Infections caused by multidrug-resistant (MDR) pathogens can lead to high morbidity and mortality among hospitalized patients. In 2019, the global mortality associated with MDR pathogens was estimated to be ∼4.95 million deaths and the most common drug-resistant pathogens that led to death were Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa. 1 Carbapenem-resistant gram-negative bacteria, such as A. baumannii and Enterobacterales, are of grave concern in the United States as well as the European continent and the Asia-Pacific region. 2–Reference Yam, Hsu and Yap4 In the era of MDR pathogens, the availability of active antimicrobial agents has become limited. Therefore, to combat these MDR pathogens, several strategies must be incorporated such as improved infection prevention and control, implementation of antimicrobial stewardship (ASP), active surveillance, and development of new antimicrobial agents. In well-developed countries like the United States, it is well recognized that the ASP team should consist of multidisciplinary healthcare personnel (eg, infectious diseases physician, clinical pharmacist, infection control nurse, and clinical microbiologist) who can influence appropriate antimicrobial prescriptions. Integration of the pharmacist into the ASP team has been shown to increase appropriateness of antimicrobial prescriptions and reduction in antimicrobial consumption, hospital antimicrobial expenditure, and in hospital length of stay. Reference Apisarnthanarak, Lapcharoen, Vanichkul, Srisaeng-Ngoen and Mundy5–Reference Saha, Hawes and Mazza7
Guidelines from the Infectious Diseases Society of America (IDSA), the American Society of Health-System Pharmacist (ASHP), and a consensus statement on ASP from Asia recommended that a pharmacist should be a coleader of the ASP. 8–Reference Barlam, Cosgrove and Abbo10 The clinical pharmacist member of the ASP team should have some training in infectious disease. Clinical pharmacists are core members of the ASP team and have an important role in ensuring appropriate antimicrobial prescriptions. According to an ASHP statement, a pharmacist involved in the ASP has responsibilities to promote optimal antimicrobial use, to reduce the transmission of infections, and to educated other healthcare professionals, patients, and the public. 8 The role of the ASP clinical pharmacist includes developing local guidance and formulary choices with the physician coleader, recommending switching from intravenous to oral formulations, identifying de-escalation opportunities, optimizing antimicrobial dose, monitoring drug–drug and drug–food interactions, and monitoring the outcomes of antimicrobial usage (eg, clinical outcomes and antimicrobial consumption). In addition, clinical pharmacists can help educate healthcare personnel on the appropriate use of antimicrobials as part of the processes of patient care. Reference Garau and Bassetti11 A study in the United States showed that an infectious-diseases–trained pharmacist on the ASP team is associated with reduced antimicrobial consumption, reduced mortality associated with sepsis and respiratory tract infections, and reduced antimicrobial costs. Reference Yu, Rho and Morcos12 However, barriers to the integration of the clinical pharmacist into the ASP team in general, include the lack of financial support for dedicated personnel, lack of time, lack of career promotion for the pharmacist, and lack of knowledge and experience. Therefore, continued recommendations for clinical pharmacist engagement in the ASP team is essential. We reviewed the literature on the role and effectiveness of the clinical pharmacist in ASP in Asia, with a primary focus on the adult inpatient setting. The relevant studies were identified by searching the PubMed, ScienceDirect, and EMBASE databases from their inception date through August 2022. In this review, included studies were limited to English-language publications. The search strings “pharmacist,” “asia OR bangladesh OR brunei OR bhutan OR darussalam OR cambodia OR china OR guam OR hong kong OR india OR indonesia OR japan OR korea OR lao people’s democratic republic OR macao OR malaysia OR mongolia OR myanmar OR nepal OR northern mariana islands OR palau OR papua new guinea OR philippines OR singapore OR taiwan OR thailand OR timor-leste OR vietnam,” and “antimicrobial stewardship OR antibiotic stewardship” were used to identify papers that met the inclusion criteria. Our outcomes of interest were clinical outcome, proportion of appropriate antibiotic prescription, antibiotic consumption, antibiotic resistance rates, and pharmacist’s rate of recommendation acceptance. We only included papers that mainly emphasized pharmacist-led antimicrobial stewardship interventions. Letters to the editor, editorials, commentaries, review articles, and conference abstracts were excluded. The title and abstracts were screened for eligibility, and data extraction was conduted by K.J. and A.A.
Role of the clinical pharmacist in the ASP team
Several studies from around the world have reported that clinical-pharmacist–led interventions have been successfully implemented. These interventions included performing a prospective audit and feedback, educating healthcare professionals, developing guidance for treatment a specific infection (eg, urinary tract infection and respiratory tract infection) and a specific pathogen (eg, Staphylococcus aureus and Clostridioides difficile), encouraging penicillin allergy delabeling, and facilitating real-time pathogen identification and feedback to the treating physician. Reference Jantarathaneewat, Montakantikul, Weber, Nanthapisal, Rutjanawech and Apisarnthanarak13–Reference Heyerly, Jones, Bokhart, Shoaff and Fisher20 The infectious diseases (ID) pharmacist is the ideal role model for pharmacist-led ASP interventions. Various studies have supported the effectiveness of ID pharmacist-led ASP interventions. Reference Yu, Rho and Morcos12,Reference Jantarathaneewat, Montakantikul, Weber, Nanthapisal, Rutjanawech and Apisarnthanarak13 However, most successful clinical-pharmacist–led interventions have been performed in high-income countries such as the United States, Canada, Australia, and Japan, as well as the European continent.
Evidence on the efficacy of pharmacist-driven ASPs in Asia
Several studies have reported the effectiveness of ID-pharmacist–driven ASPs in Asian countries such as Japan, Thailand, China, Korea, and India (Table 1). In Japan, ID pharmacist involvement in the ASP of a tertiary-care hospital increased the rate of appropriate blood-culture collection and de-escalation therapy (71% vs 85%; P < .001). Reference Uda, Ebisawa and Sakon21 A community hospital ASP with clinical pharmacist involvement decreased the duration of antimicrobial treatment in uncomplicated gram-negative bacteremia (8 vs 14 days; P < .001) and resulted in an increase in de-escalation, as reported in the study by Nakamura et al (10.2% vs 30.8%; P < .05). Reference Fukuda, Tanuma, Iio, Saito, Komura and Yamatani22,Reference Nakamura, Arima and Tashiro23 A clinical-pharmacist–led ASP intervention among patients with methicillin-resistant Staphylococcus aureus (MRSA) bacteremia led to an increase in targeted antimicrobial administration duration (at least 14 days for uncomplicated bacteremia and 28 days for complicated bacteremia; 44.8% vs 72.1%; P = .027) and early use of anti-MRSA drugs within 24 hours after MRSA was detected (62.3% vs 82.4%; P =.038). Reference Ohashi, Matsuoka and Shinoda24 A timeout intervention for vancomycin showed a decrease in weekly vancomycin days of therapy (DOT) per 1,000 patient day (coefficient, −0.49; P = .007) as well as a decrease antimicrobial usage in the pharmacist-led arm (coefficient, −0.77; P = .007) compared to an ID physician arm. Reference Hasegawa, Tagashira and Murakami25 Finally, a study in Japan reported that clinical pharmacist involvement in an ASP team reduced the volume of antimicrobial prescriptions in a skilled nursing facility (incidence rate ratio [IRR], 0.885; P < .001) (Figure 1). Reference Takito, Kusama, Fukuda and Kutsuna26

Fig. 1. Barriers and solutions for clinical pharmacist-driven ASP intervention in Asia.
Table 1. Summary of studies on ASP with clinical pharmacist involvement in Asia classified by countries
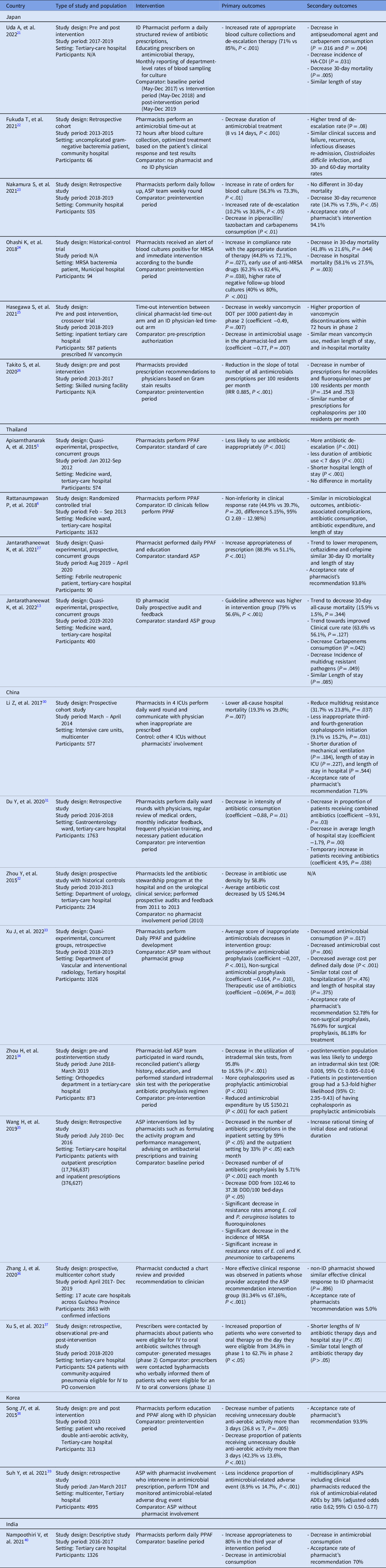
DDD, defined daily dose; HA-CDI, hospital-acquired Clostridioides difficile infection; ID, infectious diseases; IRR, incidence rate ratios; IV-PO conversion, intravenous to oral antibiotic conversion ; MRSA, Methicillin-resistant Staphylococcus aureus; TDM, therapeutic drug monitoring; PPAF, prospective audits and feedback.
Several clinical-pharmacist–led ASP studies have been conducted in Thailand. A randomized controlled trial in Thailand found that an ASP clinical-pharmacist–led prospective audit and feedback was not inferior to that of an ID fellow in terms of clinical response (difference, 5.15%; 95% CI, 2.69%–12.98%). This finding confirms the concept that a clinical pharmacist can be an alternative to a physician-led ASP implementation strategy. Reference Rattanaumpawan, Upapan and Thamlikitkul6 This research was followed by a study conducted in a medicine ward at a tertiary-care hospital that showed that an ID-pharmacist–led ASP intervention, especially in conjunction with an ID physician, can further increase guideline adherence (79% vs 56.6%; P < .001), as well as decrease carbapenem consumption (mean defined daily dose [DDD], 191.94 vs 256.14; P = .042). Reference Apisarnthanarak, Lapcharoen, Vanichkul, Srisaeng-Ngoen and Mundy5,Reference Jantarathaneewat, Montakantikul, Weber, Nanthapisal, Rutjanawech and Apisarnthanarak13 In the specific setting of febrile neutropenic patients, a clinical-pharmacist–led ASP intervention was associated with more appropriate prescriptions (88.9% vs 51.1%; P < .001) without affecting the mortality rate. Reference Jantarathaneewat, Apisarnthanarak, Limvorapitak, Weber and Montakantikul27 Finally, clinical pharmacist involvement in the multidisciplinary team support improved adherence to a vancomycin dosing protocol (90.8% vs 55%; P < .001), and the clinical pharmacist can help the ASP team implement antibiotic heterogeneity through periodic antibiotic monitoring and supervision strategy in both medicine and surgical departments in Japan and Thailand. Reference Katawethiwong, Apisarnthanarak, Jantarathaneewat, Weber, Warren and Suwantarat28,Reference Chaononghin, Jantarathaneewat, Weber, Warren and Apisarnthanarak29
In China, a multicenter study conducted in 8 intensive care units (ICUs) at 4 university hospitals resulted in a lower all-cause hospital mortality in 4 ICUs where clinical pharmacists were involved (19.3% vs 29%; P = .007). Reference Li, Cheng and Zhang30 But this study had limitations including a short study period, potential selection bias of the clinical cohort, and a small sample size. Reference Li, Cheng and Zhang30 Studies conducted in a gastroenterology ward and a urology ward, respectively, reported reductions in antimicrobial consumption during the respective study periods in which clinical pharmacists were involved (coefficient, −0.88; P = .01). Reference Du, Li and Wang31,Reference Zhou, Ma, Zhao, Tian, Sun and Cui32 A quasi-experimental study on the efficacy of an ID pharmacist with a concurrent control group in the department of vascular and interventional radiology reported a decrease in inappropriate prescriptions, particularly in perioperative antimicrobial prophylaxis (coefficient, −0.207; P < .001), nonsurgical antimicrobial prophylaxis (coefficient, −0.164; P = .010), and therapeutic use of antimicrobials (coefficient, −0.069; P = .003). Reference Xu, Huang and Yu33 In an orthopedic department, a pharmacist-led intervention was performed on perioperative antibiotic prophylaxis by standardizing the cephalosporin intradermal skin test. Reference Zhou, Liu and Sun34 This intervention achieved a reduction the utilization of intradermal skin tests from 95.8% to 16.5% (P < .001) and reduced the cost of antimicrobials by $150.21 (P < .001) for each patient. Reference Zhou, Liu and Sun34 A pharmacist-led ASP, hospital-wide intervention in China improved antibiotic consumption and achieved a corresponding decrease in the incidence of drug-resistant gram-negative bacteria. 35 A multicenter study also showed that adherence to clinical pharmacist’s recommendations improved clinical response compared with rejection of the clinical pharmacist’s recommendation. Reference Zhang, Li and He36 Among patients with community-acquired pneumonia, a pharmacist-led intervention converting intravenous to oral administration of antibiotic integrated with computer decision support led to an increase in the proportion of patients who were converted to oral therapy from 34.8% to 62.7% (P < .05) and shortened the lengths of intravenous antibiotic therapy and hospital stay (P < .05). Reference Xu, Wang, Song, Han and Zhang37
In South Korea, a clinical pharmacist-led intervention that focused on patients receiving antimicrobials with redundant activity. Reference Song, Kim and Huh38 The ID-pharmacist–led intervention achieved a decrease in the number (26.8 vs 7; P = .005) and proportion (42.3% vs 13.6%; P < .001) of patients receiving unnecessary antimicrobials for >3 days. Reference Song, Kim and Huh38 Another retrospective study in South Korea reported that an ASP with clinical pharmacist involvement was associated with a decrease in the incidence of antimicrobial-related adverse events (8.9% vs 14.7%; P < .001). Reference Suh, Ah and Chun39 Finally, similar to previous studies, a study on clinical pharmacist involvement in the ASP in India showed an increase in the proportion of appropriate prescriptions during the third year of the intervention period. Reference Nampoothiri, Sudhir and Joseph40
Similar to those reported in Western developed countries like the United States, the acceptance rates of clinical pharmacist recommendations in Asia range from 70% to 94%. In high-income Asian countries, such as Japan and South Korea, the acceptance rates of pharmacist recommendations tend to be higher (∼90%), whereas in middle-income countries, such as China and India, tend to report lower acceptance rates (70%–90%). The most effective interventions feature daily prospective audit and feedback by the clinical pharmacist along with the ID physician. Reference Molloy, McGrath, Thomas, Kaye and Rybak41 The relevant outcomes in Asia include adherence to the intervention or bundle, appropriateness of antimicrobial use, antimicrobial consumption, and clinical outcomes such as clinical improvement and mortality. An ASP that includes clinical pharmacists can increase the appropriateness of antimicrobials prescribed and decrease unnecessary antimicrobial use. However, rare reports of the impact of intervention on the development of antimicrobial resistance have emerged, and they may indicate the need to integrate effective infection prevention. The other relevant outcomes, such as cost-effectiveness of clinical pharmacist involvement in the ASP team, should be the subject of future research. Clinical-pharmacist–led ASP interventions in other settings such as ambulatory care, emergency room, high dependency unit, pediatrics, solid-organ transplant unit, patients with cytomegalovirus infection, and patients with invasive candidiasis have been conducted in the United States, Canada, and Europe. Reference Westerhof, Dumkow, Hanrahan, McPharlin and Egwuatu42–Reference Burns, Johnson, Pham, Egwuatu and Dumkow48 The addition of delabeling penicillin allergies through penicillin skin testing by the clinical pharmacist to current ASP interventions has effectively reduced antimicrobial-therapy–related costs, clarification of allergies, and optimized antimicrobial therapy. Reference Kurtz, Heyerly, Bokhart and Simpson49,Reference Harmon, Richardson, Simons, Monforte, Fanning and Harrington50 Additionally, during the COVID-19 pandemic, ASP interventions were essential to optimizing antimicrobial use when rates of bacterial coinfection were relatively low. Reference Pierce and Stevens51 In Canada, a clinical trial of a clinical pharmacist-led ASP team on COVID-19 patients is ongoing. Reference Chen, Hoang and Yaskina52 Interventions on COVID-19 patients have not been well recognized in Asia; thus, longer follow-up periods with larger sample sizes are needed.
A study in the United States showed that the use of procalcitonin levels to guide interventions by clinical pharmacists led to discontinuation of vancomycin therapy in patients with lower respiratory tract infections and normal procalcitonin levels. Reference Watkins, Van Schooneveld, Reha, Anderson, McGinnis and Bergman53 Rapid identification of gram-positive bacteria through diagnostic systems like the VITEK-2 automated system, matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI-TOF MS), and rapid polymerase chain reaction (PCR) with real-time ASP interventions by a clinical pharmacist have been implemented successfully in the United States. Reference Wong, Bauer, Mangino and Goff19,Reference Heyerly, Jones, Bokhart, Shoaff and Fisher20,Reference Beganovic, Costello and Wieczorkiewicz54,Reference Mahrous, Thabit, Elarabi and Fleisher55 Identification of antimicrobial susceptibility using Verigene along with ASP guidance can improve time to initiation of appropriate antimicrobials as well as reduce mortality rates and 30-day readmission rates. Reference Mahrous, Thabit, Elarabi and Fleisher55 Therefore, clinical pharmacist-led ASP interventions should be integrated with rapid diagnostic test.
Barriers and possible solutions
In Asia, some barriers to the implementation of the ID pharmacist-led ASP remain. These include the lack of dedicated personnel, a labor-intensive pharmacist workload, lack of time, lack of knowledge and perception toward ASP by clinicians, shortage of clinical pharmacists with advanced training in infectious diseases, and the lack of integration of the pharmacists’ role with other healthcare professionals when delivering care. Reference Jantarathaneewat, Montakantikul, Weber, Nanthapisal, Rutjanawech and Apisarnthanarak13 A survey in Malaysia revealed that administration support, commitment from leadership, and perseverance are essential to facilitate the role of clinical pharmacists in the ASP. Reference Lai, Islahudin, Ambaras Khan and Chong56 Another survey in South Korea revealed that ASPs were limited only to top-tier general hospitals. The study concluded that a clinical pharmacist is essential to successful implementation of the ASP along with adequate financial reimbursement for the professionals who perform and maintain the ASP. Reference Park, Kang and Choi57 Data from the survey of gaps and opportunities in ASPs in Asia revealed that 80% of respondents had a pharmacist working on ASP activities; however, almost half of the respondents reported no financial support such as salary support, training, or information technology services at their hospitals. Reference Chang, Chuang and Veeraraghavan58 Furthermore, a survey conducted in the Asia-Pacific region revealed that 41% of hospitals had no trained ID pharmacists even though most respondents worked in large hospitals. Reference Lee, Lye and Chung59 A nationwide survey from the United States revealed that pharmacists with formal ASP responsibilities dedicated 0.6 full-time equivalents (FTE), whereas pharmacists without formal ASP responsibilities spent an average of 0.125 FTE on ASP activities, even though the average program should have 1 FTE allocated for an ASP pharmacist. Reference Dionne, Wagner, Chastain, Rosenthal, Mahoney and Bland60 The lack of data on clinical-pharmacist FTE among ASPs in Asia may be a barrier to implementing effective ASPs in this region.
To successfully encourage pharmacist involvement in their respective ASPs, each institution should identify its own gaps and challenges. Reference Apisarnthanarak, Kwa and Chiu9 Prioritizing formal support and approval from hospital leadership and making a case for the return on investment of clinical pharmacist involvement in ASP activities are essential. Reference Apisarnthanarak, Kwa and Chiu9 Illustrating to administrators that the role of the clinical pharmacist in the ASP team is that of an expert in antimicrobial delivery can potentially lead to a modification in their role in routine activities in the hospital pharmacy. A full-time antimicrobial stewardship pharmacist has been well recognized as a viable advanced career path for clinical pharmacists in the United States, Canada, and Europe.
In Asia, especially in developing countries, most ID-trained clinical pharmacists perform their ASP activities along with their routine work without additional renumeration. Without financial support from hospital administration, the ID-trained clinical pharmacist will not be able to complete their tasks on the ASP team. A possible solution is to require administrative support before hospitals are granted accreditation. The pharmacy council should promote the clinical pharmacist’s role in the ASP team and support the continuing education for pharmacist who are interested in or work for the ASP team. Furthermore, the role of the clinical pharmacist on the ASP team should be integrated into the curricula of doctor of pharmacy programs to encourage participation of pharmacists in the multidisciplinary ASP team. A recent study identified a trend toward increased clinical pharmacist involvement in ASP teams in Japan. These researchers also recommended that policy makers and stakeholders promote and support the evidence-based activities of clinical-pharmacist–led ASPs for small to medium-sized hospitals. Reference Maeda, Miyake and Inose61
This review had several limitations. It was difficult to determine which factors were important to the success of the ASP because each study involved a multifaceted intervention and different outcomes were measured. Our review is mostly applicable to the adult inpatient population rather than other settings such as the outpatient setting and pediatric population. Publication bias may exist, especially in the middle- and low-income countries. The standardized definitions for processes and outcome measures in ASP is necessary for future studies. Future studies that stratify interventions based on the income level of the country would provide more insight and would improve the homogeneity of the data. The role of clinical pharmacist in ASPs in Asia is limited to the inpatient setting. Further studies on other settings are needed before any recommendations can be made on pediatric and ambulatory settings.
According to previous studies, clinical pharmacist involvement in the ASP team can be implemented successfully in Asia. However, some barriers to widespread implementation of clinical-pharmacist–driven ASPs remain. The approval of and financial support from hospital leadership are important steps in initiating an ASP strategy. Integrating a clinical pharmacist’s role in an ASP team as a policy and financial support along with career advancement for the clinical pharmacist will encourage the proliferation and acceptance of clinical-pharmacist–driven ASPs. Training in infectious diseases is essential for clinical pharmacists on ASP teams. Moreover, the role of clinical pharmacist can be expanded if ASPs are implemented in other areas such as ambulatory care, the emergency departments, and units housing immunocompromised patients. Integrated rapid diagnostic methods and real-time ASP interventions are attractive strategies that will expand the clinical pharmacy service.
Acknowledgments
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.




