Implication
Alpaca is an important fiber-producing species with 22 different natural hair colors. Understanding the molecular mechanism of hair color formation in this species is of interest to the alpaca breeders. This study revealed a functional role of miR508-3p in melanogenesis in alpaca melanocytes providing a new candidate gene for producing natural hair colors in fine fiber-producing species such as alpaca by transgenic approach.
Introduction
Many coding genes have been reported to play important roles in hair color formation in mammalian species. microRNAs (miRNAs) are non-coding 21 to 25 nt RNA molecules, that play important roles in regulating gene expression in development, physiology and disease of animals (Bartel, Reference Bartel2004). The expression and function of miRNAs in the skin of mammalian species including mouse (Andl et al., Reference Andl, Murchison, Liu, Zhang, Yunta-Gonzalez, Tobias, Andi, Seykora, Hannon and Millar2006; Yi et al., Reference Yi, O’Carroll, Pasolli, Zhang, Dietrich, Tarakhovsky and Fuchs2006; Dong et al., Reference Dong, Wang, Xue, Dong, Yang, Fan, Yu, Tian, Ma and Smith2012), goat and sheep (Wenguang et al., Reference Widlund and Fisher2007), and alpaca (Zhu et al., Reference Zhu, He, Jia, Jiang, Bai, Yu, Lv, Fan, He, Geng, Dong, Qiao, Lee, Smith and Dong2010; Yang et al., Reference Yan, Liu, Zhu, Li, Yue, Zhao, Gong and Wang2015) have been reported. For example, overexpression of miR-137 in mice causes a change in skin color from black to brown (Dong et al., Reference Dong, Wang, Xue, Dong, Yang, Fan, Yu, Tian, Ma and Smith2012). Other miRNAs, such as miR-340, miR182 and miR-25, were shown to regulate pigmentation by targeting microphthalmia transcription factor (MITF) messenger RNA (mRNA) (Bemis et al., Reference Bemis, Chen, Amato, Classen, Robinson, Coffey, Erickson, Shellman and Robinson2008; Segura et al., Reference Tachibana2009; Goswami et al., Reference Goswami, Tarapore, TeSlaa, Grinblat, Setaluri and Spiegelman2010). miR508-3p is one of the members of the miR-506 family, which are located on Xq27.3 (Zhou et al., Reference Zhou, Chen, Li, Li, Hu, Huang, Zhao, Liang, Wang, Sun and Shi2010). miR508-3p plays an important role as a tumor suppressor during tumor formation (Zhai et al., Reference Zhai, Zhou, Zhao, Wan, Yu, Guo, Qin, Chen and Lu2012). Overexpression of miR508-3p and miR-509-3p suppresses the proliferation of renal cell carcinoma, induces cell apoptosis, and inhibits cell migration in vitro (Zhai et al., Reference Zhai, Zhou, Zhao, Wan, Yu, Guo, Qin, Chen and Lu2012). From the miRNA profiles of alpaca skins with white hair color v. brown hair color, we found miR508-3p was expressed differentially (Tian et al., Reference Vachtenheim and Borovanský2012), which suggested that miR508-3p might be related to hair color. Moreover, bioinformatics analysis revealed that one of the predicted target genes of miR508-3p is MITF. MITF is a basic helix-loop-helix leucine zipper transcription factor that regulates melanocyte cellular differentiation and the transcription of melanogenic enzymes (tyrosinase (TYR), tyrosinase-related protein TYRP1 and TYRP2) (Levy et al., Reference Livak and Schmittgen2006). MITF not only controls expression of pigmentation-related genes but also conveys hormonal and stress responses to pigmentation genes (Vachtenheim and Borovanský, Reference Kennell, Cadigan, Shakhmantsir and Waldron2010). Therefore, we hypothesized that miR508-3p may regulate melanin production by targeting MITF. This study was undertaken to determine the role of miR508-3p in melanogenesis using cultured alpaca melanocytes.
Material and methods
In situ hybridization
Alpaca melanocytes used in this study were established by our lab and were maintained as previously described (Bai et al., Reference Bai, Sen, Yu, Yang, Wang, Fan, Lv, Lee, Smith and Dong2010). For in situ hybridization, cells were fixed in 4% paraformaldehyde for 30 min, followed by washing in 0.1 M PBS (Phosphate Buffer Saline) (pH 7.4) three times. After treatment with proteinase K (40 g/ml; Roche Applied Science, Indianapolis, IN, USA), cells were fixed in 1% paraformaldehyde for 10 min. A quantity of 3 pmol of digoxigenin-labeled miR508-3p probe were diluted into 20 μl of hybridization buffer, applied to the slides, and allowed to hybridize at 37°C overnight. Slides were then washed at 37°C with 2×SSC (Saline Sodium Citrate) solution and incubated with 1 : 1000 diluted alkaline phosphatase-conjugated mouse anti-digoxigenin antibody (Roche Applied Science) for 2 h at 37°C. Alkaline phosphatase reaction was carried out with diaminobenzidine (DAB). The miR508-3p probe sequence is 5′-ATTGTCACTTTTTGGAGTAGA-3′, and scrambled sequence is 5′-GTGTAACACGTCTATACGCCCA-3′(Bio-High Technology, Shijiazhuang, Hebei, China).
Construction of plasmids
The miR508-3p expression plasmid was constructed by inserting an oligonucleotide corresponding to the sequence of the pre-miR508-3p into a mammalian expression vector, pcDNA6.2-GW/EmGFPmiR (Invitrogen, Shanghai, China), which contains a CMV (Cytomegalovirus) promoter driving the expression of GFP (Green Fluorescent Protein) and miR508-3p. A negative control (NC) plasmid was also constructed using scrambled sequence of pre-miR508-3p. The luciferase reporter plasmid was constructed by cloning 3′UTR (untranslated regions) of alpaca MITF into a dual luciferase pmirGL0 vector (Promega, Madison, USA). 3′UTR sequence of alpaca MITF containing the miR508-3p binding site was obtained by PCR using alpaca skin complementary DNA (cDNA) as a template with primers containing XbaI and NotI sites (Table 1). The PCR product and the vector were digested with XbaI and NotI and ligated together to obtain the pmirGL0-MITF-wt construct. The miR508-3p binding site in 3′UTR of MITF region was mutated using a site-directed gene mutagenesis kit (Beyotime, Beijing, China) according to the manufacturer’s instructions to obtain the pmirGL0-MITF-mut construct. All constructs were confirmed by sequencing.
Table 1 Primers used in this study

MITF=microphthalmia transcription factor; F=forward; R=reverse; RT=real time; RT-PCR=reverse transcript PCR; wt=wild type; mut=mutant; TYR=tyrosinase; TYRP2=tyrosinase-related protein 2.
Cell culture and transfection
Melanocytes were transfected with the pcDNA6.2-miR508-3p plasmid or the NC plasmid using lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. After 3 days of transfection, melanocytes were collected. Cell lysates and total RNA were prepared and subjected to western blot and quantitative real-time PCR analysis, respectively.
Dual luciferase assay for microRNA target validation
For the luciferase reporter assay, 2 μg of pmirGL0-MITF-wt or pmirGL0-MITF-mut plasmid were co-transfected into 293T cells with miR508-3p plasmid or NC miRNA plasmid using lipofectamine 2000 (Invitrogen). Luciferase activities in the transfected cells were measured with a dual luciferase reporter assay kit (Promega, Fitchburg, WI, USA) 2 days after co-transfection. Firefly luciferase activity was normalized to renilla luciferase activity to adjust for transfection efficiency. Data are expressed as relative luciferase activities (n=3, mean±SE).
Quantitative real-time PCR for microRNA and messenger RNA
Total RNA from melanocytes was extracted using TRIzol reagent (Invitrogen) or an RNeasy kit (Qiagen, Dusseldorf, Germany) according to the manufacturer’s instructions and treated with DNase I (Sigma). For mRNA quantification, 1 μg of total RNA was converted to cDNA using a cDNA synthesis kit (Takara, Dalian, China) according to the manufacturer’s instructions, and quantitative real-time PCR was performed using SYBR Green PCR master mix (Takara). For miRNA quantification, cDNA was generated using a cDNA synthesis kit (Takara), a specific stem-loop RT primer and a common reverse primer according to a previously established method for real-time quantification of miRNAs (Chen et al., Reference Chen, Ridzon, Broomer, Zhou, Lee, Nguyen, Barbisin, Xu, Mahuvakar, Andersen, Lao, Livak and Guegler2005), and quantitative real-time PCR was performed using SYBR Green PCR master mix. The primer sequences are listed in Table 1. Real-time PCR was performed on a 7500 Fast Real-Time PCRTM system (Applied Biosystems, Foster City, CA). Melting curves for each sample were analyzed to validate specificity of amplification. All samples were run in triplicate, and the relative amount of miRNA and mRNA was normalized to the amount of U6 and β-actin mRNA, respectively. Quantification of miRNA and mRNA transcript abundance was performed using the comparative threshold cycle (C T ) method (Livak and Schmittgen, Reference Segura, Hanniford, Menendez, Reavie, Zou, Alvarez-Diaz, Zakrzewski, Blochin, Rose, Bogunovic, Polsky, Wei, Belitskaya-Levy, Lee, Bhardwaj, Osman and Hernando2001).
Western blot analysis
Western blot analysis was performed as previously described (Dong et al., Reference Dong, Wang, Xue, Dong, Yang, Fan, Yu, Tian, Ma and Smith2012) with the following primary antibodies: rabbit anti-MITF at 1 : 200 dilution (Santa Cruz, Finnell Stree tDallas, Texas, USA), rabbit anti-TYR at 1 : 1000 dilution (Santa Cruz), rabbit anti-TYRP1 at 1 : 1000 dilution (Abcam, Cambridge, UK), rabbit anti-β-actin (Sigma). Anti-rabbit IgG secondary antibody was purchased from Invitrogen. Immunoblots were scanned on a ChemiDOCTM XRS+ imager (Bio-Rad, Hercules, CA), and protein levels were quantified using the Image-Pro Plus software (Olympus, Tokyo, Japan).
Immunocytochemistry
Melanocytes were washed three times in 0.1 M PBS for 3 min each, fixed in 4% paraformaldehyde, and then incubated at room temperature in 3% hydrogen peroxide for 15 min to block the action of any endogenous peroxidase. After washing with 0.1 M PBS three times for 5 min each, cells were immersed in BSA (Bovine Serum Albumin) at 37°C for 40 min. Cells were then incubated at 4°C overnight in anti-MITF antibody solution. Following washing three times in 0.1 M PBS for 5 min each, cells were incubated with HRP (Horseradish Peroxidase)-conjugated anti-rabbit IgG for 30 min at 37°C, and then incubated with the substrate 3,3′-DAB for 20 min at room temperature to produce a brown precipitate. The immunostaining of the cells was then observed under a microscope (Leica, Germany). For NCs, PBS was used to substitute the primary antibody.
Melanin measurement
Melanocytes were harvested and rinsed with PBS followed by addition of l ml of 1 N NaOH to dissolve the melanin. For assay of alkali-soluble melanin, melanin content was measured spectrophotometrically at an absorbance of 475 nm. For the spectrophotometric assay of eumelanin, cells were hydrolyzed in hot 30% hypophosphoric acid and hydroiodic acid. After cooling, 50% ethanol was added and cell samples were centrifuged at 2234×g for 10 min. Insoluble eumelanic pigments were selectively solubilized in hot sodium hydroxide and hydrogen peroxide; they were cleared by centrifugation at 10 700×g for 1 min with a Sorvall Ultracentrifuge (Waltham, Massachusetts, USA). Supernatants were analyzed for absorbance at 350 nm. For the spectrophotometric assay of pheomelanins, cells were solubilized in a phosphate buffer (pH 10.5) and cleared by centrifugation at 10 700×g for 10 min. and then analyzed for absorbance at 400 nm. The melanin measurement was normalized to total amount of cells. All experiments were performed in triplicate.
Statistical analysis
Data, which were reported as mean±SE from three replicates, were analyzed using ANOVA and Fisher’s protected LSD test. The differences in abundance of miRNA, mRNA, protein, relative luciferase activities and melanin production between control and experimental groups were determined by ANOVA using SPSS 11.5 software (SPSS, Chicago, IL, USA).
Results
miR508-3p targets the predicted microRNA binding site in the 3′UTR of microphthalmia transcription factor
To validate the specificity of miR508-3p targeting MITF through the predicted miRNA binding site in 3′UTR of MITF with high similarity among camel, horse, cattle and human (Figure 1a and b), luciferase reporter assays were performed using luciferase reporter constructs containing either the wild type MITF (pmirGL0-sGC-wt) or the mutant MITF (pmirGL0-sGC-mut). The constructs were co-transfected into 293T cell with the miR508-3p expression plasmid or NC plasmid. Dual luciferase reporter assays showed that the reporter activity in cells co-transfected with pmirGL0-MITF-wt and miR508-3p plasmid was decreased by >40%, compared with the cells co-transfected with pmirGL0-MITF-wt and the NC plasmid (Figure 1c). Luciferase reporter activity in cells co-transfected with pmirGL0-MITF-mut and miR508-3p plasmid was similar to the reporter activity in cells co-transfected with pmirGL0-MITF-mut and the NC miRNA plasmid (Figure 1d). These data indicate that miR508-3p can bind and regulate MITF in a sequence specific fashion through the predicted binding site in 3′UTR of MITF.
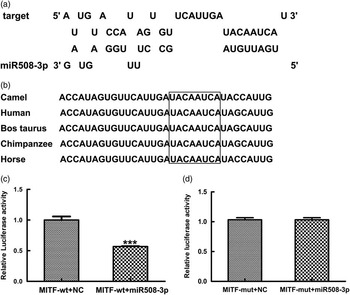
Figure 1 Specificity of miR508-3p targeting 3′UTR of microphthalmia transcription factor (MITF) transcript. (a) Predicted miR508-3p binding site in the 3′UTR of MITF transcript. (b) Alignment of MITF sequences around the miR508-3p binding site (the highlighted boxes indicate the miR508-3p seed region). (c) Luciferase reporter activities in 293T cells co-transfected with the reporter construct containing the wild type (wt) 3′UTR of MITF (pmirGL0-MITF-wt) and the miR508-3p or the negative control (NC) plasmid. (d) Luciferase reporter activities in 293T cells co-transfected with the mutant reporter construct (pmirGL0-MITF-mut) and the miR508-3p or the NC plasmid.
Localization of miR508-3p in alpaca melanocytes
To identify the localization of miR508-3p in alpaca melanocytes in vitro, in situ hybridization was performed. The assay showed that no specific hybridization signal was detected in the cells hybridized with the NC probe (Figure 2a) and specific hybridization signal for miR508-3p in the cytoplasm of alpaca melanocytes (Figure 2b).
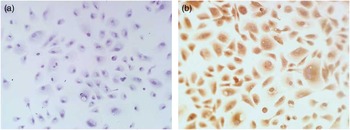
Figure 2 Localization of miR508-3p in alpaca melanocytes by in situ hybridization analysis. (a) Melanocytes hybridized with negative control probe. (b) Melanocytes hybridized with digoxigenin-labeled miR508-3p probe.
Effect of miR508-3p overexpression on messenger RNA and protein abundance of microphthalmia transcription factor
To determine whether MITF is a target of miR508-3p, the miR508-3p expression plasmid was used to transfect alpaca melanocytes to test the effect of miR508-3p on expression of MITF. Quantitative real-time PCR analysis showed increased accumulation of miR508-3p in melanocytes transfected with the miRNA expression plasmid in comparison with the cells transfected with the NC plasmid (P<0.01, Figure 3a). Overexpression of miR508-3p resulted in a decrease in the abundance of both MITF mRNA (P<0.01, Figure 3b) and protein as analyzed by western blotting and immunocytohistology (Figure 3c to e). These data indicate that MITF is regulated by miR508-3p.
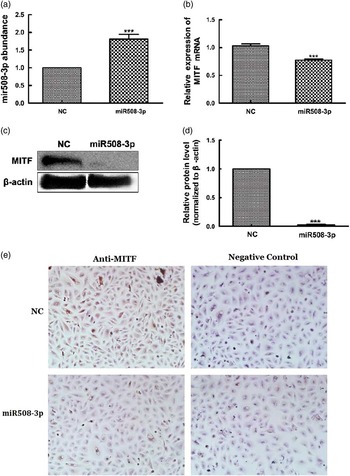
Figure 3 Effect of miR508-3p on messenger RNA (mRNA) and protein abundance of microphthalmia transcription factor (MITF). (a) miR508-3p expression in melanocytes transfected with the miR508-3p expression plasmid. (b) MITF mRNA expression in melanocytes transfected with the miR508-3p expression plasmid. (c–e) MITF protein expression in melanocytes transfected with the miR508-3p expression plasmid analyzed using Western blot and immunocytochemical detection, respectively. Data are expressed as mean±SE from three replicates. ***P<0.001. NC=negative control.
Effect of miR508-3p overexpression on the expression of melanogenic genes
The abundance of both mRNA and protein for TYR and TYRP2 genes was examined in melanocytes transfected with the miR508-3p expression construct relative to cells transfected with the NC plasmid. As shown in Figure 4a, overexpression of miR508-3p in melanocytes significantly decreased the mRNA abundance for TYR and TYRP2 genes (P<0.01 and P<0.05, respectively). Western blot analysis showed that the expression of TYR and TYRP2 proteins was significantly reduced in melanocytes overexpressing miR508-3p (P<0.01, Figure 4b and c).
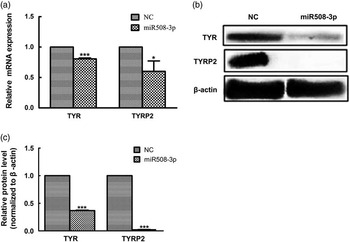
Figure 4 Effect of miR508-3p on the expression of coat color genes in melanocytes. (a) tyrosinase (TYR) and tyrosinase-related protein 2 (TYRP2) messenger RNA (mRNA) expression in melanocytes transfected with the miR508-3p expression plasmid. (b and c) TYR and TYRP2 protein expression in melanocytes transfected with the miR508-3P expression plasmid. Data were normalized to β-actin and expressed as relative fold change. Data are expressed as mean±SE from three replicates. *P<0.05; ***P<0.001. NC=negative control.
Effect of miR508-3p overexpression on the melanin production
To determine whether miR508-3p overexpression affects melanin production, the production of total alkali-soluble melanogenesis (ASM), eumelanogenesis (EM) and pheomelanogenesis (PM) were measured in alpaca melanocytes transfected with the miR508-3p expression plasmid. As shown in Figure 5, overexpression of miR508-3p in alpaca melanocytes reduced ASM production by 45% (P<0.01, Figure 5a), EM production by about 45% (P<0.01, Figure 5b) and PM production by about 60% (P<0.01, Figure 5c).
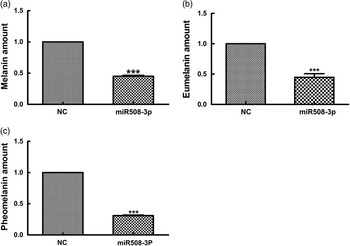
Figure 5 Effect of miR508-3p on melanin production. (a) Alkali total melanin production in melanocytes overexpressing miR508-3p. (b) Eumelanin production in melanocytes overexpressing miR508-3p. (c) Pheomelanin production in melanocytes overexpressing miR508-3p. Data are expressed as mean±SE from three replicates. ***P<0.001. NC=negative control.
Discussion
Melanocytes are specialized cells in which melanin synthesis (eumelanins and pheomelanins) takes place. Melanin is responsible for skin and hair color in mammalian species. The phenotype of coat color depends on two types of melanin (black to brown eumelanin and yellow to reddish brown pheomelanin) produced by melanocytes resident in the skin (Ito et al., Reference Ito and Wakamatus2000; Ito and Wakamatus, Reference Ito, Tashiro, Muta, Ozawa, Chiba, Nishizawa, Yamamoto, Kuhara and Sabaki2008). The quality and ratio of eumelanin to pheomelanin dictate the coat color (Ito et al., Reference Ito and Wakamatus2000). Despite considerable knowledge of the genetic regulation of coat color in mice and identification of loci involved in coat color regulation in fiber-producing species, the molecular and cellular mechanisms that regulate coat color are not completely understood. A number of miRNAs involved in melanogenesis have been reported in different animal species. Such miRNAs include miR-137 in mouse (Dong et al., Reference Dong, Wang, Xue, Dong, Yang, Fan, Yu, Tian, Ma and Smith2012), miR-429 in fish (Yan et al., Reference Yang, Fan, Shi, Ji, Zhang, Wang, Herrid, Zhang, Yao, Smith and Dong2013) and miR-8 in Drosophila (Kennell et al., Reference Kobayashi, Viera, Potterf, Sakai and Imokawa2012). In order to identify target genes of miRNA, which are specifically associated with hair color formation, we have screened miRNA expression profile in alpaca skin. As a result, several miRNA that are differentially expressed in skin were identified, such as miR508-3p (Tian et al., Reference Vachtenheim and Borovanský2012). In addition, the functional role of miRNA on melanogenesis was also investigated, highlighted with the studies on the effect of lpa-miR-nov-66 (Yang et al., Reference Yan, Liu, Zhu, Li, Yue, Zhao, Gong and Wang2015) and miR-25 (Zhu et al., Reference Zhu, He, Jia, Jiang, Bai, Yu, Lv, Fan, He, Geng, Dong, Qiao, Lee, Smith and Dong2010) on melanin production.
Melanogenesis is mainly determined by genes on several pathways such as the traditional c-kit and cyclic AMP (cAMP) pathways, which merge at MITF (reviewed by Tachibana, Reference Tian, Jiang, Fan, Wang, Meng, He, He, Geng, Yu, Song, Zhang, Yao, Smith and Dong2000). MITF is directly regulated by miRNAs such as miR-137 (Dong et al., Reference Dong, Wang, Xue, Dong, Yang, Fan, Yu, Tian, Ma and Smith2012) and miR-25 (Zhao et al., Reference Zhao, Wang, Meng, Ji, Xu, Chen, Fan, Yu, Yao and Dong2015). MITF is one of the target genes for miR508-3p. In general, the actions of miRNA are to promote degradation of mRNA or to suppress mRNA translation by binding to the 3′UTR of target mRNA (Bartel, Reference Bartel2004; Wu et al., Reference Wu, Fan and Belasco2006). Results of the luciferase reporter assay in the present study provided evidence indicating that MITF is a target gene regulated by miR508-3p.
miR508-3p is expressed higher in alpaca skins with white hair color than that with black hair color (Tian et al., Reference Vachtenheim and Borovanský2012). Here, we first found that miR508-3p was expressed in cytoplasm of alpaca melanocytes with high abundance, which suggested that it may play a role in melanocyte. Overexpression of miR508-3p in alpaca melanocytes down-regulated the expression of MITF both at the transcriptional and translational level, especially at translational level. Moreover, we found the transcriptional and transcriptional expression of TYR and TYRP2 were decreased by miR508-3p targeting MITF. The melanogenic enzymes TYR, TYRP1 and TYRP2 are regulated by the transcription factor MITF for the production of melanin (Widlund and Fisher, Reference Wenguang, Jianghong, Jinquan and Yashizawa2003; Levy et al., Reference Livak and Schmittgen2006). However, it has been reported that there is no transcriptional regulation of TYR, TYRP1 and TYRP2 by the cAMP pathway in human melanocytes (Busca and Ballotti, Reference Busca and Ballotti2000). So, the results suggests that the regulation of miR508-3p on the melanogenesis by targeting MITF may not be through the cAMP pathway.
It was reported that MITF is not a principal regulator of the TYRP2 gene (Tachibana, Reference Tian, Jiang, Fan, Wang, Meng, He, He, Geng, Yu, Song, Zhang, Yao, Smith and Dong2000). TYR is absolutely required for both eumelanin and pheomelanin synthesis, whereas TYRP1 and TYRP2 seem to be more crucial for eumelanin synthesis (Busca and Ballotti, Reference Busca and Ballotti2000). Here, we found that eumelanin production was decreased 45% by miR508-3p overexpression. Therefore, decreased eumelanin synthesis in cells overexpressing miR508-3p is due to lower expression of TYR and TYRP2, and decreased pheomelanin synthesis is due to reduced expression of TYR. This finding is similar to the observation that agouti signal protein decreases eumelanin synthesis but promotes pheomelanin synthesis in melanocytes, due to inhibition of TYR activity and the loss of TYRP1 and TYRP2 expression (Kobayashi et al., Reference Levy, Khaled and Fisher1995).
In conclusion, we provided evidence supporting an important functional role for miR508-3p in alpaca melanocytes by targeting 3′UTR of MITF. The post-transcriptional expression of TYR and TYRP2 is decreased consequently, thereby resulting in decreased melanin production. The observed effect of miR508-3p on melanin production is via down-regulation of MITF expression, suggesting a functional role for miR508-3p in the regulation of melanogenesis. Hence, miR508-3p is a potential candidate gene for producing natural coat colors in fiber-producing species by transgenic approach.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (NSFC, No. 31201868), 131 expert project of Shanxi Province, Special Fund for Agro-Scientific Research in the Public Interest of China (No. 201303119) and Youth Foundation of Shanxi Province (2014021028-2).








