Introduction
Increasing attention has been paid to dietary fibre (DF) fermentation, including non-starch polysaccharides (NSP) and resistant starch (RS), in the large intestine of pigs during the past several years. Indeed, DF lowers the energy value of the diet since their digestibility varies from 0.40 to 0.60 compared with the other nutrients (protein, fat, sugars or starch), which are above 0.80 (Noblet and Le Goff, Reference Noblet and Le Goff2001). On the other hand, the short-chain fatty acids (SCFA) produced by intestinal bacteria due to fibre fermentation can be used by the host animal for his own energy supply. This can cover up to 15% of the maintenance energy requirements in growing pigs and 30% in sows (Varel and Yen, Reference Varel and Yen1997). The knowledge of the contribution of DF to energy supply is also important for smallholders in the tropics, since the latter feed their pigs with unconventional fibrous ingredients, such as tree leaves (Leterme et al., Reference Leterme, Botero, Londoño, Bindelle and Buldgen2006).
The bulking effect of fibre and the prebiotic influence on some intestinal bacterial strains can also benefit the animals by improving satiety, quietness and intestinal health (Williams et al., Reference Williams, Verstegen and Tamminga2001). It has been shown, however, that some types of DF may increase diarrhoea in piglets (Montagne et al., Reference Montagne, Pluske and Hampson2003).
The bacterial growth supported by DF fermentation induces a shift of N excretion from urea in urine to bacterial protein in faeces and lowers the pH of the latter (Zervas and Zijlstra, Reference Zervas and Zijlstra2002; Martinez-Puig et al., Reference Martinez-Puig, Pérez, Castillo, Andaluz, Anguita, Morales and Gasa2003). The protein catabolism in the distal part of the colon and the NH3 emission from the manure are therefore reduced (Nahm, Reference Nahm2003). The relationship between DF fermentability and N excretion shift is still poorly documented although the source of DF is suspected to influence the growth of the bacterial population (Kreuzer et al., Reference Kreuzer, Machmüller, Gerdemann, Hanneken and Wittman1998; Zervas and Zijsltra, Reference Zervas and Zijlstra2002).
The aim of the present study was to determine in vitro, the amount of protein synthesis by faecal microbes, when (1) starch and different sources of purified NSP, or (2) ingredients differing in DF content, are available as the energy source for microbial fermentation. The kinetics of fermentation and SCFA production were also measured in order to evaluate their relationship with microbial protein synthesis.
Material and methods
Animals and diets
The experiments were carried out using three Belgian Landrace sows (weighing from 226 ± 12 to 257 ± 14 kg) as sources of bacterial inoculum. The animals were kept in one group and received daily, in two meals (0800 and 1500 h), 3 kg of a commercial diet (ZENA-D, Quartes, Deinze, Belgium) with the following chemical composition (g/kg dry matter (DM)): crude protein (CP), 164; ash, 67; neutral-detergent fibre (NDF), 253; acid-detergent fibre (ADF), 144; acid-detergent lignin (ADL), 29; total DF, 312. The sows had free access to water for the duration of the experiments. The collection of faeces started after 4 weeks of adaptation to the diet.
Substrate
Experiment 1: fermentation of purified carbohydrates. Five purified sources of fermentable carbohydrates were chosen according to their differences in soluble and insoluble fibre content, constituent sugars and glucosidic bonds (Table 1): potato starch (Fluka 85650), fibrous cellulose (Sigma C-6663), inulin (Fibruline, Cosucra, Warcoing, Belgium), citrus pectin (Sigma P-9135) and xylan from oat spelts (Fluka 95590).
Table 1 Chemical composition of the purified carbohydrate sources (experiment 1) and the raw and the pepsin–pancreatin hydrolysed substratesFootnote † (experiment 2) (g/kg dry matter)

Abbreviations are: DM = dry matter; NDF = neutral-detergent fibre; ADF = acid detergent fibre; ADL = acid-detergent lignin.
† Hydrolysed substrates are the residues of the raw substrates after they have undergone a pepsin-pancreatin hydrolysis according to Boisen and Fernández (Reference Boisen and Fernández1997).
Experiment 2: fermentation of sources of fermentable carbohydrates. Sugar-beet pulp (Beta vulgaris), raw potato (Solanum tuberosum) and wheat bran (Triticum aestivum), ground to pass a 1-mm mesh screen by means of a Cyclotec 1093 Sample Mill (FOSS Electric A/S, Hilleroed, Denmark), were used as substrates for in vitro fermentation.
Prior to fermentation, the substrates underwent an in vitro pepsin–pancreatin hydrolysis following the protocol of Boisen and Fernández (Reference Boisen and Fernández1997). In this method, 2 g samples were weighed in conical flasks. A phosphate buffer solution (100 ml, 0.1 mol/l, pH 6.0) and an HCl solution (40 ml, 0.2 mol/l) were poured into the flasks. The pH was adjusted to 2.0 with 1 mol/l HCl or 1 mol/l NaOH and 2 ml of a chloramphenicol solution (Sigma C-0378, 0.5 g per 100 ml ethanol) was added. Finally, a solution of pepsin (4 ml, 25 g/l, porcine pepsin: 2000 FIP-U/g, Merck no. 7190) was added to the mixture. The flasks were closed with a rubber stopper and placed for 2 h under gentle agitation in a water-bath at 39 ± 0.5°C.
After pepsin hydrolysis, 40 ml of a phosphate buffer solution (0.2 mol/l, pH 6.8) and 20 ml of a NaOH solution (0.6 mol/l) were added. The pH was adjusted to 6.8 with 1 mol/l HCl or 1 mol/l NaOH and a solution of pancreatin (2 ml, 100 g/l, pancreatin: Sigma P-1750) was added. The flasks were then closed with a rubber stopper and placed for 4 h under gentle agitation in a water-bath at 39 ± 0.5°C. After hydrolysis, the residues were collected by filtration on a Nylon cloth (42 μm), washed with ethanol (2 × 25 ml 95% ethanol) and acetone (2 × 25 ml 99.5% acetone), dried for 24 h at 60 ± 1°C and weighed. Enzymatic hydrolysis was performed from 40 to 51 times (8 replicates × 6 or 7 periods, according to the substrate). The hydrolysis residues from the different replicates and periods were pooled for subsequent in vitro fermentation.
The DM disappearance (dDM) during the pepsin–pancreatin hydrolysis was calculated as follows:
The chemical compositions of the raw and hydrolysed substrates are detailed in Table 1.
In vitro fermentation
In vitro fermentation was performed using the gas test method described by Menke and Steingass (Reference Menke and Steingass1988) and adapted to the pig by Bindelle et al. (Reference Bindelle, Buldgen, Boudry and Leterme2007). Briefly, an inoculum was prepared from fresh faeces of the three experimental sows. Faeces (50 g/l) were mixed to a buffer solution composed of salts and minerals (Menke and Steingass, Reference Menke and Steingass1988). The N source in the buffer solution (NH4HCO3) was replaced by an equimolar quantity of 15N-labelled NH4Cl (2% of enrichment, ISOTEC no. T85-70216; Isotech, Miamisburg, OH, USA). The fermentation at 39°C started when 200 mg of one of the substrates and 30 ml of the inoculum were introduced into 100-ml glass syringes.
The experimental scheme was as follows: for experiment 1: 5 substrates × 9 replicates + 3 blanks (containing only inoculum), repeated over two periods; and for experiment 2: 3 substrates × 9 replicates + 3 blanks, repeated over three periods.
The gas volumes of three syringes per substrate were recorded at regular intervals until 72 h. The six remaining syringes were stopped by quenching in an iced water-bath for 20 min, at half-time to asymptotic gas production, T/2 according to the model of France et al. (Reference France, Dhanoa, Theodorou, Lister, Davies and Isac1993) presented below. At this moment, half of the final gas volume shown in Tables 2 and 3, was produced in the syringes. This time was determined during a preliminary fermentation run since it differed according to the substrate. At the half-time to asymptotic gas production, the rate of gas production and bacterial growth is in a linear phase, near its maximum.
Table 2 Kinetics parameters of the gas accumulation curves recorded for the purified carbohydrates incubated with sows faecal inoculum and sugars disappearance, bacterial nitrogen incorporation, total short-chain fatty acid production and molar ratios at half-time to asymptotic gas production (experiment 1)

Abbreviations are: BNI = bacterial nitrogen incorporation; SCFA = short-chain fatty acid; d.f. = degrees of freedom.
a,b,c For one parameter, means followed by different superscripts in the columns differ at a significance level of 0.05.
†L, lag time (h); T/2, half-time to asymptotic gas production (h); μt =T/2, fractional rate of degradation at t = T/2 (per h); Gf, maximum gas volume (ml/g DM).
‡Proportion of sugars disappeared from the syringe content at half-time to asymptotic gas production.
Table 3 Dry matter disappearance during the pepsin–pancreatin hydrolysis, kinetics parameters of the gas accumulation curves recorded for the hydrolysed feedstuffs incubated with sows faecal inoculum and sugars disappearance, bacterial nitrogen incorporation, total short chain fatty acid production and molar ratios at half-time to asymptotic gas production (experiment 2)

Abbreviations are: dDM = dry matter disappearance; DM = dry matter; BNI = bacterial nitrogen incorporation; SCFA = short-chain fatty acid; d.f. = degrees of freedom.
a,b,cFor one parameter, means followed by different superscripts in the columns differ at a significantly (P < 0.05).
†L, lag time (h) level of 0.05; T/2, half-time to asymptotic gas production (h); μt =T/2, fractional rate of degradation at t = T/2 (per h); Gf, maximum gas volume (ml/g DM).
‡Proportion of sugars disappeared from the syringe content at half-time to asymptotic gas production.
The syringes were subsequently emptied and rinsed with distilled water (2 × 5 ml). The fermentation residue of three syringes were pooled and freeze-dried for further determination of residual sugars. The content of the three other syringes were centrifuged (12 000 × g, 20 min, 4°C). An aliquot of the supernatant (approximately 10 ml) was taken for SCFA analysis and the rest was discarded. The pellet was suspended in distilled water (30 ml) to dilute traces of 15N-labelled NH4Cl originating from the buffer, centrifuged (12 000 × g, 20 min, 4°C) and the supernatant was discarded. The resulting pellet concentrating the bacterial and the undigested substrate was freeze-dried, weighed and analysed for total N and 15N-enrichment. For each period, three samples of the inoculum were also taken, centrifuged for further 15N and SCFA analysis.
Kinetics of gas production
Gas accumulation curves recorded during the 72 h of fermentation were modelled according to France et al. (Reference France, Dhanoa, Theodorou, Lister, Davies and Isac1993):
where G denotes the gas accumulation at time (t), Gf (ml/g DM) the maximum gas volume for t = ∞ and L (h) the lag time before the fermentation starts. The constants b (per h) and c (per h1/2) determine the fractional rate of degradation of the substrate μ (per h), which is postulated to vary with time as follows:
The kinetics parameters (Gf, L, μt =T/2 and T/2) were compared in the statistical analysis. T/2 is the half-time to asymptotic gas production when G = Gf/2. The syringes that suffered an accidental leakage of gas were discarded.
Measurement of SCFA production at half-time to asymptotic gas production
Supernatants prepared as described above were filtered using 0.2 μm nylon 13 mm HPLC syringe filter no. 2166 (Alltech Associates Inc., Deerfield, IL, USA) and analysed for SCFA with a Waters 2690 HPLC system (Waters, Milford, MA, USA; 30°C, with iso-caproic acid as the internal standard) fitted with an HPX 87H column (Bio-Rad, Hercules, CA, USA) combined with a UV detector (210 nm).
Measurement of N incorporation into microbial cells
Total N and 15N-enrichment in the freeze-dried pellets were measured by means of an elemental analyser coupled to an isotope-ratio mass spectrometer (Europa Scientific Ltd, Crewe, UK). Bacterial N incorporation (BNI, corresponding to N in the pellet incorporated from the buffer solution into the bacteria) per amount of incubated substrate at T/2 was calculated as follows:
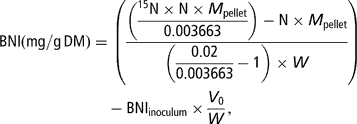
where N (g/g) denotes the concentration of N in the pellet, M pellet (mg) the dry weight of the pellet, 0.003663 the natural enrichment in 15N of the substrates and the faeces used to prepare the inoculum, 0.02 the enrichment of the mineral buffer in 15N, 15N (g/g) the concentration of 15N in total N of the pellet, V 0 (ml) the volume of inoculum transferred in the syringes at the start of the fermentation and W (gDM) the amount of substrate placed in the syringe.
Chemical analysis
The raw and hydrolysed substrates and the diet, ground to pass a 1-mm mesh screen by means of a Cyclotec 1093 Sample Mill (FOSS Electric A/S, Hilleroed, Denmark), were analysed for their content in dry matter (105°C for 24 h), ash (550°C for 8 h), nitrogen (Kjeldahl method, CP = 6.25 × N content), ether extract (Soxhlet method, using ether), NDF (using Na2SO3 and Termamyl, Novo Nordisk, Bagsværd, Denmark) and ADF and lignin, using the Fibercap system (Foss Electric, Bagsvaerd, Denmark). Starch was determined using amyloglucosidase according to the method of Faisant et al. (Reference Faisant, Planchot, Kozlowski, Pacouret, Colonna and Champ1995). Total and soluble dietary fibre (SDF) contents were measured by means of the AOAC 991.43 method (Association of Official Analytical Chemists, 1995), after grinding the samples through a 0.5-mm mesh screen. Constituent sugars of cellulose and xylan were determined as alditol acetates by gas–liquid chromatography (Englyst et al., Reference Englyst, Quigley, Hudson and Cummings1992). The glucose and fructose contents of inulin and the uronic acid content of pectin were determined by high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) on a Dionex DX500 chromatrography system (Dionex Corporation, Sunnyville, CA, USA) after enzymatic hydrolysis with endo- and exo-inulinase (50°C, 24 h) and Viscozyme (Realco, Louvain-la-Neuve, Belgium, 50°C, 15 h), respectively.
Statistical analysis
Statistical analyses were performed using the MIXED procedure of the SAS 8.02 software (Statistical Analysis Systems Institute, 1999) using the following general linear model: for experiment 1:
where Y is the result, α the mean, Si the fixed effect of the substrate (i = 1, …, 5), Pj the random effect of the period (j = 1, 2) and ε the error term; and for experiment 2:
where Y is the result, α the mean, Si the fixed effect of the substrate (i = 1, 2, 3), Pj the random effect of the period (j = 1, 2, 3) and ε the error term.
Results
Experiment 1
The gas accumulation curves recorded during the fermentation of the purified carbohydrates are illustrated in Figure 1 and fermentation kinetics parameters, BNI and SCFA productions of the five carbohydrates are shown in Table 2.

Figure 1 Mean values and standard deviations of the gas production curves recorded during the fermentation of purified carbohydrates incubated with sow faecal inoculum (experiment1).
With the lowest lag (L) and half-time to asymptotic gas production (T/2), inulin, starch and pectin were the most rapidly fermented substrates (P < 0.001). Inulin and cellulose showed lower fractional rates of degradation, compared with starch, pectin and xylan. The final gas production (Gf) also differed between the substrates (P < 0.001): pectin yielded the highest production and xylan the lowest.
Sugar disappearance ranged from 0.8 to 0.9 for inulin, pectin and starch. This indicates that almost all the substrate initially present in the syringe was fermented at T/2 for these fibre sources. For cellulose and xylan, only half of the substrate had been fermented when the fermentation was stopped at T/2. BNI measured at T/2 were higher (P < 0.05) for inulin and starch when compared with xylan, cellulose and pectin. The SCFA production for starch and inulin was higher compared with that of pectin, xylan and cellulose (P < 0.025). However, cellulose and xylan produced more SCFA per gram of fermented sugars compared with starch and inulin. Pectin yielded the lowest SCFA per gram of fermented sugars. The molar ratio of SCFA showed that a higher proportion of acetate was produced for pectin and xylan compared with the other carbohydrates.
Experiment 2
The gas accumulation curves recorded during fermentation of the hydrolysed feedstuffs are shown in Figure 2 and kinetics parameters, BNI and SCFA productions are given in Table 3.
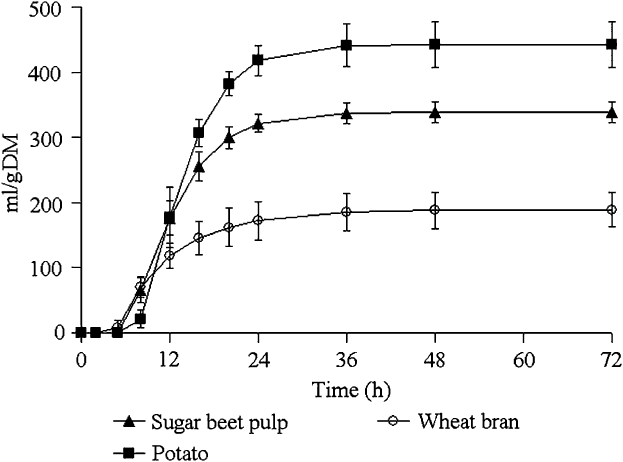
Figure 2 Mean values and standard deviations of the gas production curves recorded during the fermentation of pepsin–pancreatin hydrolysed feedstuffs incubated with sow faecal inoculum (experiment 2).
Potato yielded the greatest final gas volume (P < 0.001) followed by sugar-beet pulp and wheat bran. The latter showed a lower fractional rate of degradation compared with potato and sugar-beet pulp (P = 0.008) and had the shortest lag and the earliest half-time to asymptotic gas production (P < 0.001).
The rate of sugar disappearance at T/2 ranged from 0.17 for wheat bran to 0.50 for potato. BNI expressed per gram of incubated substrate was the highest for potato and sugar-beet pulp compared with wheat bran (P < 0.01). When BNI was expressed per gram of fermented sugars, the difference observed between wheat bran, potato and sugar-beet pulp became insignificant due to a reduction in the number of observations. Conversely, in experiment 1, there was a difference in BNI between the substrates, even when expressed per gram of fermented sugars. SCFA productions per gram of incubated substrate were the highest for potato followed by sugar-beet pulp and wheat bran (P < 0.001). Wheat bran yielded higher SCFA per gram fermented sugars, compared with sugar-beet pulp and potato (P < 0.05). The molar ratio also differed between the substrates: potato yielded more butyrate, and sugar-beet pulp and wheat bran produced more acetate. Branched SCFA were not detected during SCFA analysis.
Correlation and regression
Correlation and regression were calculated throughout experiments 1 and 2. When pectin was discarded from the database, BNI (mg/g sugar fermented) was correlated to the half-time of asymptotic gas production (T/2) (r = −0.61, P = 0.143) and to SCFA (mg/g sugar fermented) (r = −0.72, P = 0.068) with levels approaching significance. BNI was also correlated to sugar disappearance (r = 0.80, P = 0.029), to insoluble dietary fibre (IDF) (Table 1) (r = −0.77, P = 0.043) and to SDF (starch included in SDF) content of the substrates (r = 0.79, P = 0.039). A regression equation was calculated linking BNI to SCFA and to T/2. The equation is: BNI (mg/g sugar fermented) = 40.7–0.71 T/2 (h)–0.247 SCFA (mg/g sugar fermented) (r 2 = 0.91, P = 0.004).
Discussion
The results clearly indicate that the source of dietary carbohydrate influences bacterial protein synthesis during colonic fermentation in pigs. The primary function of microbial carbohydrate fermentation is to release ATP, which is required for bacterial cell basal and growth metabolism (Karsli and Russell, Reference Karsli and Russell2001). BNI will thus depend on the efficiency of ATP generation from the substrate and on the use of this ATP for the maintenance or growth of the bacteria. The rate of fermentation of carbohydrates depends on their composition and on their structure. Generally, solubility is also linked to rapid fermentation (Hoover and Stokes, Reference Hoover and Stokes1991) since the swelling and the high water-binding capacity of SDF increase the surface area accessible to bacteria. This is highlighted in this study by the trends towards negative and positive correlation linking BNI to IDF and to SDF (starch included in SDF), respectively.
Two major steps are involved: the hydrolysis of the polysaccharide and the fermentation of the released oligo- and monosaccharides.
BNI is firstly a consequence of the hydrolysis kinetics. As indicated by the negative correlation between BNI and T/2, high BNI were found among the substrates with high fermentation rates as measured by the low L and T/2 values and the high μt = T/2 (Tables 2 and 3,): inulin and starch in experiment 1 and sugar-beet pulp and potato in experiment 2. Cellulose, xylan and wheat bran had lower rates of fermentation and yielded lower BNI values. Hydrolysis is the rate-limiting step of microbial fermentation for cellulose (Noike et al., Reference Noike, Endo, Chang, Yaguchi and Matsumoto1985; Lynd et al., Reference Lynd, Weimer, van Zyl and Pretorius2002), xylans and arabinoxylans (Strobel and Russell, Reference Strobel and Russell1986; Hopkins et al., Reference Hopkins, Englyst, Macfarlane, Furrie, Macfarlane and McBain2003), contrary to starch, inulin and pectin. When hydrolysis is slow, more energy is required for cell maintenance per unit of time and less energy is available for growth.
The differences in the use of the energy coming from fermentation of substrates for bacterial growth is also reflected by the amount of SCFA produced per gram of fermented substrate (Blümmel et al., Reference Blümmel, Makkar and Becker1997). When pectin was discarded from the database, BNI values tended to be negatively correlated with SCFA production (r = −0.72, P = 0.068). Higher SCFA productions for cellulose and xylan compared with inulin and starch indicate that a lower proportion of the energy content of the substrate was used for growth, since this energy is found in the SCFA. This observation is also applicable when sugar-beet pulp, potato and wheat bran are compared in experiment 2.
Once the polysaccharides are hydrolysed, the chemical composition of the substrates also influences the pathways of SCFA production, ATP generation and, as a consequence, the BNI. The released monosaccharides support microbial growth with little differences in efficiency (Hoover and Stokes, Reference Hoover and Stokes1991) since the intestinal bacteria use mainly the Embden–Meyerhof–Parnas pathway, also known as glycolysis, that degrades glucose to pyruvate via glucose-6-phosphate (Prescott et al., Reference Prescott, Harley and Klein1996), for ATP production (Miller and Wolin, Reference Miller and Wolin1996). However, xylans and pectins are first metabolised by the pentose–phosphate pathway (Macfarlane and Macfarlane, Reference Macfarlane and Macfarlane2003) starting from the pentose to fructose-6-phosphate and glyceraldehyde-3-phosphate via xylulose-5-phosphate (Prescott et al., Reference Prescott, Harley and Klein1996). This reduces the bacterial growth rates as reported by Crittenden et al. (Reference Crittenden, Karppinen, Ojanen, Tenkanen, Fagerström, Mättö, Saarela, Mattila-Sandholm and Poutanen2002) who compared xylose and xylo-oligosaccharides with glucose. It explains, beyond the rate of hydrolysis, the lowest BNI values observed for xylan (515 g xylose per kg DM) and pectin (672 g glucuronic acid per kg DM) in experiment 1 and wheat bran (251 g xylose per kg DM) in experiment 2.
The SCFA profiles confirm that different fermentation strategies were used by the bacteria. The production of acetate and butyrate yields 34 mmol ATP per gram SCFA compared with 40 mmol ATP per gram of propionate (Blümmel et al., Reference Blümmel, Makkar and Becker1997). Acetate is typical of fermentation under C-limiting conditions (Macfarlane and Macfarlane, Reference Macfarlane and Macfarlane2003). The high production of acetate observed with pectinolytic and xylanolytic bacteria indicates that they recover less energy from the substrate compared with bacteria fermenting starch or fructans which use faster and more energy efficient cycles, leading to higher propionate production. In the case of xylan and wheat bran, high acetate levels are a consequence of low hydrolysis rates that limit the release of monosaccharides. For pectin, this is due to the production of extracellular pectinolytic enzymes (Drochner et al., Reference Drochner, Kerler and Zacharias2004). The extracellular release of oligosaccharides benefit microbial competitors and reduce the growth of pectinolytic bacteria. On the contrary, Bacteroides, the most abundant saccharolytic genus in the intestine, bind starch to their outer membrane prior to hydrolysis, which prevents any competition with other bacteria (Hooper et al., Reference Hooper, Midtvedt and Gordon2002).
The low BNI yield recorded for pectin, despite a low T/2, a high μt = T/2 and low SCFA production, was an exception compared with the other substrates. This is due to the high degree of methoxylation of the pectin used in this study (Sigma-Aldrich, 2006). Methoxylation decreases the growth rate of pectin-fermenting bacteria (Olano-Martin et al., Reference Olano-Martin, Gibson and Rastall2002). The methyl group can be esterified with a release of methanol (Drochner et al., Reference Drochner, Kerler and Zacharias2004) but, unlike glucuronic acid, the methyl group is unusable by pectinolytic bacteria (Hall and Herejk, Reference Hall and Herejk2001) for ATP generation and growth.
The N content of pectin (32.5 g CP per kg DM) and that of the hydrolysed wheat bran (96.0 g/kg DM) decreased the mineral N uptake from the buffer solution compared with the other fast-fermenting substrates. Intestinal bacteria use ammonia as a major source of N (Younes et al., Reference Younes, Demigné, Behr and Rémésy1995). However, microbes that ferment substrates with high levels of CP recycle the N content of the substrate protein (Hoover and Stokes, Reference Hoover and Stokes1991; Cone et al., Reference Cone, Jongbloed, Van Gelder and De Lange2005).
Finally, the in vitro classification of potato, sugar-beet pulp and wheat bran in terms of potential impact on urinary v. faecal N excretion patterns should be combined with in vivo data such as the retention time of the feed in the gastro-intestinal tract. In this study, fermentation was stopped at T/2. This time differs from in vivo residence time in the large intestine.
It can be concluded that the rate of fermentation of the carbohydrates and the chemical composition, the soluble fibre content, the constituent sugars and the CP content, were the major factors that affected bacterial energy production, BNI and SCFA production by the colonic bacteria of pigs. These in vitro observations need to be confirmed through in vivo experiments.
Acknowledgements
The authors gratefully acknowledge the personnel of the Faculty of Gembloux and the ‘Centre wallon de Recherches agronomiques’ for their technical assistance. The authors also wish to thank Dr Nicolas Gengler, Dr Marc Culot and Dr Yves Beckers for their help in the statistical analysis and the interpretation of the results and Laura Eastwood for careful correction of the final manuscript.







