.045, range = 0.0206–1) and 0.2333 (SE = 0.0243, range = 0.0046–0.7676).
Factors affecting distribution of WNCs
Overall, WNC were present in 35% of the surveyed area (20 of 57 sampled blocks) in 2001, and 14% (8 of 57 sampled blocks) in 2011. Average (± SD) NDVI value per survey blocks in 2001 (4,521 ± 556) was significantly greater (t = 6.20, P < 0.001) than for 2011 (3,916 ± 481; Figure 3). For the pooled data across years, the final model indicated strong influence of the NDVI and proximity to soums on the WNC distribution (Table 4). Estimated probability of WNC presence increased as vegetation productivity and distance to soums increased. In other words, WNCs preferred areas with greater vegetation productivity but they tended to avoid areas closer to soums (Figure 4). The estimated variance of the random effect (e.g. year) was nearly zero (Table 4). There was no indication of significant spatial autocorrelation. The AUC for the final model was 0.75, indicating that the model had good discrimination ability.
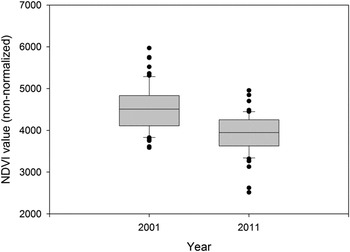
Figure 3. Comparison of average NDVI (non-normalized) values per 25 km2 survey block (n = 57) along the Ulz River in Eastern Mongolia between 2001 and 2011.
Table 4. Parameter estimates of the mixed model explaining distribution of White-naped Cranes along the Ulz River during 2001 and 2011, Eastern Mongolia.

Significance codes: ‘***’ 0.001; ‘**’ 0.01; ‘*’ 0.05
Random effect: year, SD = 8.747e-09
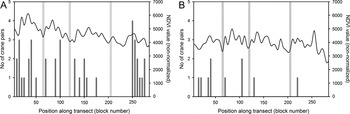
Figure 4. Number of White-naped Crane pairs (dark grey bars) and average NDVI (non-normalized) values (black line) per 25 km2 block (n = 57) along the Ulz River survey transects in 2001 (A) and 2011 (B) in Eastern Mongolia. Grey bars indicate location of soums along the survey transects.
Discussion
The number of TPs recorded along the 270 km Ulz River transect was considerably lower in both 2010 and 2011, than the numbers reported a decade earlier. Even the conservative number of 31 BPs recorded along 255 km of the Ulz Basin in 2001 greatly exceeded the seven and three BPs recorded along the full 270 km transect in 2010 and 2011, respectively. Given the geographic focus of the 2001 surveys, and the limitations of available historic data from other locations, we are unable to confirm whether the reduction in WNC numbers along the Ulz River transect is due to a decline in WNC population numbers, or to the relocation of birds to more suitable sites. However, (with the exception of the Khurkh Khuiten Valley) the relatively low numbers of WNCs observed during the generalized surveys, which encompassed sections of the Kherlen and Onon Rivers that were formerly considered strongholds of WNCs, do suggest that crane populations may indeed be in decline. The higher number of TPs recorded in 2011 was likely due to more intensive survey methods, rather than a true increase in crane numbers. However, the apparent reduction in breeding pairs from 2010 to 2011 implies that the proportion of cranes attempting to breed in a given year may vary, although the factors influencing this were unclear.
The reduction in numbers of TPs across the Ulz River transect was not uniform, with encounter rates of TPs as high as seven per 10 km of river basin in 2010 and 2011 comparing favourably to the 4.7 and 8.8 pairs per 10 km recorded in 2000 and 2001, respectively. The almost complete withdrawal of cranes along eastern portions of the transect in 2010 and 2011, therefore had a disproportionate impact on the total count in these years (Figure 3). This was likely due to the exceptionally dry conditions in the eastern third of the transect, as no water was present in the river channel, and several lakes described by Bradter et al. (Reference Bradter, Gombobaatar, Uuganbayar, Grazia and Exo2005) were either severely depleted, or completely dry. The importance of ground moisture was supported by the findings of the occupancy model (based on a visual estimate of wet meadow coverage), and by the GLMM (using vegetation productivity based on NDVI). The decline in habitat suitability throughout the Ulz River transect between 2001 and 2011 is clearly illustrated by comparing the NDVI measured across the transect in 2001, with that measured a decade later (Figure 3).
Both the occupancy model and the GLMM found that TPs are selecting areas away from habitation, where grazing intensity is lower. This is consistent with the findings of Bradter et al. (Reference Bradter, Gombobaatar, Uuganbayar, Grazia and Exo2005). However, this factor is likely to have remained relatively constant between 2001 and 2011 as both human population and livestock numbers in the survey districts have remained relatively stable during this period (National Statistical Office 2012). Both methods of estimating detectability obtained very similar results. The distance sampling method applied to the Ulz River and Onon Balj datasets provided detection probabilities of 0.27 and 0.24, respectively, with the lower detection probability in the latter likely to relate to the increase in woody vegetation in the taiga habitat of Onon Balj. The detection probability of 0.23 estimated by the top-ranked occupancy model based on the Onon Balj dataset was remarkably similar to the estimate obtained using the distance sampling method for this site. Together, these results suggest that approximately 75% of WNCs are not detected during count surveys in Mongolian breeding habitat. This finding is surprising for a large and distinctive bird, occupying relatively open terrain, but the consistency of results using different datasets and methods is compelling. Based on these detection probabilities, the estimated minimum count of 234 cranes observed in 2011 across the Ulz River transect and areas covered within the generalized survey, may actually represent 867 to 1,017 WNCs. These figures are equivalent to approximately 74–87% of the mean maximum WNC counts obtained at the main Chinese wintering site at Poyang Lake, between 2006 and 2012. While attempts to count cranes in the vast wetland of Poyang Lake presents its own challenges (Li et al. Reference Li, Wu, Harris and Burnham2012), counts obtained at large wintering aggregations are less likely to be impacted by the low detection probabilities we found in Mongolian breeding sites, suggesting the sites we surveyed support a substantial portion of the western population.
Despite the reduction in WNC numbers recorded in the Ulz River transect, our findings indicate that BPs are successfully raising chicks to the point of fledging. However, across the transect the number of fledglings observed in 2010 and 2011 was merely 55% and 18%, respectively of that recorded in 2001. This might seem to contradict the high fledging success recorded in 2010 and 2011, but we suspect these estimates are unrealistically high. It is more likely that surveys that began from 19 to 22 May would not have detected breeding attempts that failed prior to this date, and these high fledging success estimates likely reflect a moderate underestimate of BPs in the 2010 and 2011 surveys.
In conclusion, while the limitations of historic data prevent us from assessing the extent of any spatial shift in overall breeding populations, it is clear that the number of territorial WNCs present within the most intensively studied habitat have declined substantially in the 10 years since 2001. In the context of estimated detection probabilities, the areas surveyed within this study are likely to support a large proportion of the western breeding population of WNCs. This finding, together with the negative trend in counts at the primary wintering location in Poyang Lake, suggests that the western WNC population is undergoing a marked decline. Surveys confirm the importance of shallow wetlands, in areas with low human and livestock pressure as breeding habitat for WNCs. Careful management of these wetland systems (in such areas as the Ulz River basin and the Khurkh Khuiten Ramsar Site) will be vital to maintaining the productivity of breeding WNCs. Such protection should be managed sensitively, as these resources are also critical to the livestock tended by nomadic herders, elevating the potential for conflict, particularly during periods of drought.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270915000301
Acknowledgements
We would like to extend special thanks to N. Tseveenmyadag, Ute Bradter, and Sundev Gomboobaatar for their generosity in sharing their expertise on Mongolian cranes. We are also grateful to Chris Bowden, Farah Ishtiaq, Ruth Tingay, Sue Cardillo, Peter Zahler, and the attendees of the Onon Balj 2011 White-naped Crane Transboundary Working Group workshop for their assistance during surveys. Funding for this project was provided by the National Institute of Allergy and Infectious Disease, National Institutes of Health, Department of Health and Human Services (Contract No. HHSN2662007 00009C); the United States Agency for International Development (USAID) Scaling up Conservation Success with Sustainable Conservation Approaches in Priority Ecosystems Cooperative Agreement (No. EEM-A-00-09-0007-00); and the Wildlife Conservation Society.