Introduction
The White-winged Flufftail is regarded as the rarest and most threatened rallid species in Africa (Taylor Reference Taylor1994, BirdLife International 2018, Evans et al. Reference Evans, Smit-Robinson, Tarboton, Taylor, Peacock and Wanless2015). It is currently listed as ‘Critically Endangered’ (CR) (BirdLife International 2018), with an estimated global population size of 700 mature individuals. However, due to data deficiency, confidence in the population estimate is low and it is believed that the population could be significantly smaller (Evans et al. Reference Evans, Smit-Robinson, Tarboton, Taylor, Peacock and Wanless2015). A study describing the Toll-like receptor genetic diversity in White-winged Flufftail confirms low genetic diversity in the innate immune regions similar to that observed in other bird species that have undergone population bottlenecks (Dalton et al. Reference Dalton, Vermaak, Smit-Robinson and Kotzé2016). This species is largely known from the Ethiopian Highlands and eastern South Africa, with isolated historical sightings also noted in Zambia and Zimbabwe (Taylor Reference Taylor, Hockey, Dean and Ryan2005). Records across the two primary regions display significant temporal separation, with Ethiopian and South African records being largely confined to June–September and October–March respectively (Taylor Reference Taylor1994, Taylor and van Perlo Reference Taylor and van Perlo1998). Furthermore, despite numerous surveys, all breeding records have been exclusively confined to the Ethiopian Highlands (Mendelsohn et al. Reference Mendelsohn, Sinclair and Tarboton1983, Taylor Reference Taylor1994, Taylor and van Perlo Reference Taylor and van Perlo1998, Taylor Reference Taylor, Hockey, Dean and Ryan2005, Allan et al. Reference Allan, McInnes and Wondafrash2006, Davies et al. Reference Davies, Smit-Robinson, Drummond, Gardener, Rautenbach, Van Stuyvenberg, Nattrass, Pretorius, Pietersen and Symes2015). These two factors, together with the lack of subspeciation between the two regional populations (Dalton et al. Reference Dalton, Smit-Robinson, Vermaak, Jarvis and Kotzé2018), has suggested that the species is migratory between the northern breeding range in the Ethiopian highlands and non-breeding austral summer range in South Africa (Taylor Reference Taylor, Hockey, Dean and Ryan2005, Sande et al. Reference Sande, Ndang’ang’a, Wakelin, Wondafrash, Drummond and Dereliev2008).
In the context of southern African rallids, the White-winged Flufftail is one of the smallest species occurring in the region (length 13.5–14.5 cm, mass 32 g; Taylor Reference Taylor, Hockey, Dean and Ryan2005). It is noted as a habitat specialist, inhabiting palustrine wetland habitats at typically higher elevations (1,100–1,900 m asl), but three vetted records have been noted at lower elevations in the South Africa (e.g. 150 m) (Taylor and van Perlo Reference Taylor and van Perlo1998). South African records have generally originated from dense and short (50 cm) sedge Carex spp. dominant vegetation, but have also been noted in mixed stands of sedge and taller species (Phragmites and Typha spp.) (Taylor Reference Taylor, Hockey, Dean and Ryan2005, Evans et al. Reference Evans, Smit-Robinson, Tarboton, Taylor, Peacock and Wanless2015). Furthermore, one commonality across studies suggests a strong preference for shallow water within wetland habitats, with an increase in water level through flooding (natural or anthropogenic) generally leading to the abandonment of the respective site (Taylor Reference Taylor1994, Reference Taylor, Hockey, Dean and Ryan2005). Unlike other African rallid species, White-winged Flufftail has a noted lack of vocalisation across its range (Allan et al. Reference Allan, McInnes and Wondafrash2006, Davies et al. Reference Davies, Smit-Robinson, Drummond, Gardener, Rautenbach, Van Stuyvenberg, Nattrass, Pretorius, Pietersen and Symes2015). Although auditory surveys have been used previously for this species (Taylor 1995), recent surveys (Allan et al. Reference Allan, McInnes and Wondafrash2006. Davies et al. Reference Davies, Smit-Robinson, Drummond, Gardener, Rautenbach, Van Stuyvenberg, Nattrass, Pretorius, Pietersen and Symes2015) suggest discrepancies exist. Until clarified, the implications thereof impede the use of auditory surveys as a suitable/reliable monitoring method. Therefore, the exact vocalisation and use by White-winged Flufftail remain relatively ambiguous.
The low density, localised distribution, small body size, cryptic colouration, aquatic and densely vegetated habitat preference, extremely elusive behaviour and lack of auditory cues have made this species notoriously difficult to study (Mendelsohn et al. Reference Mendelsohn, Sinclair and Tarboton1983, Taylor Reference Taylor1994, Reference Taylor, Hockey, Dean and Ryan2005, Allan et al. Reference Allan, McInnes and Wondafrash2006, Davies et al. Reference Davies, Smit-Robinson, Drummond, Gardener, Rautenbach, Van Stuyvenberg, Nattrass, Pretorius, Pietersen and Symes2015). Subsequently, there are significant data deficiencies with regard to facets of the species’ population status, biology, distribution, habitat use, activity patterns, migratory status and dispersal behaviour (Evans et al. Reference Evans, Smit-Robinson, Tarboton, Taylor, Peacock and Wanless2015). A recent study based on the sequencing of mitochondrial (COI, Cytb, 12S/Val/16S) and nuclear (ADH-5, GPD3-5 and bfib7) markers affirms that South African and Ethiopian birds are genetically similar (Dalton et al. Reference Dalton, Smit-Robinson, Vermaak, Jarvis and Kotzé2018), supporting the hypothesis that these two regions do not host different species or subspecies but rather one migrating population, having separate ranges during the different seasons in Ethiopia and South Africa. Given the current conservation status of the species, it is of utmost importance that fundamental gaps in our understanding of this species be addressed in order to better direct conservation efforts.
Until recently, the most common method utilised to confirm the presence of this species was an invasive form of sampling that elicits a flushing response of individuals inhabiting the target habitat type through walked transects and/or rope dragging (Green Reference Green1985, Bibby et al. Reference Bibby, Jones and Marsden1998). However, the elusive and skulking nature of many rallid species, particularly those of the Sarothrura (flufftail) genus, combined with their reluctance to take flight, has been noted as a potentially significant limitation to the efficiency of rope dragging for these species (Davies et al. Reference Davies, Smit-Robinson, Drummond, Gardener, Rautenbach, Van Stuyvenberg, Nattrass, Pretorius, Pietersen and Symes2015). In addition to the detection bias and limited efficacy associated with rope dragging for some rallid species, the potential impact of rope dragging (e.g. trampling and disturbance) on both the target species and associated habitat has elicited concern (Davies et al. Reference Davies, Smit-Robinson, Drummond, Gardener, Rautenbach, Van Stuyvenberg, Nattrass, Pretorius, Pietersen and Symes2015, Colyn et al. Reference Colyn, Campbell and Smit-Robinson2017). However, a recent study utilising a non-invasive array of camera traps proved efficient at registering the presence and activity of multiple cryptic and elusive rallid species (including one flufftail species) within palustrine wetland habitat in South Africa (Colyn et al. Reference Colyn, Campbell and Smit-Robinson2017). The primary aim of our study was to ascertain if a non-invasive array of camera traps could be used to document the first estimate of site occupancy, fine scale habitat use, and activity patterns of White-winged Flufftail across an austral summer season. Furthermore, we aimed to assess the site occupancy, habitat use and activity patterns of other rallid species recorded and determine if inter-species influences were noted.
Methods
Study site
Our study area is located between the Dullstroom (25°25’6.18“S 30° 6’14.66”E) and Belfast (25°40’28.67“S 30° 4’47.71”E) areas of Mpumalanga, South Africa. This area has yielded the most frequent and consistent number of White-winged Flufftail records in South Africa (Taylor Reference Taylor1994) and has been the focal area for a recent species-specific study (Davies et al. Reference Davies, Smit-Robinson, Drummond, Gardener, Rautenbach, Van Stuyvenberg, Nattrass, Pretorius, Pietersen and Symes2015). A particular palustrine wetland system within the given region, namely Middelpunt Wetland (MW), has been actively surveyed for White-winged Flufftail presence and activity using rope dragging in 2005, 2006, 2011, 2014 and 2015 (Davies et al. Reference Davies, Smit-Robinson, Drummond, Gardener, Rautenbach, Van Stuyvenberg, Nattrass, Pretorius, Pietersen and Symes2015). Given the existing survey history of MW and recorded presence of the species across some of the respective years surveyed (e.g. Davies et al. Reference Davies, Smit-Robinson, Drummond, Gardener, Rautenbach, Van Stuyvenberg, Nattrass, Pretorius, Pietersen and Symes2015), occasioned MW as being an ideal study site.
The broader study site is largely comprised of high-altitude palustrine wetland and adjacent highland grassland. MW is part of the Lakenvlei wetland system, which is currently listed as an the Steenkampsberg Important Bird and Biodiversity Area (IBA; SA 016) (Marnewick et al. Reference Marnewick, Retief, Theron, Wright and Anderson2015) and forms part of the recently declared Greater Lakenvlei Protected Area. The palustrine wetland vegetation communities present at MW include three dominant habitat types, namely sedge meadows (Cyperaceae spp.), and Phragmites and Typha beds (Davies et al. Reference Davies, Smit-Robinson, Drummond, Gardener, Rautenbach, Van Stuyvenberg, Nattrass, Pretorius, Pietersen and Symes2015). Additionally, six notable habitat patches incorporating mixed vegetation (sedges, forb, reed spp., etc.) have been documented (Davies et al. Reference Davies, Smit-Robinson, Drummond, Gardener, Rautenbach, Van Stuyvenberg, Nattrass, Pretorius, Pietersen and Symes2015). Apart from White-winged Flufftail, MW is known to host six other rallid species across the respective years surveyed (Davies et al. Reference Davies, Smit-Robinson, Drummond, Gardener, Rautenbach, Van Stuyvenberg, Nattrass, Pretorius, Pietersen and Symes2015).
Survey design
The survey design in terms of camera placement was tailored as per Colyn et al. (Reference Colyn, Campbell and Smit-Robinson2017). However, given the primarily species-specific focus and aim to estimate site occupancy, the survey design had to be altered to maintain occupancy assumptions (MacKenzie et al. Reference MacKenzie, Nichols, Royle, Pollock, Bailey and Hines2006). Given the absence of data related to territory size of White-winged Flufftail, the territory size of a sympatric species known to co-occur in similar habitats, namely Red-chested Flufftail Sarothrura rufa (Mendelsohn et al. Reference Mendelsohn, Sinclair and Tarboton1983, Taylor Reference Taylor1994), was used to inform camera spacing. Red-chested Flufftail territory size in continuous habitat such as MW is noted as 0.10–0.45 ha, resulting in a potential average territory diameter of 10–50 m (Taylor Reference Taylor1994). Therefore, in order to maintain independence and prevent spatial auto-correlation, camera spacing was larger than the diameter of the average territory size (Rovero et al. Reference Rovero, Zimmermann, Berzi and Meek2013).
A further consideration incorporated into our survey design was the noted efficiency of surveying extensively when studying rare species (MacKenzie and Royle Reference MacKenzie and Royle2005). Extensive surveying incorporates surveying at as many sites as feasible to increase spatial coverage (MacKenzie and Royle Reference MacKenzie and Royle2005). Similarly, MacKenzie et al. (Reference MacKenzie, Nichols, Lachman, Droege, Royle and Langtimm2002) note that increasing the number of sampling occasions (temporal coverage), increases both the precision and accuracy of derived occupancy estimates. Although the majority of sightings have been documented in sedge-dominant vegetation at MW (Mendelsohn et al. Reference Mendelsohn, Sinclair and Tarboton1983, Taylor Reference Taylor1994, Davies et al. Reference Davies, Smit-Robinson, Drummond, Gardener, Rautenbach, Van Stuyvenberg, Nattrass, Pretorius, Pietersen and Symes2015), Taylor (Reference Taylor1994) includes records obtained in South Africa from Phragmites, Typha and mixed vegetation habitats. Additionally, MacKenzie and Royle (Reference MacKenzie and Royle2005) recommend that sites are not exclusively selected based on historical or pre-existing knowledge of occurrence alone, as this can produce inflated occupancy estimates. Therefore, the resulting survey design incorporated a stratified grid of nine Ltl Acorn 6540MC camera traps spaced at a minimum distance of 50 m across the core wetland habitats at MW (Figure 1). The grid allowed for camera placement across all dominant vegetation communities present at MW. These included sedge-dominant, Phragmites-dominant, Typha-sedge and sedge-forb mixed vegetation communities (Table S1 in the online supplementary material). Due the unreliability and stochasticity of White-winged Flufftail presence, survey duration was maximised to include a consecutive 72-day survey across the entire peak temporal period known to host records in South Africa, namely December to February (Taylor Reference Taylor, Hockey, Dean and Ryan2005).
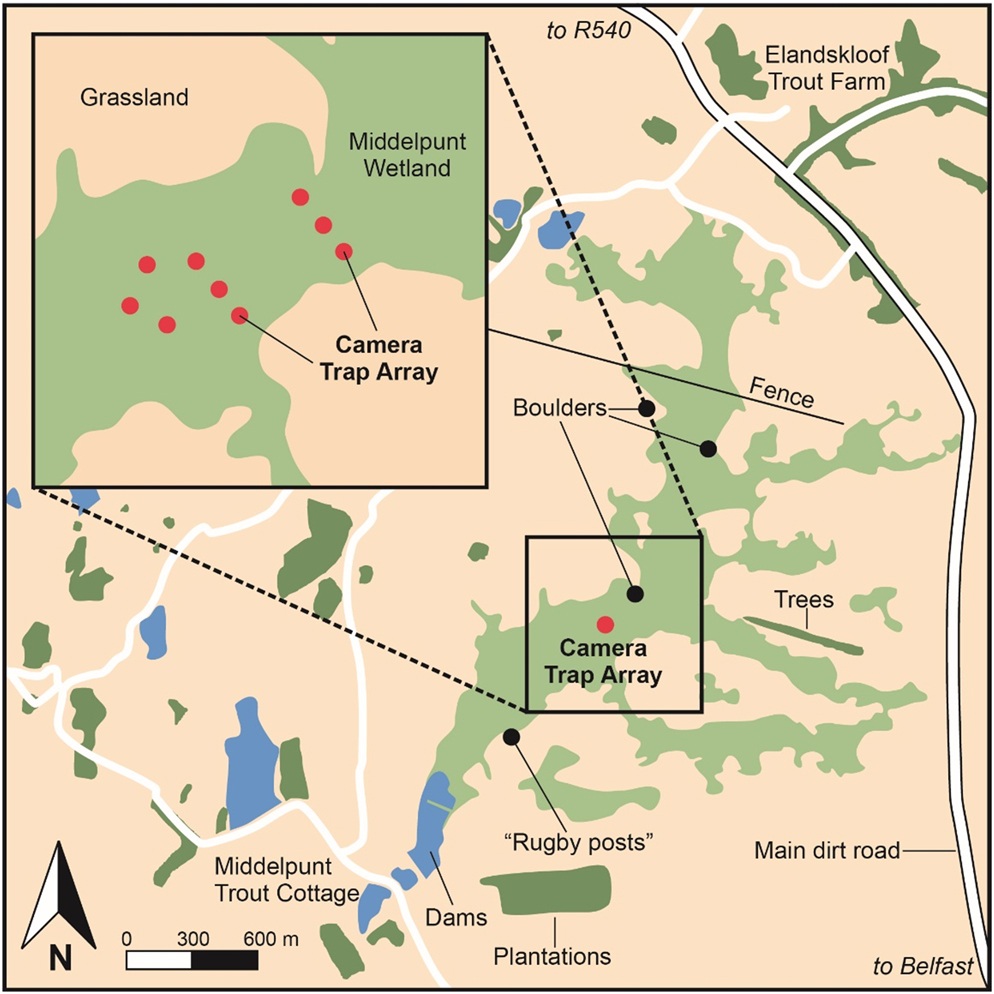
Figure 1. The survey design layout of camera traps across core wetland habitat at the study site, Middelpunt Wetland.
Camera trap site characteristics
Rainfall and fire history are two drivers known to influence the state of wetland microhabitats (Rogers et al. Reference Rogers, Ellery, Winternitz and Dohmeier1989). Microhabitat characteristics known to influence White-winged Flufftail presence includes water depth, vegetation structure and vegetation community (Davies et al. Reference Davies, Smit-Robinson, Drummond, Gardener, Rautenbach, Van Stuyvenberg, Nattrass, Pretorius, Pietersen and Symes2015). Broad vegetation communities were mapped out by recording coordinates of community boundaries whilst walking through the wetland (Davies et al. Reference Davies, Smit-Robinson, Drummond, Gardener, Rautenbach, Van Stuyvenberg, Nattrass, Pretorius, Pietersen and Symes2015), which was further supplemented by high resolution aerial (drone) photographs. Water depth was logged using a yardstick within each camera trap detection arc (i.e. photo boundary) and recorded as a categorical variable: 1 (< 3 cm), 2 (3–10 cm) and 3 (> 10 cm).
The period surveyed (December 2016–February 2017) produced 690.4 mm of rainfall, which was higher than the average (585 mm) for the area (South African Weather Services 2017). As part of management practices by landowners, MW was burnt during the dry wintering months preceding the survey. Additional data related to rainfall, water depth and fire history across the respective study site are provided in Table S1 and Figure S1.
Data analyses
In addition to the assumption of independence addressed through our survey design above, closure is another assumption requiring consideration with regard to occupancy modelling (MacKenzie et al. Reference MacKenzie, Nichols, Lachman, Droege, Royle and Langtimm2002, Reference MacKenzie, Nichols, Royle, Pollock, Bailey and Hines2006). Closure assumes that all sites sampled remain closed to changes in occupancy state over the course of sampling (MacKenzie et al. Reference MacKenzie, Nichols, Lachman, Droege, Royle and Langtimm2002, Reference MacKenzie, Nichols, Royle, Pollock, Bailey and Hines2006, O’Connell and Bailey 2011, Rovero et al. Reference Rovero, Zimmermann, Berzi and Meek2013). Closure can be impacted on by immigration, emigration, births and deaths and is particularly relevant to studies focusing on wide ranging and/or migratory species (MacKenzie et al. Reference MacKenzie, Nichols, Royle, Pollock, Bailey and Hines2006). Due to the unpredictable nature of White-winged Flufftail presence across its range and therefore at MW, closure across the entire peak season (December to February) could not be assumed. Immigration into and emigration out of the site could occur at any given period and the average length of use/stay within an occupied wetland in unknown. Kendall (Reference Kendall1999) addresses the immigration or emigration of a species during the study period by pooling datasets, but if both movements are expected, the associated bias will remain (MacKenzie et al. Reference MacKenzie, Nichols, Royle, Pollock, Bailey and Hines2006). Therefore, in order to maintain closure for White-winged Flufftail or any other migratory rallid recorded during the respective survey, data were truncated to include samples from the first and last detection only (MacKenzie et al. Reference MacKenzie, Nichols, Lachman, Droege, Royle and Langtimm2002). Furthermore, for any resident/non-migratory rallid species assessed, truncation was not utilised and closure was assumed as per single-season occupancy models (MacKenzie et al. Reference MacKenzie, Nichols, Lachman, Droege, Royle and Langtimm2002, O’Connell and Bailey 2011, Hamel et al. Reference Hamel, Killengreen, Henden, Eide and Roed-Eriksen2013, Rovero et al. Reference Rovero, Zimmermann, Berzi and Meek2013).
Data were collated into binary (presence/absence) detection history matrices for the consecutive 72 days surveyed. Data collation included all ground-dwelling avian species and medium-to-large (> 0.2 kg) mammal species. Key identification characteristics of White-winged Flufftail during data collation included primarily white secondaries and chestnut-barred tail, as well as hind neck, mantle and breast colouration (Taylor Reference Taylor1994). In order to increase the detection probability of each individual survey period, every three consecutive days were further collated and synthesised to represent one survey (Ramesh and Downs Reference Ramesh and Downs2013). If camera trap malfunction, interference and/or theft prevented data collection at a given site and over a given sample/s, these were included into the detection history matrix as missing observations (MacKenzie et al. Reference MacKenzie, Nichols, Lachman, Droege, Royle and Langtimm2002). However, given the increase in standard error associated with data loss and associated missing observations (MacKenzie et al. Reference MacKenzie, Nichols, Lachman, Droege, Royle and Langtimm2002), if missing samples for a given site exceeded 20% of the total expected samples to be collected, the site was removed from the dataset utilised for analyses. Furthermore, given the low capture rate of the focal species and resultant low naïve occupancy estimate (< 0.2), site localities were grouped to represent habitat patches (Ehlers Smith et al. Reference Ehlers Smith, Ehlers Smith, Ramesh and Downs2017). The software package Presence was utilised to run occupancy models for respective species and included both site and sample specific covariate data. Categorical covariates were translated into multiple series of binary indicator variables depicted as 0 or 1 (MacKenzie Reference MacKenzie2012). All continuous covariate data were standardised using the z-transformation equation (MacKenzie Reference MacKenzie2012). Covariate data collected and/or collated per camera trap site included average vegetation height, basal cover, canopy cover, dominant fine-scale vegetation community, water depth, trail presence/absence, trail width and trail type (Colyn et al. Reference Colyn, Campbell and Smit-Robinson2017). Model fit was assessed and ranked using the Akaike Information Criterion (AIC) model selection criteria, which included AIC, delta AIC, AIC weight and Model Likelihood values (Anderson and Burnham Reference Anderson and Burnham2002). Additionally, the Pearson’s chi-square statistic and parametric bootstrapping (1,000 runs) as per MacKenzie and Bailey (Reference MacKenzie and Bailey2004) were used to further test model fit.
Activity pattern analysis was conducted to estimate activity peaks and overlap between associated species of interest across two temporal periods, namely a conventional 24-hour cycle and a seasonal cycle (December to February) (Meredith and Rideout Reference Meredith and Rideout2017). Consecutive sightings of the same species at the same camera trap site were deemed independent when separated by a 30 min interval (Rovero et al. Reference Rovero, Jones and Sanderon2005). Furthermore, capture frequency was defined as the number of independent sightings per 100 camera days (Kelly and Holub Reference Kelly and Holub2008). Kernel density estimation (KDE), a non-parametric method of evaluating the density function (Rideout and Linkie Reference Rideout and Linkie2009), was utilised to assess activity patterns in software R (R Development Core Team 2008). Furthermore, the R package “overlap” was used to assess the measure of overlap between peak activity periods of species assessed (Meredith and Rideout Reference Meredith and Rideout2017). Due to the influence of sample size on the performance of estimators of coefficient of overlapping, sample sizes smaller than 50 were allocated formula “Dhat1” (Linkie and Rideout Reference Linkie and Rideout2011, Meredith and Rideout Reference Meredith and Rideout2017). Furthermore, 95% confidence intervals were obtained from 10 000 bootstrap samples per overlap interaction assessed (Linkie and Rideout Reference Linkie and Rideout2011, Meredith and Rideout Reference Meredith and Rideout2017). Due to detection bias, very small avian species (< 20 g) and species utilising an alternative foraging strategy to that of rallids (e.g. foliage gleaners), were listed as non-target avian species and excluded from all analyses (Colyn et al. Reference Colyn, Campbell and Smit-Robinson2017).
Results
Capture frequency and occupancy estimation
Our study was effective in confirming the presence of White-winged Flufftail at MW, with eight independent sightings and an associated capture frequency (cf) of 1.28 (Table 1, Figure 2). The study accumulated a survey effort of 626 camera days from nine camera traps over the survey period, December 2016–February 2017. White-winged Flufftail were exclusively recorded at one of the nine survey locations, a Typha-sedge mixed vegetation community. Furthermore, out of the total survey duration, seven (88%) of the independent sightings were recorded across a consecutive 10-day period from late December to early January, whilst the remaining one independent sighting was recorded 18 days later (late January). Mean occupancy (ψ) and detection probability (P) estimates over the truncated period were 0.20 + 0.18 and 0.22 + 0.07 (Table 2). Results indicated that basal and canopy cover both had strong positive influences on site occupancy (β = 27.28 and β = 29.51), whilst water depth negatively influenced detection probability (β = -96.95). The resultant SE of occupancy estimates (0.20 + 0.18) and the goodness-of-fit value (ĉ = 1.5) for the global model, highlight the influence of the short temporal period during which the species was present and narrow resultant detection-history data obtained.
Table 1. All target (rallid) and non-target (non-rallid) avian species recorded during the camera trap survey at Middelpunt Wetland. Species classified as regionally threatened (Taylor et al. 2015) are highlighted in bold, whilst all non-rallid species are highlighted in grey below the dotted line.


Figure 2. Selected images of a female White-winged Flufftail Sarothrura ayresi recorded during the study (indicated by arrow) displaying a distinctive white patch (i.e. secondaries) whilst walking with wings folded in (above) and conducting an apparent territorial wing-flapping display (below).
Table 2. Summary of the Akaike Information Criterion (AIC) model selection criteria for the two best model fits per species assessed. *Mean occupancy (ψ) and detection (P) probabilities displayed represent the best model fit.

The vegetation community surrounding the presence locality was comprised of c.30% sedge (mean height = 30 cm), 60% Typha (mean height = 170 cm) and 10% forbs (mean height = 20 cm). During the core period at which 88% of independent sightings were made (i.e. late December to early January), the average vegetation height, water depth, basal cover and canopy cover at the exclusive presence locality were 150 cm, 15 cm, 80% and 45% respectively. This area differed significantly (P < 0.05) in relation to average canopy (P = 0.01) and basal (P = 0.003) cover when compared to the other sampled units. Furthermore, the respective sites varied significantly in average water depth across three (P = 0.006, P = 0.02, P = 0.01) of the surveyed localities. Water depth across surveyed localities were not significantly associated with dominant vegetation communities sampled (P = 0.17), but seemingly a factor of local topographic relief (e.g. depressions, channels and sediment compaction). Conversely, as expected, dominant vegetation types sampled varied significantly in average height (P < 0.05).
Three changes in environmental covariate data were noted during the absence of White-winged Flufftail records between the core group of sightings recorded up to early January and the solitary and last sighting logged in late January. Firstly, the last sighting preceded a significant rainfall (flooding) event that increased water depths across all surveyed localities at the study site. At the exclusive presence locality, water depth preceding the rainfall event (mean 15 cm) varied significantly to that directly after the event (mean 30 cm, P = 0.004). Water depth remained relatively high for 12 days from the onset of the rainfall event, after which it became comparable (P = 0.28) to when White-winged Flufftail were first recorded at the locality. Secondly, basal and canopy cover reduced notably following the core group of White-winged Flufftail sightings (early January). Basal cover was significantly reduced by 40% (P < 0.05), whilst canopy cover was marginally reduced by 10% (P = 0.07). Potential disturbance factors of vegetation cover noted during this period included primarily flooding of basal cover and disturbance of sections of canopy cover through the respective rise in water levels. Southern Reedbuck Redunca arundinum were observed moving through this camera locality over the same period, but the associated disturbance impact was thought to be negligible. Significantly, following the absence of White-winged Flufftail records over this period when water depth and vegetative cover (basal and canopy) were altered, a solitary and final sighting was recorded. At the time of the sighting the water depth was comparable to when White-winged Flufftail were first recorded, whilst basal and canopy cover were still significantly lower (P < 0.05, P = 0.07) following the flooding event. Surveying commenced for a further 279 camera days within the survey period (December–February) following this sighting and yielded no additional records.
Three other rallid and four non-rallid avian species were recorded during the survey (Table 1). Other rallid species recorded included Red-chested Flufftail, Spotted Crake Porzana porzana and African Rail Rallus caerulescens with 14, 12 and 303 independent sightings recorded respectively. Spotted Crake (cf = 1.92) and Red-chested Flufftail (cf = 2.24) were relatively comparable in capture frequency to White-winged Flufftail (cf = 1.28), whilst African Rail yielded a significantly greater capture frequency (cf = 48.40) than the other rallid species. Mean occupancy (ψ) and detection (P) probability estimates for other rallid and non-rallid species assessed were in the range 0.20–0.67 and 0.22–0.67 (Table 2). Vegetation height influenced both the occupancy (β = 1.62) and detection probability (β = 2.06) of African Rail positively, whilst canopy (β = 89.02) and basal (β = 65.08) cover strongly influenced the occupancy of Spotted Crake. Red-chested Flufftail occupancy was influenced positively by canopy cover (β = 3.14), whilst detection probability was influenced negatively by water depth (β = -5.45). The last species assessed, African Snipe Gallinago nigripennis, yielded occupancy estimates that responded negatively to vegetation height (β = -1.98) and detection probability that responded positively water depth (β = 0.45).
Spotted Crake was only recorded across a consecutive three-day period in mid-December. However, during this very brief period, it exhibited a high level of activity and associated captures when compared to other species present across the entire study period (Table 1). Furthermore, Spotted Crake was only recorded at the same exclusive camera trap that recorded White-winged Flufftail. The temporal difference at the exclusive spatial location being occupied by Spotted Crake and White-winged Flufftail was six days. The environmental covariate data with regards to water depth and vegetative cover were comparable (P > 0.05) over the peak temporal period of occupancy across the two species. Additionally, both species occupancy estimates were influenced positively by basal and canopy cover (Table 2).
Non-rallid species recorded included ‘Vulnerable’ African Grass Owl Tyto capensis, Marsh Owl Asio capensis, African Snipe and Yellow-billed Duck Anas undulata (Table 1). Non-target species recorded included 13 bird, five mammal and one reptile species (Table S2).
Activity patterns, inter-species interactions, demographics and behaviour
White-winged Flufftail kernel density estimates of activity patterns display peak periods of activity at dawn and dusk (Figure 3). Based on the distribution of sightings recorded, a strong preference for activity surrounding sunrise was displayed, with 60% of sightings registered between 05h00 and 06h00. Of the rallid species assessed, Spotted Crake yielded the highest degree of overlap with White-winged Flufftail, with a resultant overlap coefficient of 0.62 (0.33–0.86) (Figure 4). Red-chested Flufftail and African Rail yielded lower and comparable levels of overlap with White-winged Flufftail: 0.44 (0.17–0.71) and 0.39 (0.17–0.59) respectively (Figure 4). Confidence intervals were relatively wide as a result of the small sample size obtained for White-winged Flufftail (Linkie and Ridout Reference Linkie and Rideout2011). White-winged Flufftail yielded the highest kernel density estimates over the sunrise activity period when compared to the other three rallid species (Figure 4).

Figure 3. Kernel density estimates of daily activity patterns of White-winged Flufftail in high altitude palustrine wetland habitat, South Africa. Dotted lines indicate approximate time of sunrise and sunset over the respective period.

Figure 4. Estimated daily activity patterns (lines) and coefficient of overlap (grey-shaded area) of White-winged Flufftail (solid line) and three other rallid species (dotted line) recorded during the study, namely Spotted Crake, Red-chested Flufftail and African Rail.
When activity patterns were assessed over the entire seasonal cycle of the study period (i.e. December– February), our results displayed a spatial niche avoidance between White-winged Flufftail and Red-chested Flufftail (Figure 5). Although both species occurred at the respective camera site by day 16 of the survey, once White-winged Flufftail activity peaked, Red-chested Flufftail activity diminished. Furthermore, Red-chested Flufftail activity was once again noted and increased significantly following the absence of White-winged Flufftail from day 35 of the survey (Figure 5). This significant increase in activity by Red-chested Flufftail led to a breeding attempt and successfully fledged juvenile that was recorded frequently following day 60 of the survey.
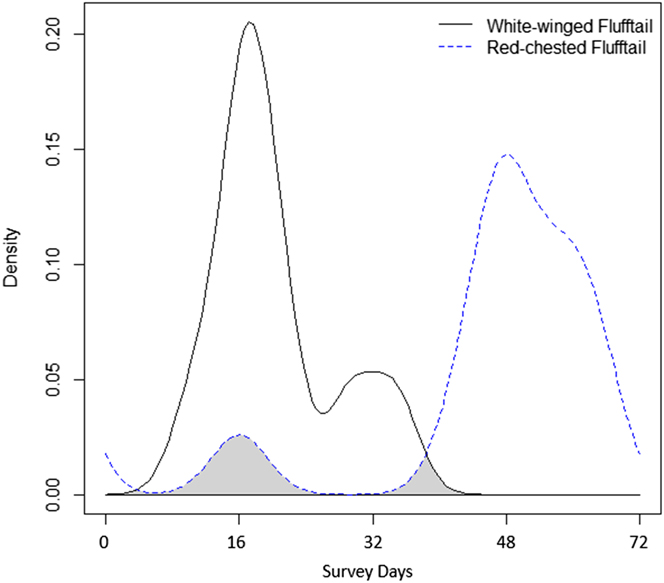
Figure 5. Kernel density estimates of sighting distribution across the study period (December–February) for White-winged (solid line) and Red-chested Flufftail (dotted line) at the individual camera trap where the focal species (White-winged Flufftail) was exclusively recorded.
Demographics of White-winged Flufftail sightings included an adult female, adult male and juvenile (sex unknown). Noted behaviour included a female utilising a perch site to presumably display. These displays incorporated wing-flapping from the perch, followed by the display of white secondaries while walking in the immediate area. The voluntary display of white secondaries whilst the wings were folded in, seemed comparable to that of pectoral tuft displays recorded in other species (Evans and Hatchwell Reference Evans and Hatchwell1992). The voluntary nature of this display was evident in that comparable images of other adults failed to produce these white patches. This wing-flapping and voluntary display of secondaries whilst walking was noted twice from the same perch site over a three-day period. Furthermore, following both these sightings, a male was observed coming into the area shortly after the female had left (8 min and 4 min respectively).
Discussion
The ecological application of technological advances has yielded increased capacity and ongoing improvements for surveying rare and elusive species worldwide (Nichols et al. Reference Nichols, Karanth, O’Connell, O’Connell, Nichols and U Karanth2011). Our study confirms camera trapping as the first non-invasive method of surveying the CR White-winged Flufftail. Results from this study represent more independent sightings of this species than that accumulated through four consecutive years of rope dragging surveys at the same respective site (Davies et al. Reference Davies, Smit-Robinson, Drummond, Gardener, Rautenbach, Van Stuyvenberg, Nattrass, Pretorius, Pietersen and Symes2015). Additionally, camera trapping provided the first assessment of site occupancy and fine-scale activity patterns of this species. This study also accumulated the first photographic material of this species behaving and interacting within its natural habitat in an undisturbed state. Building on the foundational work conducted using traditional survey methods (Taylor Reference Taylor1994, Allan et al. Reference Allan, McInnes and Wondafrash2006, Davies et al. Reference Davies, Smit-Robinson, Drummond, Gardener, Rautenbach, Van Stuyvenberg, Nattrass, Pretorius, Pietersen and Symes2015), this study demonstrates the applicability and potential of camera trapping as a non-invasive, accurate and reliable survey method for elusive rallids.
Habitat covariates most influencing White-winged Flufftail occupancy included basal and canopy cover (Table 2). Although the species has a known association with dense wetland vegetation (Evans et al. Reference Evans, Smit-Robinson, Tarboton, Taylor, Peacock and Wanless2015), our study confirms the importance that intact canopy and basal cover could have on species occupancy. This further highlights the potential threat of activities that lower vegetative cover, such as overgrazing and excessive trampling by domestic stock, could have on species persistence in a given area (Evans et al. Reference Evans, Smit-Robinson, Tarboton, Taylor, Peacock and Wanless2015). Furthermore, our results confirm the noted preference of White-winged Flufftail for shallower water levels within wetlands, as seen by water level negatively influencing detection probability within our study. Also corroborated by our study is the sensitivity of this species to microhabitat alteration, whereby any significant reduction in vegetative cover and increase in water level could lead to the local abandonment of that site. The local abandonment of a microhabitat noted in our study was caused by a natural flooding event and therefore also highlights the need for sufficiently sized wetlands and patch connectivity across the landscape to accommodate this species. The low resultant capture frequency and occupancy estimate of White-winged Flufftail from our study corroborate the supposition that this species is rare and currently represented by very low densities (Evans et al. Reference Evans, Smit-Robinson, Tarboton, Taylor, Peacock and Wanless2015). These results are further supported by low genetic diversity confirmed in the innate immune regions (Dalton et al. Reference Dalton, Vermaak, Smit-Robinson and Kotzé2016). The low diversity, rendering the species more vulnerable to changes in the environment, needs to be further elucidated as it may ultimately contribute to the extinction of the species.
Many rallids are known to be territorial in both the breeding and non-breeding range (Taylor Reference Taylor1987, Reference Taylor1994, Taylor and van Perlo Reference Taylor and van Perlo1998). Spotted Crake, the only other migratory rallid recorded in our study, has been noted to display aggressive territorial behaviour in the non-breeding stop-over sites (Taylor Reference Taylor1987). Furthermore, Mendelsohn et al. (Reference Mendelsohn, Sinclair and Tarboton1983) noted that although Red-chested and White-winged Flufftail were found at the same wetland, there seemed to be little overlap between them. Our study confirms that spatial avoidance between the two species does seem to occur, with Red-chested Flufftail seemingly abandoning the local microhabitat after the arrival and peak activity of White-winged Flufftail (Figure 5). Other studies have noted that White-winged Flufftail occur singly, in pairs or small family groups (Taylor Reference Taylor1994), but our study only recorded solitary birds. The peak period hosting the majority of sightings yielded one intra-specific interaction. Two consecutive sightings of a female White-winged Flufftail utilising a perch to presumably display, followed by a male moving into the area shortly thereafter, but never overlapping in site utilisation. Although based on a limited sample size, the inter-species spatial avoidance and lack of intra-species spatial overlap, together with noted displays, suggest that White-winged Flufftail exhibit territorial behaviour in the respective austral summer range.
The migration strategy employed by most migratory species involves alternation between flights and stopover foraging periods (Schaub et al. Reference Schaub, Pradel, Jenni and Lebreton2001, Strandberg and Alerstam Reference Strandberg and Alerstam2007). Most migratory ground-foraging species undertake a full stop incorporating intensive foraging activity to accumulate fuel reserves along the migration journey (Schaub et al. Reference Schaub, Pradel, Jenni and Lebreton2001, Lindstrom Reference Lindström, Berthold, Gwinner and Sonnenschein2003). Although White-winged Flufftail is thought to be a migratory species based on the available data (Taylor Reference Taylor, Hockey, Dean and Ryan2005), the exact status is disputable (Davies et al. Reference Davies, Smit-Robinson, Drummond, Gardener, Rautenbach, Van Stuyvenberg, Nattrass, Pretorius, Pietersen and Symes2015). Furthermore, DNA analysis of mitochondrial (COI, Cytb, 12S/Val/16S) and nuclear (ADH-5, GPD3-5 and bfib7) markers revealed only three interspecific genetic variations between South African and Ethiopian birds, suggesting that these birds are not different species or subspecies but are rather one migrating population (Dalton et al. Reference Dalton, Vermaak, Smit-Robinson and Kotzé2016). Our study provides further support for the postulation that White-winged Flufftail is a migratory species. The species was only recorded across a restricted temporal period, whereby the bulk of the sightings occurred across a consecutive 10-day period. Furthermore, the level of activity during the short temporal period of occupancy was higher than other non-breeding resident rallid species, suggestive of typical intensive stopover foraging exhibited by migratory species (Schaub et al. Reference Schaub, Pradel, Jenni and Lebreton2001, Lindstrom Reference Lindström, Berthold, Gwinner and Sonnenschein2003). Furthermore, the short temporal period of intensive activity closely resembled that of Spotted Crake documented in this study, which is a known Palearctic breeding migrant (Taylor Reference Taylor1987).
White-winged Flufftail are known to prefer short sedge, grass and Asteraceae meadows in the Ethiopian breeding range (Taylor and van Perlo Reference Taylor and van Perlo1998, Taylor Reference Taylor, Hockey, Dean and Ryan2005). Similarly, in the non-breeding austral range the species seemingly shows a preference for shorter (< 60 cm) sedge and grass meadows (Taylor and van Perlo Reference Taylor and van Perlo1998, Taylor Reference Taylor, Hockey, Dean and Ryan2005, Davies et al. Reference Davies, Smit-Robinson, Drummond, Gardener, Rautenbach, Van Stuyvenberg, Nattrass, Pretorius, Pietersen and Symes2015). However, the species has been noted to occupy taller vegetation habitats in the austral summer range, including mixed stands of sedge and taller reeds (Taylor Reference Taylor, Hockey, Dean and Ryan2005, Evans et al. Reference Evans, Smit-Robinson, Tarboton, Taylor, Peacock and Wanless2015). Our results exclusively recorded the species within a taller (mean 170 cm) mixed Typha–sedge community, but failed to detect the species in the preferred sedge meadows in which 66% of the camera localities were situated. Further studies are required to ascertain if the absence of sightings within sedge meadows is a reflection of true absence or pseudo-absence. Survey design potentially requires refinement to accommodate alternative microhabitat camera placement methods that prevent bias in detection probability and increase the reliability of detection histories accumulated across camera localities.
Management recommendations for existing habitat should limit the excessive reduction of vegetative cover within wetland habitats by maintaining low stocking densities to prevent overgrazing and/or extensive trampling within the respective management units. Given the species’ noted preference for good quality peat wetlands (Davies et al. Reference Davies, Smit-Robinson, Drummond, Gardener, Rautenbach, Van Stuyvenberg, Nattrass, Pretorius, Pietersen and Symes2015), frequent (annual) intense fires in the wintering months should be avoided as these will adversely impact on peat condition and accumulation (Hamilton Reference Hamilton, Cohen, Casagrande, Andrejko and Best1984, Kirkman et al. Reference Kirkman, Goebel, West, Drew and Palik2000). Furthermore, fire not only impacts on peat characteristics, but can influence the development of dominant wetland vegetation communities (Hamilton Reference Hamilton, Cohen, Casagrande, Andrejko and Best1984, Timmins 1992, Kirkman et al. Reference Kirkman, Goebel, West, Drew and Palik2000). A complete lack of fire could potentially alter grass-sedge wetland communities to taller dense stands of vegetation, whilst more frequent fires could benefit prevalence of grass-sedge community (Kirkman et al. Reference Kirkman, Goebel, West, Drew and Palik2000).
Conclusion
White-winged Flufftail was noted in our study as a low-density, habitat specialist species that proved sensitive to habitat alteration, particularly reduction in vegetative cover (basal and canopy) or increase in water level. Furthermore, the species’ short temporal period of occupation across an austral summer season within our study site suggests a reliance on multiple wetland sites across its migratory journey. Our study supports the need for conservation initiatives focused on securing contiguous sections of suitable wetland habitat in order to accommodate the persistence of this ‘Critically Endangered’ species. Imperative elements of conservation networks would include replication and representation (Redford et al. Reference Redford, Amato, Baillie, Beldomenico and Clum2011), whereby numerous local wetland sites are secured at multiple localities across the species’ range.
In order to progress our understanding of site occupancy and population trends for this species, further refinement of the survey technique and/or survey design is required to increase detection probabilities and subsequent datasets obtained. One particular area of concern is the lack of any sightings at any of the camera traps located in sedge meadows, which is a preferred habitat type for the species (Davies et al. Reference Davies, Smit-Robinson, Drummond, Gardener, Rautenbach, Van Stuyvenberg, Nattrass, Pretorius, Pietersen and Symes2015). We postulate that this possibly was not a reflection of occupancy, but an artefact of the current survey design. Sedge meadows yielded a significantly shorter and denser vegetation structure than in other mixed vegetation units sampled, which could necessitate an alternate camera placement strategy. Furthermore, given the noted relationship observed in our study between site occupancy and vegetation cover, vegetation cropping in front of cameras as part of the camera placement technique would need to be carefully considered. Lack of cropping would not allow for efficient camera surveying as vegetation would obscure the detection arc (Colyn et al. Reference Colyn, Radloff and O’Riain2018), whilst cropping more than is absolutely needed could bias resultant detection probabilities. Identifying the optimal camera placement technique for ground-dwelling species inhabiting dense short wetland habitats therefore requires further investigation.
A survey technique that can reliably and accurately determine presence and be replicated across the species range would significantly aid the understanding of the species’ population status and more efficiently direct conservation initiatives. Furthermore, this method would be applicable to other rare and elusive rallid species for which data deficiencies exist, such as ‘Endangered’ Slender-billed Flufftail Sarothrura watersi, ‘Vulnerable’ Yellow Rail Coturnicops noveboracensis and ‘Vulnerable’ Swinhoe’s Rail Coturnicops exquisites.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270918000400
Acknowledgements
We would like to acknowledge the Department of Environmental Affairs, Airports Company South Africa and the Ingula Partnership, particularly Eskom and the Middelpunt Wetland Trust, for their support and involvement in the project. Furthermore, we would like to acknowledge KEM-JV for supporting the BirdLife South Africa Fellow of Conservation post of R. B. Colyn. Special thanks to co-workers and collaborators Malcolm Drummond, Warwick Tarboton, Greg Davies, Adam Riley and David Allan. We would like to thank and acknowledge Faansie Peacock for the design of the locality map (Figure 1) presented in this manuscript.









