Viral pandemics have historically been associated with secondary bacterial infections, and coronavirus disease 2019 (COVID-19) has been no exception. Subsequent bacterial infections, particularly lower respiratory tract infections, which are the leading cause of infectious disease mortality worldwide, Reference Lozano, Naghavi and Foreman1,2 have been associated with increased mortality both during the 1918 Spanish influenza pandemic and during seasonal influenza outbreaks. Reference Morens, Taubenberger and Fauci3,Reference Morris, Cleary and Clarke4 However, differentiating viral versus bacterial infection is a challenge for clinicians, which has led to inappropriate or prolonged use of antibiotics in patients with COVID-19. As previously described, the overuse of antibiotics increases the risk of antimicrobial resistance (AMR) Reference Ventola5–Reference Strathdee, Davies and Marcelin7 which can cause severe infections and complications, such as disruption of the gut microbiota leading to outbreaks of Clostridium difficile infection. Reference Llor and Bjerrum8,Reference Imwattana, Knight and Kullin9
In this review, we examined the prevalence of secondary bacterial infections and bacterial coinfections in patients with COVID-19 and the use of antibiotics associated with these infections. A literature search of PubMed and Embase was conducted to identify relevant studies published up to June 2, 2021 (Supplementary Table 1). The main types of bacterial infections studied were (1) coinfections or community-acquired (CA) infections prior to or within the first 3 d of hospitalization, (2) secondary or hospital-acquired (HA) infections on or after day 4 of hospitalization, according to the National Healthcare Safety Network definition, 10 and (3) both CA and HA infections.
We also reviewed the impact of secondary bacterial infections and bacterial coinfections on clinical outcomes (eg, length of hospitalization, intensive care unit [ICU] admission and mortality), the etiology of these bacterial infections, the antibiotic treatment approaches, and discuss the development of AMR.
Prevalence of secondary bacterial infections and bacterial coinfections in patients with COVID-19
Most studies have reported an estimated rate of secondary bacterial infections and bacterial coinfections <20% (Tables 1–3). Reference Silva, Lima and Magalhaes11–Reference Sogaard, Baettig and Osthoff16 However, CA bacterial infection has been less commonly reported, with rates ranging between 1% and 7.5% (Table 1). Reference Karaba, Jones and Helsel13,Reference Vaughn, Gandhi and Petty14,Reference Thelen, Buenen, van Apeldoorn, Wertheim, Hermans and Wever17,Reference Wang, Yang, Li, Wen and Zhang18 The rates of HA bacterial infections were variable and ranged from 9.3% to 32% for overall secondary bacterial infections (Table 3). Reference Ripa, Galli and Poli19,Reference Pink, Raupach and Fuge20 Although the heterogeneity of the methodologies and populations (eg, moderate-to-severe COVID-19 cases, outpatients vs inpatients) make it difficult to compare rates of bacterial infections, in general, HA infection rates tended to be higher than CA infection rates in studies that recorded data on both (Table 2). Reference Garcia-Vidal, Sanjuan and Moreno-Garcia15,Reference Sogaard, Baettig and Osthoff16,Reference Kubin, McConville and Dietz21,Reference Langford, So and Raybardhan22
Table 1. Summary of Included Studies Involving Patients With COVID-19 and Community-Acquired (CA) Bacterial Coinfections

Note. CABP, community-acquired bacterial pneumonia; ICU, intensive care unit; LOS, length of stay; NR, not reported; w/o, without.
a Based on published information, including clinical details, or on the time of infection diagnosis: outpatient/≤3 d of hospitalization = community acquired infection, unless otherwise stated in the source.
b Rates were reported per total number of patients with COVID-19.
c Data for hospital LOS, ICU admission rates in patients with COVID-19 who had coinfections.
d Outcomes were reported in total patient population.
Table 2. Summary of Included Studies Involving Patients With COVID-19 and Community-Acquired (CA) Bacterial Coinfections or Hospital-Acquired (HA) Secondary Bacterial Infections

Note. BSI, bloodstream infections; CA, community acquired; CABP, community-acquired bacterial pneumonia; CPE, carbapenemase-producing Enterobacterales; CRKp, carbapenem-resistant Klebsiella pneumoniae; CRPA, carbapenem-resistant Pseudomonas aeruginosa; ED, emergency department; EU, European Union; HA, hospital-acquired; HAP, hospital-acquired pneumonia; ICU, intensive care unit; IPD, invasive pneumonococcal disease; LOS, length of stay; MDR, multidrug resistant; MV, mechanical ventilation; NR, not reported; OBD, occupied bed days; OR, odds ratio; patients, patients; VAP, ventilator-associated pneumonia; w/o, without.
a Based on published information, including clinical details, or on the time of infection diagnosis: outpatient/≤3 d of hospitalization = community acquired infection; ≥4 d of hospitalization = hospital-acquired infection, unless otherwise stated in the source.
b Rates were reported per total number of patients with COVID-19.
c Data for hospital LOS, ICU admission rates in patients with COVID-19 who secondary bacterial infections or coinfections.
d Outcomes were reported in total patient population.
Table 3. Summary of Included Studies Involving Patients With COVID-19 and Hospital-Acquired (HA) Secondary Bacterial Infections

Note. BSI, bloodstream infections; CPE, carbapenemase-producing Enterobacterales; CR-Kp, carbapenem-resistant Klebsiella pneumoniae; CRPA, carbapenem-resistant Pseudomonas aeruginosa; ED, emergency department; EU, European Union; HA, hospital-acquired; HAP, hospital-acquired pneumonia; HFNT, high flow nasal therapy; ICU, intensive care unit; LOS, length of stay; MDR, multidrug resistant; MV, mechanical ventilation; NR, not reported; OBD, occupied bed d; OR, odds ratio; patients, patients; VAP, ventilator-associated pneumonia; w/o, without.
a Based on published information, including clinical details, or on the time of infection diagnosis: ≥4 d of hospitalization = hospital-acquired infection, unless otherwise stated in the source.
b Rates were reported per total number of patients with COVID-19.
c Data for hospital/ICU LOS, ICU admission rates, MV rates, MV duration in patients with COVID-19 who secondary bacterial infections.
d Outcomes were reported in total patient population.
Respiratory tract infections and bloodstream infections were the most common bacterial HA infections observed. Reference Vaughn, Gandhi and Petty14,Reference Hughes, Troise, Donaldson, Mughal and Moore23 Specifically, in a case–control study of 50 COVID-19 patients with bacterial infections, 56% had HA bacterial pneumonia versus 16% with CA pneumonia. Reference Nasir, Rehman and Omair24 The higher rates of HA infections may be linked to ICU admission, ventilator-associated infections, and prolonged hospital stay. Reference Sogaard, Baettig and Osthoff16 Indeed, a single-center study of hospitalized patients with COVID-19 in the United States reported that ICU stay and mechanical ventilation were independent predictors of HA infection in patients hospitalized with COVID-19. Reference Kubin, McConville and Dietz21 In several studies the rates of HA and/or ventilator-associated pneumonia (VAP) infection were >50% (Table 3). Reference Martinez-Guerra, Gonzalez-Lara and de-Leon-Cividanes12,Reference Nasir, Rehman and Omair24–Reference Sharov26
Although we specifically looked at bacterial infections, a few studies reported rates both for bacterial and fungal infections together. Reference Kubin, McConville and Dietz21,Reference Chen, Zhu and Xiao27–Reference Buehler, Zinkernagel and Hofmaenner30 Rates reported in hospitalized patients with COVID-19 varied from 3.6% up to 42.2% (Tables 2–3). Reference Nori, Cowman and Chen28,Reference Buehler, Zinkernagel and Hofmaenner30 In a meta-analysis including 9 studies, 8% of patients with COVID-19 experienced bacterial or fungal coinfections during hospital admission. Reference Rawson, Moore and Zhu31
Etiology of bacterial infections
Common microorganisms causing secondary bacterial infections and/or bacterial coinfections in patients with COVID-19 are shown in Figure 1. No clear pattern of preponderant pathogens was observed; however, the most frequently reported pathogens associated with both CA and HA infections were Escherichia coli, Streptococcus pneumoniae, Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae, Haemophilus influenzae, Acinetobacter baumannii, Mycoplasma spp, M. pneumoniae, Stenotrophomonas maltophilia, and Acinetobacter spp (Fig. 1). Reference Langford, So and Raybardhan22,Reference Nasir, Rehman and Omair24,Reference Sharov26,Reference Chen, Zhu and Xiao27,Reference Foschi, Zignoli and Gaibani32–Reference Lansbury, Lim, Baskaran and Lim34 Notably, only 2 additional microorganisms were observed to only cause CA infection: Enterobacter cloacae (2 cases among 5 patients with bacterial respiratory infections) Reference Wang, Yang, Li, Wen and Zhang18 and Proteus mirabilis (18 (8%) of 221 cultures from 183 patients with COVID-19 and CA infections). Reference Kubin, McConville and Dietz21 Each was reported in 1 study. A wide range of other pathogens were reported to only cause HA infection across a range of studies (Fig. 1). Reference Silva, Lima and Magalhaes11,Reference Martinez-Guerra, Gonzalez-Lara and de-Leon-Cividanes12,Reference Garcia-Vidal, Sanjuan and Moreno-Garcia15,Reference Nori, Cowman and Chen28,Reference Buehler, Zinkernagel and Hofmaenner30,Reference Posteraro, De Angelis and Menchinelli35–Reference Lee, Koh and Kim38
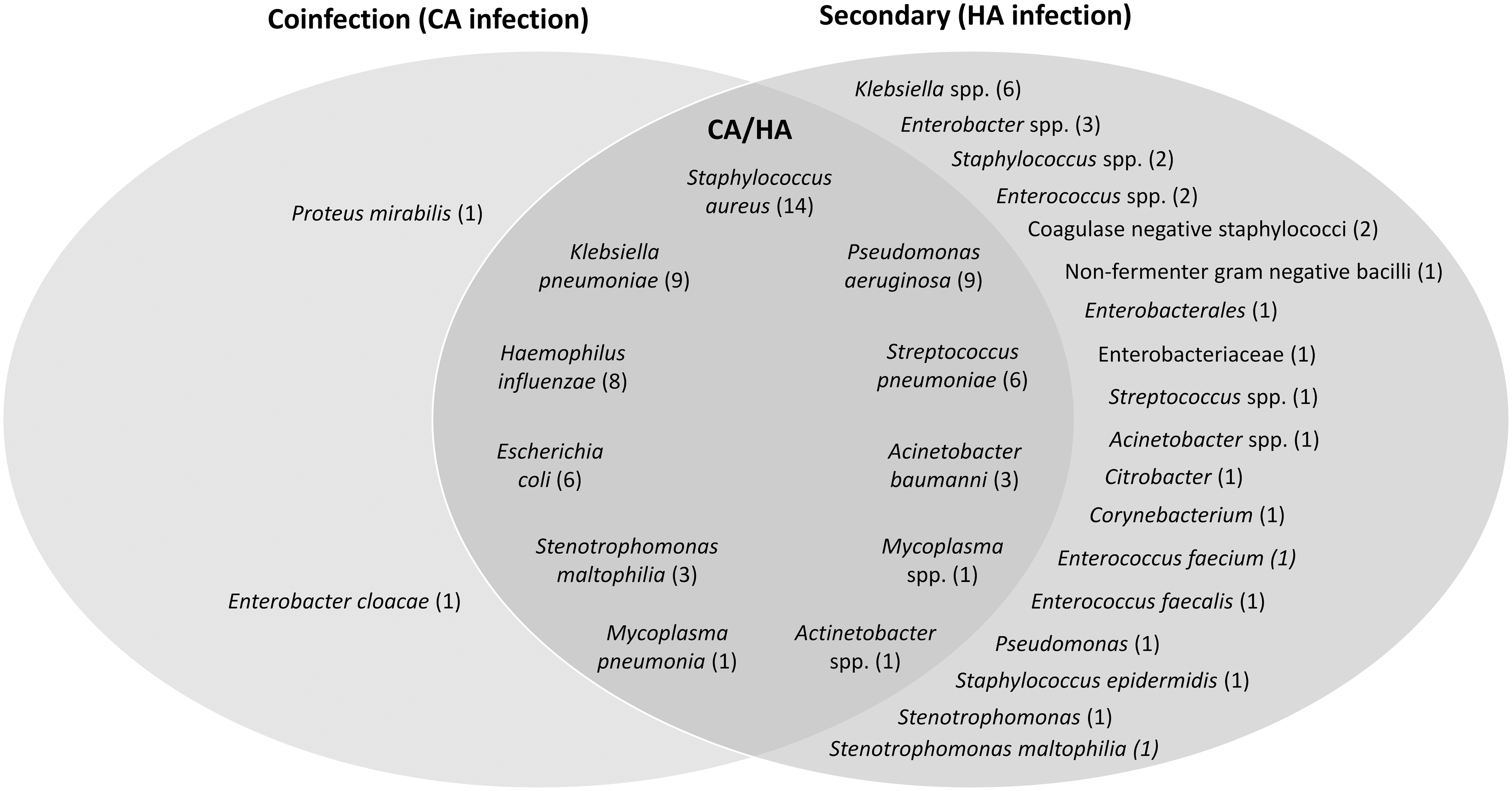
Fig. 1. Common etiologies of bacterial coinfections and/or secondary bacterial infections in patients with COVID-19. The most frequently reported bacterial microorganisms from 22 studies (up to 5 of the most common bacterial microorganisms) were included for each type of infection from each study: 6 studies for CA infection, Reference Thelen, Buenen, van Apeldoorn, Wertheim, Hermans and Wever17,Reference Wang, Yang, Li, Wen and Zhang18,Reference Kubin, McConville and Dietz21,Reference Chen, Zhu and Xiao27,Reference Baskaran, Lawrence and Lansbury36,Reference Hoshiyama, Wada and Nihonyanagi63 12 studies for HA infection, Reference Silva, Lima and Magalhaes11,Reference Martinez-Guerra, Gonzalez-Lara and de-Leon-Cividanes12,Reference Garcia-Vidal, Sanjuan and Moreno-Garcia15,Reference Pink, Raupach and Fuge20,Reference Chen, Zhu and Xiao27,Reference Nori, Cowman and Chen28,Reference Buehler, Zinkernagel and Hofmaenner30,Reference Posteraro, De Angelis and Menchinelli35–Reference Lee, Koh and Kim38,Reference Chong, Chieng and Tiwari40 and 6 studies for both CA and HA Reference Langford, So and Raybardhan22,Reference Nasir, Rehman and Omair24,Reference Sharov26,Reference Foschi, Zignoli and Gaibani32–Reference Lansbury, Lim, Baskaran and Lim34 (studies could report >1 type of infection). The number of studies that reported each organism are shown in parenthesis. Note. CA, community-acquired; COVID-19, coronavirus disease 2019; HA, hospital-acquired.
In a retrospective study of 254 hospitalized patients with COVID-19, the proportion of pathogens detected increased with the duration of ICU stay, consisting mainly of gram-negative bacteria, particularly K. pneumoniae and E. coli. Reference Baskaran, Lawrence and Lansbury36 In contrast, S. aureus and S. pneumoniae were the pathogens most commonly detected within 48 hours of hospital admission. Reference Baskaran, Lawrence and Lansbury36 Similarly, a retrospective study of 3,028 hospitalized COVID-19 patients showed that the proportion of gram-negative bacteria causing HA infections increased with longer hospital stay, whereas staphylococci were more commonly isolated within the first 14 days of hospitalization. Reference Kubin, McConville and Dietz21 Beyond day 14 of hospitalization, Enterobacterales and Pseudomonas spp predominated. Reference Kubin, McConville and Dietz21 Overall, this finding reflects an increased acquisition of pathogens and wider range of organisms with length of hospitalization.
Impact of secondary bacterial infections and bacterial coinfections on outcomes in patients with COVID-19
Mortality rates reported in patients with COVID-19 who had bacterial coinfections and/or secondary bacterial infections ranged between 6.5% and 66.7%; however, observation periods and population differed, which may account for the wide variation in rates (Tables 1–3).
In several studies, mortality rates were significantly higher in COVID-19 patients with bacterial coinfections or secondary bacterial infections compared with those without. Reference Martinez-Guerra, Gonzalez-Lara and de-Leon-Cividanes12,Reference Vaughn, Gandhi and Petty14,Reference Garcia-Vidal, Sanjuan and Moreno-Garcia15,Reference Ripa, Galli and Poli19,Reference Nasir, Rehman and Omair24,Reference Posteraro, De Angelis and Menchinelli35,Reference Adelman, Bhamidipati and Hernandez-Romieu37–Reference Rouze, Martin-Loeches and Povoa39 Notably, in one study, high 14-day mortality rates (54.8%) and 30-day mortality rates (66.7%) were reported among 42 hospitalized patients with COVID-19 and S. aureus bacteremia. Reference Cusumano, Dupper and Malik25 In a second study of 1,705 patients with COVID-19, mortality rates were significantly higher in patients with CA bacterial infections compared to those without (47.5% vs 18.0%; P < .001). Reference Vaughn, Gandhi and Petty14 However, in other studies, no difference in mortality rates between patients with and without bacterial coinfection or secondary bacterial infection was reported, with an overall mortality rate at 28 days of 31.5% in patients with COVID-19. Reference Chong, Chieng and Tiwari40,Reference Russell, Fairfield and Drake41 These discrepancies may be due to low sample size in some studies, leading to inadequate power to detect a mortality difference. Reference Chong, Chieng and Tiwari40 In other studies, most deaths occurred early during hospitalization; therefore, less time was available to collect microbiological samples. Reference Russell, Fairfield and Drake41 This finding suggests that the rate of bacterial infections in patients with COVID-19 might be underestimated.
Along with increased mortality, other noteworthy trends among COVID-19 patients with bacterial coinfection or secondary bacterial infection included prolonged length of hospital stay, Reference Vaughn, Gandhi and Petty14,Reference Garcia-Vidal, Sanjuan and Moreno-Garcia15,Reference Kubin, McConville and Dietz21,Reference Nasir, Rehman and Omair24,Reference Chong, Chieng and Tiwari40 more frequent ICU admission, Reference Karaba, Jones and Helsel13,Reference Garcia-Vidal, Sanjuan and Moreno-Garcia15,Reference Ripa, Galli and Poli19,Reference Kubin, McConville and Dietz21,Reference Nasir, Rehman and Omair24,Reference Chong, Chieng and Tiwari40 and use of invasive mechanical ventilation. Reference Kubin, McConville and Dietz21,Reference Nasir, Rehman and Omair24,Reference Nori, Cowman and Chen28,Reference Lee, Koh and Kim38,Reference Chong, Chieng and Tiwari40,Reference Bogossian, Taccone and Izzi42–Reference Gomez-Simmonds, Annavajhala and McConville45 In a study of 100 COVID-19 patients, patients severely or critically ill at the time of admission were 4.4 times more likely to develop a bacterial infection, Reference Nasir, Rehman and Omair24 and those with bacterial infections were more likely to be admitted to the ICU compared with patients without bacterial infections (56% vs 18%; P < .001). Reference Nasir, Rehman and Omair24 Similarly, patients with CA bacterial pneumonia (CABP) were more likely to be admitted to the ICU compared with patients without coinfections (33% vs 16%; P < .01). Reference Karaba, Jones and Helsel13 Interestingly, in a single-center retrospective study of 989 patients, hospital length of stay was only significantly increased in patients with HA bacterial infections, and not in those with CA bacterial infections. Reference Garcia-Vidal, Sanjuan and Moreno-Garcia15 Furthermore, other studies have also reported that patients with bacterial coinfections or secondary bacterial infections were older in age Reference Karaba, Jones and Helsel13–Reference Garcia-Vidal, Sanjuan and Moreno-Garcia15,Reference Thelen, Buenen, van Apeldoorn, Wertheim, Hermans and Wever17,Reference Kubin, McConville and Dietz21,Reference Singh, Upadhyay, Reddy and Granger33,Reference Baskaran, Lawrence and Lansbury36,Reference Lee, Koh and Kim38,Reference Amin-Chowdhury, Aiano and Mensah46,Reference Kimmig, Wu and Gold47 and were immunocompromised. Reference Garcia-Vidal, Sanjuan and Moreno-Garcia15,Reference Kubin, McConville and Dietz21,Reference Obata, Maeda, Do and Kuno48 These populations typically at greater risk of developing severe COVID-19 and frequently have chronic underlying conditions and comorbidities, such as diabetes, kidney disease, or cancer. Reference Vaughn, Gandhi and Petty14,Reference Garcia-Vidal, Sanjuan and Moreno-Garcia15,Reference Chen, Zhu and Xiao27
Nasir et al Reference Nasir, Rehman and Omair24 reported that a larger proportion of patients with COVID-19 and bacterial infections received treatment with systemic steroids compared with patients without bacterial infections (92% vs 62% respectively; P = .001) and that treatment with steroids was a significant risk factor for bacterial infections. Reference Nasir, Rehman and Omair24 In a study of 226 hospitalized COVID-19 patients, treatment with steroids increased the risk of bacterial infections but steroid use did not affect the mortality rate (Table 3). Reference Obata, Maeda, Do and Kuno48 In another study of 111 hospitalized COVID-19 patients, tocilizumab use was associated with patients with high risk of developing bacterial or fungal infections (Table 3). Reference Kimmig, Wu and Gold47 Although mortality in the group of patients who received tocilizumab was higher than those not receiving treatment (39.6% vs 17.4% respectively; P = .016), Reference Kimmig, Wu and Gold47 this may be due to the fact that patients in the tocilizumab group were sicker and tocilizumab use predisposes to secondary bacterial infections.
Antibiotic treatment approaches in patients with COVID-19 and secondary bacterial infections or bacterial coinfections
Considerable heterogeneity in reported treatment rates and antibiotic treatment approaches was reported across the studies included in this review, perhaps in part due to the variability in study locations and differing local and national guidelines to antibiotic treatment (Fig. 2 and Fig. 3). National US guidelines (from the National Institutes of Health), updated in April 2021, recommend empiric antibiotics if secondary bacterial pneumonia or sepsis is suspected in patients with COVID-19 but to re-evaluate patients daily and de-escalate or stop antibiotic treatment if there is no evidence of bacterial infection. 49

Fig. 2. Proportion of patients with COVID-19 receiving antibiotics: (a) in patients with COVID-19 and carbapenemase-producing Enterobacterales; (b) in patients with carbapenem-resistant Klebsiella pneumoniae; (c) in patients with COVID-19 and bloodstream infection; (d) in patients with carbapenem-resistant Pseudomonas aeruginosa for suspected bacterial superinfection; and (e) in patients with COVID-19 and bacterial infection. Note. CA, community-acquired; COVID-19, coronavirus disease 2019; HA, hospital-acquired.

Fig. 3. Most frequently used antibiotics in patients with COVID-19.a (a) Studies expressed data as percentage of patients receiving antibiotic treatments, except the study by Langford et al, Reference Langford, So and Raybardhan52 in which data were presented as percentage of prescriptions of an antibiotic class per total number of antibiotic prescriptions. Data are provided only for antibiotic classes that were used in >10% of patients or > 10% of prescriptions. Note. β-LI, β-lactamase inhibitors; COVID-19, coronavirus disease 2019.
Despite the low rates of secondary bacterial infections observed, most studies reported the use of empiric antibiotic treatment, with 33.7% to > 90% of COVID-19 patients treated (Tables 2 and 3). Reference Martinez-Guerra, Gonzalez-Lara and de-Leon-Cividanes12,Reference Buehler, Zinkernagel and Hofmaenner30,Reference Lansbury, Lim, Baskaran and Lim34,Reference Chong, Chieng and Tiwari40,Reference Guisado-Gil, Infante-Domínguez and Peñalva50,Reference Magnasco, Mikulska and Giacobbe51 However, data are limited and information was not available on the duration of treatment. Although many patients did not have a confirmed bacterial infection at the start of treatment, data were not available on patients who stopped or altered treatment once microbial testing to confirm bacterial infection was performed. The variation in the range of patients receiving antibiotics could be explained by the differences in geographic location, the diversity of the populations treated, the time when studies were done, and so on.
These findings suggest that antibiotic utilization was high in patients who did not have bacterial infection. In a study of 48 COVID-19 patients, no significant difference was reported in the use of empiric antimicrobial therapy in critically ill patients either with bacterial superinfection (88%) or without bacterial superinfection (94.7%). Reference Buehler, Zinkernagel and Hofmaenner30 Notably, all studies that included data on both infection rates and antibiotic use reported mismatch between use of antibiotics versus confirmed secondary or coinfection, regardless of whether infection was CA or HA (see Tables 1–3 and Fig. 2). Reference Martinez-Guerra, Gonzalez-Lara and de-Leon-Cividanes12,Reference Vaughn, Gandhi and Petty14,Reference Kubin, McConville and Dietz21 In a meta-analysis of patients with COVID-19, the prevalence of antibiotic prescribing was 62.4%, whereas the estimated rate of bacterial coinfection was 8.6%. Reference Langford, So and Raybardhan52 In a systematic review reporting bacterial and fungal coinfections in 806 patients with COVID-19, 72.1% received antimicrobial therapy despite only 8% of patients having bacterial or fungal coinfections during their hospitalization. Reference Rawson, Moore and Zhu31
A recent meta-analysis of antibiotic prescribing in 30,623 patients with COVID-19 reported considerable heterogeneity across regions with a prevalence of 63.1% (95% confidence interval [CI], 41.7%–80.4%) in Europe, 64.8% (95% CI, 54.0%–74.2%) in the United States, 76.2% (95% CI, 66.8%–82.3%) in China, 86.0% (95% CI, 77.4%–91.7%) in the Middle East, and 87.5% (95% CI, 47.8%–98.2%) in East and Southeast Asia (excluding China). Reference Langford, So and Raybardhan52 Only 5 (3.2%) of 154 studies included in this meta-analysis provided data on duration of antibiotic treatment. Reference Langford, So and Raybardhan52 Antibiotic stewardship strategies were reported in 3 studies (1.9%), including recommendations to avoid antibiotics in patients without suspected coinfection (n = 2) or to de-escalate antibiotics when additional data became available (n = 1). Reference Langford, So and Raybardhan52 In a retrospective study of 13,932 hospitalized patients with COVID-19 who were prescribed antibiotics in 150 hospitals in Spain from March 1 to June 23, 2020, antibiotics were prescribed for respiratory bacterial coinfections and/or secondary infections in 10.9% of patients with COVID-19 and 43.8% of total antibiotic prescriptions were considered inappropriate. Reference Calderón-Parra, Muiño-Miguez and Bendala-Estrada53 Interestingly, younger age and fewer comorbidities were independently associated with inappropriate antibiotic prescribing. Reference Calderón-Parra, Muiño-Miguez and Bendala-Estrada53 Notably, a lower percentage of inappropriate antibiotic prescribing was observed in patients hospitalized after March 2020 in this study, which suggests increased awareness of the problem among healthcare professionals and a better understanding of the disease.
The types of antibiotics prescribed differed across the studies we reviewed, although most were broad-spectrum agents, including fluoroquinolones, β-lactam and β-lactamase inhibitors, cephalosporins, macrolides, and penicillin-like agents (Fig. 3). This pattern of antibiotic prescribing likely reflects the empirical use of these agents, which tends to provide coverage of multiple organisms while awaiting culture results or confirmation of coinfection or secondary infection.
The potential overuse or misuse of antibiotics in the context of the COVID-19 pandemic could contribute to increased AMR. Reference Rawson, Moore and Castro-Sanchez54 AMR has been widely reported, including infections with multidrug-resistant (MDR) organisms, Reference Garcia-Vidal, Sanjuan and Moreno-Garcia15,Reference Sogaard, Baettig and Osthoff16,Reference Ripa, Galli and Poli19,Reference Nori, Cowman and Chen28,Reference Buehler, Zinkernagel and Hofmaenner30,Reference Posteraro, De Angelis and Menchinelli35,Reference Adelman, Bhamidipati and Hernandez-Romieu37,Reference Bogossian, Taccone and Izzi42 and methicillin-resistant Staphylococcus aureus. Reference Cusumano, Dupper and Malik25,Reference Foschi, Zignoli and Gaibani32,Reference Lansbury, Lim, Baskaran and Lim34–Reference Baskaran, Lawrence and Lansbury36,Reference Chong, Chieng and Tiwari40,Reference Obata, Maeda, Do and Kuno48 In a study of 989 COVID-19 patients, MDR gram-negative bacteria were isolated in 7 of 43 patients with HA infections: 3 had MDR P. aeruginosa infection, 2 had extended-spectrum β-lactamase E. coli, and 2 extended-spectrum β-lactamase K. pneumoniae. Reference Garcia-Vidal, Sanjuan and Moreno-Garcia15 Søgaard et al Reference Sogaard, Baettig and Osthoff16 only reported 1 MDR pathogen (Acinetobacter baumannii, Oxa-23) isolated in a case transferred from a hospital abroad. Buehler et al Reference Buehler, Zinkernagel and Hofmaenner30 reported that MDR bacteria (Pseudomonas aeruginosa, Enterobacter cloacae, and Burkholderia cepacia) were detected in 22.2% of all hospitalized COVID-19 patients.
Increased AMR leads to high exposure to antibiotic treatments, which can have detrimental consequences and can facilitate subsequent infections during ICU stay, particularly by gram-positive pathogens such as enterococci. Reference Chakraborty, Lam and Kommineni55 In a US cohort study of hospitalized patients with sepsis, inadequate broad-spectrum empiric antibiotic treatment was associated with ICU hospitalization and increased mortality. Reference Rhee, Kadri and Dekker56 Interestingly, inadequate antibiotic therapy was 4 times more likely in patients with resistant pathogens (eg, methicillin-resistant Staphylococcus aureus) than with nonresistant pathogens (P < .001), older patients, and patients with comorbidities. Reference Rhee, Kadri and Dekker56 Thus, improved treatment strategies (antimicrobial stewardship) and treatment options with newer antibiotics that have lower resistance rates are needed.
Antibiotic stewardship perspectives
The incidence of secondary bacterial infections and bacterial coinfections in patients with COVID-19 is relatively low, with lower rates of CA bacterial infections than HA infections. The incidence and variety of infecting pathogens increased with the length of hospitalization. Overall, the rates of secondary bacterial infections and bacterial coinfections in patients with COVID-19 were lower than rates of secondary bacterial infection and/or coinfection associated with other viral respiratory diseases such as influenza. Reference Klein, Monteforte and Gupta57 The relatively low incidence of bacterial coinfections and/or secondary infections reported during the COVID-19 pandemic could be a consequence of the implementation of national lockdowns and social distancing measures adopted by many countries during the pandemic, as was suggested in an international study demonstrating that COVID-19 lockdowns significantly reduced transmission of S. pneumoniae, H. influenzae, and N.meningitidis, leading to significant reductions in life-threatening invasive diseases worldwide. Reference Brueggemann, Jansen van Rensburg and Shaw58
Despite the relatively low rates of bacterial secondary infections and/or bacterial coinfections observed during the COVID-19 pandemic, high percentages of patients have been receiving antibiotic treatment. Empiric treatment was common, perhaps because COVID-19 patients are often hospitalized during the hyperinflammatory phase of the disease, making differentiation between viral and secondary bacterial infections challenging. Reference Tan, Ng and Tay59 From a clinical perspective, the mismatch between antibiotic utilization and reported rates of bacterial infection is of particular concern because it may exacerbate the development of AMR and associated complications.
Increased empiric antibiotic prescribing may have been due to the diversion of stewardship efforts to pandemic responsibilities and away from core activities. Reference Mazdeyasna, Nori and Patel60 Investigation of the optimal antimicrobial stewardship program interventions into pandemic response efforts to limit antibiotic overuse is warranted. However, despite national guidelines aiming to rationalize antibiotic use and maintain safe medication use in the ICU, 49,61,62 the emergency caused by the COVID-19 pandemic probably made it difficult to apply these guidelines, with overwhelmed wards and ICUs and busy healthcare professionals. Moreover, the diagnosis of bacterial infections remains a challenge, and it is difficult to distinguish between severe viral pneumonia and bacterial infection. Microbiological investigations, which are not routinely performed in patients with COVID-19, take several days to result and do not differentiate bacterial colonization from infection. Reference Vaughn, Gandhi and Petty14
Thus, the pandemic may have a lasting impact on AMR, and the long-term impact on antibiotic overuse during COVID-19 pandemic remains to be seen. The data reported here show multidrug-resistance pathogens and indicate that current empiric treatment strategies may not be effective. The development of newer antibiotics is urgently needed, particularly considering the increase in multidrug resistance for which there are no treatment options.
Although a strength of this review is the use of a comprehensive search strategy, several limitations must be considered. First, most of the included studies were small, retrospective, observational studies, with a large degree of heterogeneity between them in terms of patient populations, geographic locations, and treatment protocols. Many of the included studies lacked consistent bacteriological diagnostic and specific testing upon patient admission to hospital, which likely affected stratification of CA versus HA infections. Some studies did not give precise details regarding the timing of diagnosis, making the differentiation between CA and HA challenging. Finally, most studies included in this review were from Asia, Europe, and North America (United States), and regional differences in the patient populations, access to care, clinical practices among hospitals, and patient follow-up must be considered.
To conclude, recent data indicate that secondary bacterial infections and bacterial coinfections in patients with COVID-19 are associated with worse patient outcomes. Importantly, antibiotic utilization was consistently higher than bacterial infection rates, highlighting the need to improve appropriate treatment approaches to mitigate the complications of the misuse of antibiotics. Furthermore, due to the incidence of multidrug-resistant bacterial pathogens, new treatment and antibiotics that could overcome the problem of resistance are urgently needed. Implementing and following stewardship programs will be of crucial importance to prevent the development of resistance and to improve patient outcomes.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ash.2022.253
Acknowledgments
The authors thank Meridian HealthComms (Plumley, UK) for medical writing support, funded by Paratek Pharmaceuticals (King of Prussia, PA) in accordance with Good Publication Practice (GPP3).
Financial support
Support for medical writing by Meridian HealthComms was funded by Paratek Pharmaceuticals.
Conflicts of interest
E.M.G. is an employee of Dompé US (Boston, MA). All other authors declare no conflicts of interest related to this article.








