Implications
Enteric health and growth of the pre-weaning pig influences its lifelong production potential, therefore correct management of gut health during this time is essential. Considering the intensifying concerns over emerging antimicrobial resistance and that reduction in antibiotic use is a requirement in most countries, it is imperative to determine the efficacy of alternative treatments and prophylaxis such as probiotics. The current study demonstrates that administering the probiotic yeast Saccharomyces cerevisiae var. boulardii (SB) to piglets on the day of farrowing reduces occurrence of diarrhoea in the 1st week of life; therefore, application of SB may be used to improve pre-weaning enteric health.
Introduction
Historically, antibiotics have been used in pig medicine to treat and control bacterial enteric disease. In addition, use of antibiotic growth promoters is now prohibited in the European Union and, considering the intensifying concerns over emerging antimicrobial resistance, antibiotic use, both frequency and class, has become more tightly controlled in veterinary medicine (European Commission, 2011; Millet and Maertens, Reference Millet and Maertens2011). Therefore, investment in alternative methods of controlling enteric disease is essential. Saccharomyces cerevisiae var. boulardii is a non-pathogenic, non-colonising, yeast. Several studies have shown that, in humans, SB is an efficacious and safe probiotic, which has proven efficacy against some enteric diseases including, but not limited to, Clostridium difficile-associated disease and rotavirus diarrhoea (McFarland, Reference McFarland2006; Grandy et al., Reference Grandy, Medina, Soria, Teran and Araya2010; McFarland, Reference McFarland2010). Data on the effect of SB on neonatal porcine health are limited, possibly because of practical limitations of administration to individual neonates commercially; however, studies have demonstrated that SB supplementation improves growth in post-weaning pigs (Bontempo et al., Reference Bontempo, Di Giancamillo, Savoini, Dell'Orto and Domeneghini2006; Le Bon et al., Reference Le Bon, Davies, Glynn, Thompson, Madden, Wiseman, Dodd, Hurdidge, Payne, Le Treut, Craigon, Tötemeyer and Mellits2010).
Porcine neonatal diarrhoea is a welfare issue and may have an economic impact with regards to reduced production and increased mortality. Therefore, the main objective of this study was to determine if a single oral dose of SB administered to neonatal pigs within 24 h of farrowing would reduce diarrhoea in the 1st week of life.
Material and methods
Experimental design
A randomised controlled trial was undertaken (November–December 2012) on a farrow-to-wean farm in Sarthe, France, with a history of neonatal diarrhoea. Commercial parent sows due to farrow in the same week were paired according to parity, and a treatment, SB or control, was randomly allocated (via coin flip, by an independent person) to each of the pairs. The trial was conducted during 2 consecutive weeks in identical housing; each treatment involved 23 identical pens (the experimental unit) each containing a sow with her litter, yielding 299 and 307 piglets for control and SB treatment, respectively. To test the hypothesis that reduction of diarrhoea by treatment would lead to an improvement of health and growth, the number of experimental replicates required was determined using previously recorded performance data available from this farm (mean average daily gain (ADG) during the 1st week of life=0.160 kg, s.d.=0.041) to demonstrate an improvement of 15% in ADG by treatment, with a statistical power of 80% and significance of P<0.05 using the equation:
where n is the sample size, σ the standard deviation, Z β the Z value of power and Z α the Z value of significance (Steel and Torrie, Reference Steel and Torrie1996). All trial personnel, including investigators, were blinded to treatment allocation; blinding was verified by anonymous survey. Euthanasia, only where necessary for welfare reasons, was carried out by manual blunt trauma to the cranium; this was performed by trained personnel. All animals in the study were monitored daily by a veterinary surgeon; no adverse effect of control or SB administration was noted.
Animals were treated according to standard farm practices unless stated otherwise. Sows were moved to farrowing pens 5 days before their expected farrowing date; pens had a slatted floor (1.6 m×2.5 m), farrowing crate (0.8 m×2 m) and an area of solid plastic flooring (triangular: 1.2 m×0.85 m×1.5 m) with a heat lamp suspended above for piglet comfort. Room temperature and humidity was measured hourly (mean 23.5°C (range 21.5°C to 25°C) and mean 67% (range 56.5% to 74.5%), respectively). To equilibrate litter size, neonatal piglets were cross-fostered within treatment group. On day 0 (day of birth), piglets were identified with a numbered colour-coded ear tag, weighed and administered a 3.3 ml oral dose of paste. Treatment litters received an individual dose of paste containing 3.3×109 CFU of dried SB (Levucell®SB, S. cerevisiae CNCM-I 1079; Lallemand Animal Nutrition, Blagnac, France); control litters received the identical paste with no probiotic addition (paste constituents: soya oil, cristobalite, icing sugar, polysorbate 80, flavour). While being handled for paste administration, piglets also received a 1 ml, intra-muscular (IM) injection of amoxicillin (150 mg/ml, Vetrimoxin, CEVA animal health, Libourne, France); within 48 h of birth piglet teeth were filed, their tails docked via a heated cauterizer and they received an IM injection of iron (1 ml, 200 mg/ml Ferro2000; Coophavet, Saint-Herblon, France). At 4 days of age, piglets were given a 0.7 ml oral dose of the anti-parasitic product, toltrazuril (50 mg/ml; Baycox, Bayer, Kiel, Germany) and male piglets were surgically castrated, all in accordance with routine farm practice. The trial was approved by the farm’s veterinary consultant, and all procedures were compliant with French regulations and were approved by the University of Nottingham ethics committee.
Data collection
Between 0 and 7 days of piglet age, each pen floor was examined visually once daily for faecal consistency, by a single investigator blinded for treatment, and a score allocated to the entire pen using a faecal classification score system. If a score of 5 or more was allocated, the litter was determined to be diarrhoeic (Lewis and Heaton, Reference Lewis and Heaton1997; Pedersen and Toft, Reference Pedersen and Toft2011). Score 1 is classified as ‘Separate hard lumps, like nuts’, score 2 ‘Sausage-shaped, but lumpy’, score 3 ‘Like a sausage but with cracks on the surface’, score 4 ‘Like a sausage or snake, smooth and soft’, score 5 ‘Soft blobs with clear cut edges’, score 6 ‘Fluffy pieces with ragged edges, a mushy stool’ and score 7 ‘Watery, no solid pieces, entirely liquid’. To monitor production, piglets were weighed individually at day 0 and day 7; mortality, natural and euthanasia, was recorded in the same period.
Statistical methods
For all statistical analysis, the litter (pen) served as the experimental unit (n=23). For variables that recorded individual pig measurement (such as weight) the individuals were clustered for each litter and considered as random effect. Baseline characteristics of the two treatment groups were compared before administration of treatment using general ANOVA; mean and standard error of difference (s.e.d.) are reported for relevant variables (Table 1). Continuous data were found to be normally distributed and analysed using linear mixed models function in GenStat (14th Edition, GenStat, VSN International). For performance data (weight, ADG, mortality) and diarrhoea days, the statistical models included treatment as a fixed effect and litter size at day 0 as the random factor. The relationship between diarrhoea days and performances was evaluated using simple linear regression models. For categorical data: faecal scores and diarrhoeic status, the effect of treatment was analysed using χ 2 (with Yates’ correction for 2×2 tables) and χ 2 for trend (GraphPad Software Inc., USA) using primary data.
Table 1 Effect of SB treatment on production and diarrhoea in neonatal pigsFootnote 1

SB=Saccharomyces cerevisiae var. boulardii; s.e.d.=standard error of difference; ADG=average daily gain.
1 A single individual dose of control paste or SB CNCM-I 1079 (3.3×109 CFU) was administered within 24 h of birth to individual piglets. Weight, mortality and diarrhoea are presented from day 1 to 7. n=23 litters per treatment.
Results
Effect of SB supplementation on neonatal diarrhoea and production
After treatment, SB-supplemented litters had significantly fewer days of diarrhoea over the 1st week of life compared to controls (P<0.01; Table 1). This could not be attributed to sow parity, litter size or average birth weights as these were not significantly different between treatment groups (Table 1), although increasing sow parity correlated with number of diarrhoeic days (data not shown). SB litters also had significantly lower faecal scores, indicating firmer faeces, during the 1st week of life (Table 2, P<0.01). Six SB litters experienced no diarrhoeic events compared with a single litter in the control group (Table 1). Analysing days independently, there was a similar number of diarrhoeic litters per group before treatment (day 0); after treatment, diarrhoeic litters decreased in the SB group and increased in the controls (day 3, and over days 1 to 7; P<0.05, Figure 1). There was no statistically significant effect of SB on growth or mortality (Table 1). However, as expected, the number of diarrhoeic days was negatively correlated with ADG and average weight (both P<0.01; Figure 2a and b). Moreover, there was a positive correlation between number of diarrhoeic days and mortality from day 0 to day 7 (P<0.05; Figure 2c).
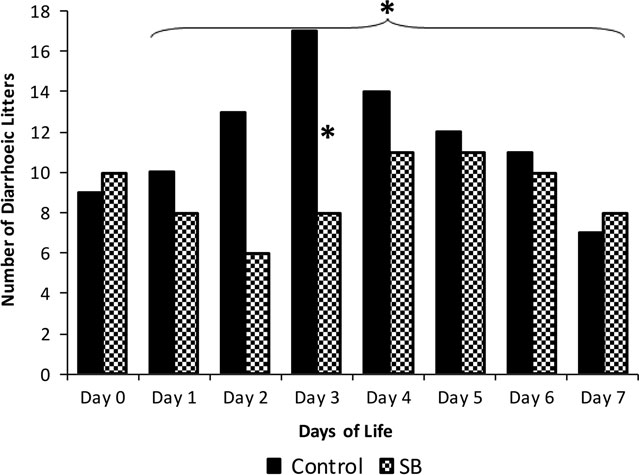
Figure 1 Effect of Saccharomyces cerevisiae var. boulardii (SB) on number of diarrhoeic litters in the 1st week of life. *P<0.05. A single individual dose of SB CNCM-I 1079 (3.3×109 CFU), or control paste was administered within 24 h of birth to individual piglets. n=23 litters per treatment.
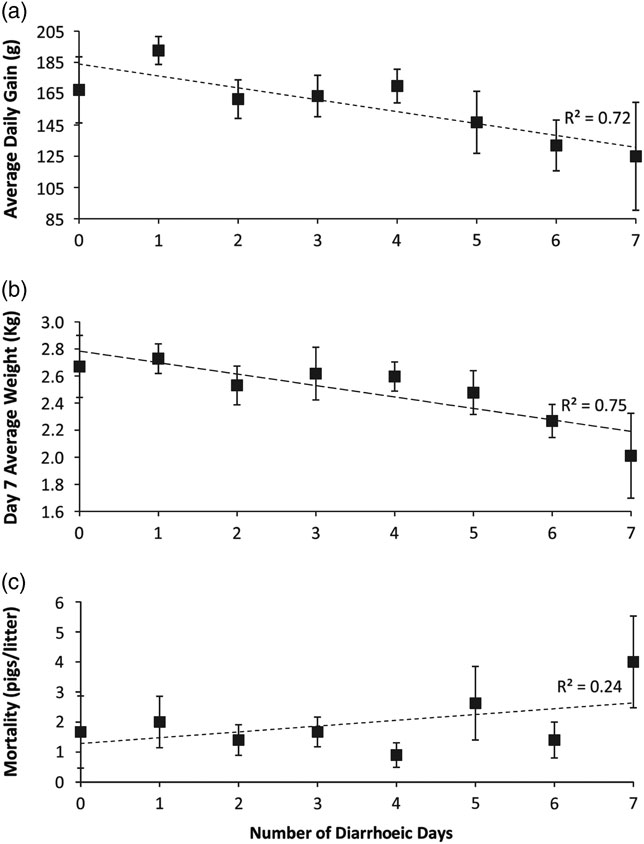
Figure 2 Effect of number of days of diarrhoea on average daily weight gain of piglets from day 0 to 7 (a); average weight at day 7 (b); and mortality from day 0 to 7 (c), independent of treatment, n=46. Significant correlations: P<0.01, P<0.01 and P<0.05, respectively. Error bars indicate standard error of the mean. Coefficients of determination (R 2) are indicated.
Table 2 Effect of SB treatment on faecal scores of neonatal pigsFootnote 1

SB=Saccharomyces cerevisiae var. boulardii.
1 A single individual dose of control paste or SB CNCM-I 1079 (3.3×109 CFU) was administered within 24 h of birth to individual piglets (n=23 litters per treatment). Pen faecal scores were recorded daily from day 1 to 7. Score 1 is classified as ‘Separate hard lumps, like nuts’, score 2 ‘Sausage-shaped, but lumpy’, score 3 ‘Like a sausage but with cracks on the surface’, score 4 ‘Like a sausage or snake, smooth and soft’, score 5 ‘Soft blobs with clear cut edges’, score 6 ‘Fluffy pieces with ragged edges, a mushy stool’ and score 7 ‘Watery, no solid pieces, entirely liquid’. Scores 1 and 2 were not detected during the study. Table shows the percentage of litters (and raw count) of each score for control and SB treatment between day 1 and 7.
*P<0.01 (χ 2 for trend on raw counts).
Discussion
The current study demonstrates that supplementation with a single dose of SB, within 24 h of birth, reduces both occurrence and severity of diarrhoea in piglets over the 1st week of life. Previous studies, in rats and humans, as well as our own unpublished data in pigs, have determined SB to be transient and non-colonising in the gastrointestinal tract (Blehaut et al., Reference Blehaut, Massot, Elmer and Levy1989); this is consistent with our findings that a significant effect of SB was not demonstrated after day 3.
SB has known positive effects on reduction of enteric pathogens of pigs including Escherichia coli (Lessard et al., Reference Lessard, Dupuis, Gagnon, Nadeau, Matte, Goulet and Fairbrother2008; Collier et al., Reference Collier, Carroll, Ballou, Starkey and Sparks2011), and in human studies C. difficile-associated disease, and rotavirus infection (McFarland, Reference McFarland2006; Grandy et al., Reference Grandy, Medina, Soria, Teran and Araya2010); however, we found no evidence of such effects (data not shown). Not all diarrhoea is caused by infectious agents; thus, the significant reduction in diarrhoea in this study, related to SB administration, could be due to the physiological effects of SB improving osmotic balance. In rat models, SB administration increases expression of sodium/glucose co-transporter-1, improving glucose, water and electrolyte re-absorption in the gut, encouraging fluid and electrolyte balance (Buts et al., Reference Buts, De Keyser, Marandi, Hermans, Sokal, Chae, Lambotte, Chanteux and Tulkens1999). Studies have also shown that SB causes an increase in activity of brush border enzymes, improving nutrient breakdown and absorption (Buts et al., Reference Buts, De Keyser and De Raedemaeker1994; Buts and De Keyser, Reference Buts and De Keyser2006).
Commercial pig production aims to produce highly prolific sows, as this provides an opportunity to be more profitable, however, problems of large litters include chilling, inadequate colostrum transfer, increased heterogeneity, low birth weight/non-viability and crushing, as well as high incidence of neonatal diarrhoea. The issue of increasing litter size has been previously reviewed, and, as it is unlikely producers will cease attempting to increase litter size, good management might negate some issues it brings (Baxter et al., Reference Baxter, Rutherford, D'Eath, Arnott, Turner, Sandoe, Moustsen, Thorup, Edwards and Lawrence2013; Rutherford et al., Reference Rutherford, Baxter, D'Eath, Turner, Arnott, Roehe, Ask, Sandoe, Moustsen, Thorup, Edwards, Berg and Lawrence2013). Thus, SB could have a role in this management, as the results of the current study shows it decreases neonatal diarrhoea.
The current study has shown the potential of SB supplementation by demonstrating the benefit of a single oral dose on enteric health of the pig. The results show that a single dose of SB on the day of farrowing significantly reduced number of diarrhoeic days experienced, and promoted lower faecal scores (firmer faeces) in the 1st week of life. It has been suggested that enteric health of the young pig can impact lifelong gut health (Mahan and Lepine, Reference Mahan and Lepine1991; McOrist and Mellits, Reference McOrist and Mellits2010). Therefore, because of the effect of SB on diarrhoea, and also the correlation between diarrhoea, mortality and growth, it may be suggested that continuous SB administration during the pre-weaning period could have significant effects on production.
Acknowledgements
This work was supported by grants from the BBSRC and Lallemand. M.L.B. and D.G. are employees of Lallemand Animal Nutrition. The authors acknowledge the technical assistance of Helen Davies, Elizabeth King and Rebecca Chandler-Bostock, and the staff of the trial farm. They thank Professor Ian Connerton, Dr Sabine Tötemeyer and Professor Julian Wiseman, for their careful and critical reading of this manuscript.






