Introduction
Early in the coronavirus disease 2019 (COVID-19) pandemic, concern for bacterial co-infections led to widespread prescribing of antibacterial drugs for patients with COVID-19 admitted to hospital. The overall prevalence of antibacterial prescribing was 75% among hospitalized patients and up to 100% in some studies of patients admitted to intensive care units (ICUs). Reference Langford, So and Raybardhan1 However, only 3%–10% of hospitalized patients with COVID-19 had a confirmed bacterial co-infection at admission, with up to 20% being diagnosed as having a secondary bacterial infection later during admission. Reference Langford, So and Raybardhan2–Reference Musuuza, Watson, Parmasad, Putman-Buehler, Christensen and Safdar4 Although a declining temporal trend in antibacterial drug prescribing among patients with COVID-19 early in the pandemic has been reported, few studies have investigated antibacterial drug use after widespread vaccination and the availability of oral antiviral drugs for individuals at high risk of severe clinical outcomes, particularly during epidemics caused by Omicron variants, which emerged in late 2021. Reference Langford, So and Raybardhan1,Reference Cong, Stuart and N5
In Hong Kong, stringent pandemic containment measures throughout 2020 and 2021 suppressed local transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Reference Cheng, Wong and Chen6–Reference Cheng, Wong and Tong8 During this period, all persons with laboratory-confirmed infections, including asymptomatic cases, were strictly isolated in public hospitals. Reference Cowling, Ali and Ng9,Reference Lin, Wu and Tsang10 The largest epidemic wave in Hong Kong was wave 5, caused by Omicron subvariants, and resulted in over one million confirmed cases and nearly 10,000 deaths within 2 months. Reference Wong, Au and Lo11,Reference Chen, Abdullah and Chan12 A large surge in COVID-19 cases in early wave 5 led to public hospitals being overloaded with patients, most of which involved older adults, and necessitated a change in government policy on 15 February 2022 to prioritize hospital admissions based on disease severity. Reference Chen, Abdullah and Chan12–Reference Wong, Cheung and Lin14 After this point, patients with COVID-19 showing mild or no symptoms were not required to isolate in hospital and could self-isolate at home.
The World Health Organization (WHO) declared the end of COVID-19 as a public health emergency in early May 2023. Quantifying antibacterial drug prescribing practices for COVID-19 inpatients throughout the pandemic allows us to establish a baseline benchmark for ongoing monitoring of antibacterial drug use and identify potential gaps in evidence to practice. Therefore, this study aimed to describe the inpatient prescribing of antibacterial drugs over time and explore factors associated with inpatient antibacterial drug use among patients with COVID-19 admitted to all public hospitals in Hong Kong up to 30 September 2022.
Methods
Data sources and ethical approval
Since the start of the COVID-19 pandemic, all officially reported COVID-19 cases have been tracked by the Centre for Health Protection (CHP) of the Hong Kong Department of Health. Data from the CHP, including age, sex, confirmation date, case classification (locally acquired or imported), and COVID-19 vaccination records, were linked using a pseudonymous identifier to electronic health records obtained from the Hospital Authority (HA), the statutory body responsible for providing public health care in the territory. Data obtained from the HA included patient demographics, prescription dispensing records (outpatient and inpatient), COVID-19 condition status, inpatient transaction records (dates of admission, discharge, and transfer and ward location), inpatient diagnoses, laboratory tests, and outpatient clinic attendance dates and diagnoses. The available data included microbiological test names and dates but not results for bacterial species or antimicrobial susceptibility. Therefore, we could not assess the prevalence of laboratory-confirmed infections. In this study, we analyzed data sets from CHP and HA extracted on 30 November 2022.
The study was approved by the institutional review board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster. Data used for this analysis were part of the public health response to the pandemic, and informed consent was waived from individual patients.
Population and setting
This cohort study included all patients with community-acquired COVID-19 who were admitted to public hospitals in Hong Kong. Community-acquired COVID-19 was defined as patients with COVID-19 who had a confirmation date of SARS-CoV-2 infection that occurred between 7 days prior to the admission date and 3 days after the admission date (Supplementary Figure 1). To permit sufficient follow-up time after hospital admission, patients with a COVID-19 confirmation date between 21 January 2020 and 30 September 2022 were eligible for inclusion. Patients without an inpatient admission, inpatients not discharged by 30 October 2022, patients with missing date of birth, and imported COVID-19 cases were excluded. For patients with multiple COVID-19-related inpatient admissions cases, we restricted our analyses to the first case closest to their confirmation date.
Baseline variables and disease severity definitions
Baseline variables included demographics, medical conditions, drugs, COVID-19 vaccination, and records of a microbiological culture result (see Supplementary Table 1 for details). As described elsewhere, dates for each epidemic wave were defined considering the daily number of cases testing positive by reverse-transcription polymerase chain reaction for SARS-CoV-2, transmissibility (measured by the effective reproduction number) and healthcare capacity. Reference Chen, Abdullah and Chan12,Reference Wong, Cheung and Lin14,Reference Mefsin, Chen and Bond15 We combined waves 1 and 2 in the analysis, given the relatively smaller number of cases that were largely being imported to Hong Kong. The fifth wave, dominated by Omicron subvariants, demonstrated distinct differences between the massive epidemic that peaked in March 2023 and the later phase of the wave when pharmaceutical interventions against COVID-19 were widely available. Reference Wong, Cheung and Lin14,Reference Mefsin, Chen and Bond15 Therefore, each patient was categorized according to their admission date as follows: waves 1–2 (1 January 2020 to 30 June 2020), wave 3 (1 July 2020 to 31 October 2020), wave 4 (1 November 2020 to 30 December 2021), early wave 5 (5E, 31 December 2021 to 22 May 2022), and late wave 5 (5L, 23 May 2022 to 30 September 2022). Reference Wong, Cheung and Lin14,Reference Mefsin, Chen and Bond15 Patients were classified into one of four hierarchical and mutually exclusive severity groups according to the outcome of the admission: fatal, critical, severe, or mild to moderate (Supplementary Table 2). Reference Lin, Wu and Tsang10,Reference Wong, Cheung and Lin14
Antibacterial drug use and outcomes
We quantified all inpatient prescriptions for drugs listed in the British National Formulary (BNF) section 5.1: Antibacterial drugs. Antibacterial drugs were further grouped according to the WHO AWaRe classification (2021) into access, watch, reserve, and not recommended groups. 16 Among patients prescribed an antibacterial drug, we extracted the initiation day of antibacterial drug(s) for each patient and classified the earliest inpatient prescription of antibacterial drug into either monotherapy (defined as the prescription of only one antibacterial drug on the initiation day of antibacterial treatment) or combination therapy (defined as the prescription of two or more antibacterial drugs on the initiation day).
Measures of drug use followed published recommendations for in-hospital antibacterial drug utilization research. Reference Stanić Benić, Milanič and Monnier17 The prevalence of antibacterial drug prescriptions in all patients with community-acquired COVID-19 during follow-up was measured as the proportion of patients with any dispensed antibacterial drug prescription from the admission date to the end of follow-up. We examined the number of days of antimicrobial therapy (DOT) for each antibacterial drug and calculated the proportion of total DOT for each antibacterial drug and AWaRe group. DOT per 1,000 patient days (PD) was the primary quantity metric of antibacterial drug use.
To minimize bias caused by outliers with extremely long hospital stays, we censored the calculation of the period at risk at 90 days. The follow-up period for an inpatient prescription ended, therefore, on the earliest of the discharge date, date of death, or inpatient day 89. Clinical outcomes reported include the number of days present in hospital and death during follow-up (ie, fatal severity).
Statistical analysis
Rates of antibacterial drug use were calculated by summing the number of DOT during a specified period divided by the number of PD during the period. We used a multivariable logistic regression model to assess associations between baseline variables and disease severity and the outcome of an inpatient antibacterial drug prescription, by estimating conditional odds ratios (ORs) and 95% confidence intervals (CI). Analyses were conducted using R version 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
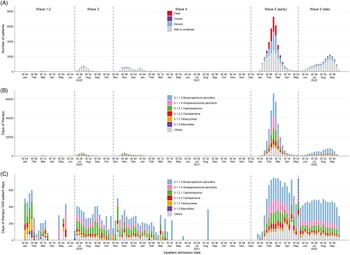
As of 30 September 2022, there were 1,765,405 officially confirmed COVID-19 cases in Hong Kong. Our analyses focused on 65,810 patients with COVID-19 who were likely infected in the local community (excluding imported cases and nosocomial cases) and admitted into a public hospital for isolation or treatment. All the included patients had either been discharged from or died in hospital at the time of analysis (Supplementary Figure 2). Over half of the patients (37,991, 57.7%) were admitted during wave 5E, which was dominated by the Omicron BA.2 subvariant (Figure 1A). Reference Chen, Abdullah and Chan12,Reference Mefsin, Chen and Bond15 The median age of the patient cohort was 70.0 years (interquartile range [IQR]: 44.0–84.0), with 7,759 (11.8%) patients aged <20 years (Table 1). Most patients had mild to moderate infections, with 1,691 (2.6%) patients admitted to ICU. In total, 7,473 (11.4%) patients died in hospital, with 90.0% (n = 6,699) of inpatient deaths occurring during wave 5E.

Figure 1. (A) Weekly number of inpatients with COVID-19 included in the study. Weekly antibacterial drug use according to BNF section in (B) days of therapy and (C) days of therapy/1,000 patient days.
Table 1. Baseline characteristics and disease severity of patients hospitalized with confirmed COVID-19 in all public hospitals stratified by epidemic wave

Note. COVID-19, coronavirus disease 2019; F, female; ICU, intensive care unit; IQR, interquartile range; M, male; NA, not applicable; RCHE, residential care home for the elderly; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Variables are shown as number of patients (%) unless otherwise indicated. Epidemic waves were defined as starting on the following dates: 1 January 2020 (waves 1–2), 1 July 2020 (wave 3), 1 November 2020 (wave 4), 31 December 2021 (wave 5E), and 23 May 2022 (wave 5L).
a Distribution of overall patient days for those admitted to an intensive care unit/high-dependency unit.
b COVID-19 vaccines became available locally beginning in February 2021 (ie, wave 4).
The number and characteristics of patients admitted to hospital varied by epidemic wave (Table 1). Patients admitted prior to wave 5 were younger and had fewer comorbidities, and more often had milder infections. The median number of days present in hospital declined over time as criteria for hospital discharge evolved and hospital capacity became limited during wave 5E. More than half of the patients admitted during wave 5E had not received any doses of COVID-19 vaccine, especially among the 15,877 patients aged over 80 years (63.1% of these patients were not vaccinated). However, by wave 5L, 78.3% of patients had received at least one dose of COVID-19 vaccine.
Antibacterial drug prescriptions
During their hospital admission, 35,507 (54.0%) patients were prescribed an antibacterial drug, with the lowest prevalence in wave 4 (28.0%) and highest in wave 5E (64.6%; Table 2). Among those receiving an antibacterial drug, most were prescribed on the day of admission (69.5%) or within 2 days of admission (89.8%). Few young people aged <20 years received an antibacterial drug prescription (n = 747, 2.1%). In contrast, adults aged ≥80 years accounted for nearly half the patients receiving antibacterial treatment. The overall prevalence of antibacterial drug use by baseline characteristic and disease severity is presented in Supplementary Table 3. Nearly all patients admitted to ICU or with critical or fatal disease severity were prescribed an antibacterial drug.
Table 2. Characteristics of patients who were hospitalized with confirmed COVID-19 and prescribed an antibacterial drug during admission stratified by epidemic wave

Note. COVID-19, coronavirus disease 2019; F, female; ICU, intensive care unit; IQR, interquartile range; M, male.
Variables are shown as number of patients (%) unless otherwise indicated. Epidemic waves were defined as starting on the following dates: 1 January 2020 (waves 1–2), 1 July 2020 (wave 3), 1 November 2020 (wave 4), 31 December 2021 (wave 5E), and 23 May 2022 (wave 5L).
a Since data are only available by day, this reflects changing drugs on the earliest prescription date, for example, from amoxicillin/clavulanic acid to ceftriaxone or piperacillin/tazobactam.
Throughout the pandemic, the weekly use of antibacterial drugs in COVID-19 cases measured by DOT closely tracked hospital admissions for COVID-19 with substantially higher prescriptions observed during wave 5E, although smaller variations in weekly prescribing rates (measured as DOT/1,000 PD) occurred across waves (Figure 1). Antibacterial drugs were prescribed at an overall rate of 550.5 DOT/1,000 PD (Supplementary Table 4). The most commonly used antibacterial drug classes were broad-spectrum penicillins (BNF 5.1.1.3), antipseudomonal penicillins (BNF 5.1.1.4), and cephalosporins (BNF 5.1.2.1) (Figure 1C). Overall, rates of use ranged from 246.9 DOT/1,000 PD in waves 1–2 to 661.2 DOT/1,000 PD in wave 5E (Figure 1C). There was limited prescribing of macrolides. Tetracyclines were prescribed for COVID-19 patients, more often in waves 1–2, and higher prescription rates for carbapenems and antipseudomonal penicillins were observed in wave 5E than in earlier waves and wave 5L (Figure 1C).
Amoxicillin/clavulanic acid, piperacillin/tazobactam, and ceftriaxone were the three most prescribed antibacterial drugs and together accounted for 67.9% of the total antibacterial DOT prescribed to patients with COVID-19 (Supplementary Table 5). Throughout each epidemic wave, the use of the most commonly prescribed antibacterial drugs was consistently higher among COVID-19 patients with fatal and critical disease severity than in patients with mild to moderate disease (Supplementary Figure 3). Amoxicillin/clavulanic acid, ceftriaxone, azithromycin, amoxicillin, cefotaxime, and doxycycline were frequently initiated within 2 days of admission, while drugs that are typically used to treat hospital-acquired bacterial infections, such as piperacillin/tazobactam, meropenem, ceftazidime, vancomycin, and linezolid, were initiated later (Supplementary Table 5).
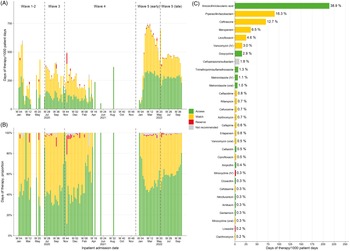
Most antibacterial drugs prescribed to patients with COVID-19 during the study period belonged to the groups of access (47.8% of DOT) and watch (49.5%), and the rest were reserve (0.9%) and not recommended (1.8%) antibacterial drugs. Rates of access and watch drug use increased rapidly in wave 5E and stabilized thereafter (Figure 2 and Supplementary Figure 4). An increasing number of days treated with watch or reserve drugs appeared in more severe infections, which was largely consistent across different epidemic waves (Supplementary Figure 5).

Figure 2. Weekly rates of antibacterial drug consumption (A), proportion of antibacterial days of therapy (B), and the 30 most prescribed antibacterial drugs (C) classified according to WHO AWaRe group. For each drug in C, the label on the right indicates the drug’s share of all antibacterial drug days of therapy. N.B. Cefoperazone/sulbactam is recommended in local treatment guidelines for the treatment of infections caused by Acinetobacter baumannii and Pseudomonas aeruginosa. Reference Ho, Wu, Chao, Hung, Lui and Lung23
Factors associated with an antibacterial drug prescription
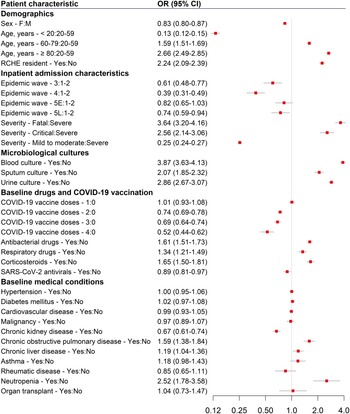
After controlling for potential confounding factors in the regression model, patients admitted during wave 3 (OR 0.61, 95% CI 0.48–0.77), wave 4 (0.39, 0.31–0.49), and wave 5L (0.74, 0.59–0.94) had lower odds of receiving an antibacterial prescription, whereas there was a similar likelihood in wave 5E (0.82, 0.65–1.03), compared with patients in waves 1–2 (Figure 3).

Figure 3. Odds ratios from the multivariable logistic regression model showing the association between baseline patient characteristics and disease severity with an inpatient antibacterial prescription. Abbreviations: 5E, early wave 5; 5L, late wave 5; CI, confidence interval; COVID-19, coronavirus disease 2019; OR, odds ratio; RCHE, residential care home for the elderly; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Factors associated with increased odds of an inpatient antibacterial drug prescription included older age (≥80 years: OR 2.66, 95% CI 2.49–2.85; 60–79 years: 1.59, 1.51–1.69, compared with 20–59 years), living in a residential care home for the elderly (2.24, 2.09–2.39), having a more severe outcome (fatal: 3.64, 3.2–4.16; critical: 2.56, 2.14–3.06, compared with cases classified as severe), and a documented record of the following during the baseline period, such as an order for blood (3.87, 3.63–4.13), sputum (2.07, 1.85–2.32), or urine culture (2.86, 2.67–3.07), a prescription for an antibacterial drug (1.61, 1.51–1.73), corticosteroid (1.65, 1.5–1.81), or respiratory drug (1.34, 1.21–1.49), and a diagnosis of chronic obstructive pulmonary disease (1.59, 1.38–1.84) or neutropenia (2.52, 1.78–3.58). Age <20 years (0.13, 0.12–0.15), female sex (0.83, 0.8–0.87), and chronic kidney disease (0.67, 0.61–0.74) appeared to be associated with decreased odds of antibacterial prescription (Figure 3). Compared with unvaccinated patients, reduced odds of having an antibacterial prescription were observed for patients with two (0.74, 0.69–0.78), three (0.69, 0.64–0.74), and four (0.52, 0.44–0.62) doses of COVID-19 vaccine administered 14 days prior to admission. Furthermore, preadmission treatment with SARS-CoV-2 antivirals was also associated with lower odds (0.89, 0.81–0.97) of an antibacterial prescription.
Discussion
This study is one of the largest analyses of inpatient prescribing of antibacterial drugs in adults and children admitted to hospital with COVID-19. Our findings demonstrate a high prescription rate of antibacterial drugs among hospitalized patients infected in the local community in Hong Kong during the pandemic, particularly in the largest wave caused by the Omicron BA.2 subvariants (wave 5E). Furthermore, we identified important variations in prescribing specific types of drugs among patients with different characteristics and highlighted the baseline factors associated with antibacterial prescriptions.
The high-quality patient data collected in Hong Kong in response to the COVID-19 pandemic allowed us to conduct a comprehensive examination of antibacterial drug prescribing throughout multiple epidemic waves. In a point prevalence survey carried out in Scotland in April 2020, 45.0% of patients with COVID-19 were prescribed antibacterial drugs. Reference Seaton, Gibbons and Cooper18 This estimate was similar to our estimate (46.7%) for waves 1–2. Despite evidence from a seminal systematic review demonstrating a low prevalence (∼15%) of bacterial co-infections or secondary infections among hospitalized patients with COVID-19 during the early days of the pandemic, Reference Langford, So and Raybardhan2 a substantial proportion of patients in our study were prescribed antibacterial drugs in later epidemic waves, revealing a clear mismatch between the prevalence of bacterial infections and antibacterial prescribing. This inconsistency has been reported in studies conducted worldwide. One of the largest studies of hospitalized patients with SARS-CoV-2 in the United Kingdom between February and June 2020 showed that 85.2% of admitted patients received an antibacterial drug prescription and that the prescribing trend was declining toward the end of the study period. Reference Russell, Fairfield and Drake19 A scoping review of studies published until March 2021 also showed that the prescribing of antibacterial drugs declined between June 2020 and March 2021. Reference Cong, Stuart and N5 This aligns with the time trends observed in our study. However, a surge in antibacterial drug use occurred in early 2022 with the Omicron variant, a concerning finding that may have had implications for the development of antimicrobial resistance (AMR) in Hong Kong. Reference Ma, Kung and Chen20,Reference Wong, Yuen, Li, Kwok, Chen and Cheng21
The pandemic response measures applied in Hong Kong required all confirmed COVID-19 cases to be isolated in designated facilities, mainly isolation wards in public hospitals, although this strict policy was abandoned during the fifth wave in February 2022 when only cases with more severe conditions could be admitted during a large Omicron outbreak in the community. 13 This policy might have resulted in a higher prevalence of antibacterial drug prescribing during early wave 5, given the changes that more severe patients could likely receive medical care with a severely stretched healthcare system.
We were able to include information on baseline COVID-19 vaccination status and SARS-CoV-2 antiviral drug treatment for patients admitted from early 2021 onward, with most hospitalizations caused by the Omicron variants, which has not been addressed in previous studies. Our findings suggested an association, but did not demonstrate causality, that patients with ≥2 doses of COVID-19 vaccine or who were prescribed SARS-CoV-2 antiviral drugs prior to hospital admission had reduced odds of receiving an inpatient antibacterial drug. Although potential causal mechanisms remain to be elucidated, this association may be explained by a reduced risk of suspected bacterial infection or perhaps different prescribing practices for such patients.
By quantifying antimicrobial prescribing using standard metrics, we have demonstrated the feasibility of conducting surveillance on antibacterial use using routinely collected inpatient data. At the global level, the WHO is undertaking a large-scale clinical platform to understand antibacterial drug prescribing in COVID-19, 22 evidence that could establish benchmarks for patients admitted with COVID-19 and other respiratory viral infections and could serve to make comparisons among treatment patterns within health systems, countries, and regions. Our research complements and supports these global efforts by providing comprehensive data on the prevalence and types of antibacterial drugs used according to the AWaRe classification among a diverse cohort of inpatients from Hong Kong.
Nonetheless, some limitations exist in this study. As a cohort study that used secondary data sources, confounding and selection bias posed potential threats to validity. The observed associations should be considered as hypothesis-generating instead of causal. Given that the available data included the entire population of patients meeting our eligibility criteria and not a sample, the risk of selection bias was minimal. The available data did not include prescription indications nor were we able to assess the appropriateness of antibacterial therapy. These limitations could be addressed in future studies that prospectively collect information on the appropriateness of therapy and data are needed to determine the occurrence of bacterial co-infection (concurrent with SARS-CoV-2 infection), superinfection, or secondary infection. In addition, the baseline data used for the analysis were collected from outpatient clinics operated by the HA and public hospitals, which might have missed information from patients who had consultations at private hospitals or clinics. However, public hospitals provide 80%–90% of hospital bed days in Hong Kong.
Conclusions
Although antibacterial drug prescribing has declined over time among inpatients with COVID-19, it is crucial to establish standardized and evidence-based diagnostic and treatment guidelines. Ongoing surveillance of antibacterial drug prescribing will allow for targeted antimicrobial stewardship activities and should be implemented as a key component of healthcare systems in response to both COVID-19 and AMR.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/ash.2023.485.
Data availability statement
Patient data used in the analysis were derived from electronic health records managed by the Hospital Authority and other patient information collected by the Centre for Health Protection during case finding and contact tracing. Access to the underlying data used in this study is subject to the approval of the two agencies.
Acknowledgments
The authors thank the Hospital Authority and the Department of Health of the Government of Hong Kong Special Administrative Region for providing the data for the analysis, Ms Kitty TM Ng (pharmacist) from the Queen Elizabeth Hospital, Hong Kong, for her comments on the manuscript, and Julie Au and Chloe Chui for their technical support.
Author contribution
Conceptualization: Joseph E Blais and Peng Wu; Resources and funding acquisition: Peng Wu and Benjamin J Cowling; Supervision: Peng Wu and Benjamin J Cowling; Writing – original draft: Joseph E Blais; Writing – review and editing: Weixin Zhang, Yun Lin, Peng Wu, Vincent CC Cheng, Celine SL Chui, and Benjamin J Cowling; Formal analysis: Joseph E Blais; Data curation: Yun Lin; Visualization: Joseph E Blais.
Financial support
This study was supported by the Research Impact Fund (R7033-18) and Collaborative Research Fund (C7123-20GF) from the Research Grants Council of the Government of Hong Kong Special Administrative Region.
Competing interests
BJC consults for AstraZeneca, Fosun Pharma, GlaxoSmithKline, Haleon, Moderna, Novavax, Pfizer, Roche, and Sanofi Pasteur. CSLC has received grants from the Food and Health Bureau of the Hong Kong Government, Hong Kong Research Grant Council, Hong Kong Innovation and Technology Commission, Pfizer, IQVIA, MSD, and Amgen; and personal fees from PrimeVigilance; outside the submitted work. The authors declare no other competing interests.