Implications
Our novel findings suggested that early intervention with maternal faecal microbiota, which contains a large number of mature and commensal microbiota, may be an effective means to improve growth performance, decrease intestinal permeability, and modulate microbial composition and metabolism of suckling piglets. Moreover, the current study also supported the view that early postnatal period is a critical window for gut microbiota manipulation to optimise the immunity and body growth of newborn piglets.
Introduction
Neonatal animals are readily colonised following birth from exposure to the environment, most notably, microbiota from their mother’s vagina, skin and faeces (Jost et al., Reference Jost, Lacroix, Braegger and Chassard2015). Increasing evidence shows that the early colonisation of gut microbiota contributes to host’s health through promoting the establishment of gut barrier function and the maturation of immune system (Gensollen et al., Reference Gensollen, Iyer, Kasper and Blumberg2016). In addition, short-chain fatty acids (SCFAs), main end-products of microbial fermentation, also play an important role in intestinal health (Tan et al., Reference Tan, Mckenzie, Potamitis, Thorburn, Mackay and Macia2014). For example, acetate has been shown to be an anti-inflammatory metabolite to maintain gut homeostasis (Fukuda et al., Reference Fukuda, Toh, Hase, Oshima, Nakanishi, Yoshimura, Tobe, Clarke, Topping, Suzuki, Taylor, Itoh, Kikuchi, Morita, Hattori and Ohno2011). Moreover, butyrate is beneficial for gut mucosal immunity and barrier function (Kelly et al., Reference Kelly, Zheng, Campbell, Saeedi, Scholz, Bayless, Wilson, Glover, Kominsky, Magnuson, Weir, Ehrentraut, Pickel, Kuhn, Lanis, Nguyen, Taylor and Colgan2015). In contrast to the stable microbiota of adult animals, the gut microbiota of neonates are less stable. Many studies have suggested that early postnatal period is a critical window for gut microbiota manipulation to optimise the immunity of newborn individual (Torow and Hornef, Reference Torow and Hornef2017). Moreover, the role of the microbiota in growth traits has attracted increasing attention (Ramayo-Caldas et al., Reference Ramayo-Caldas, Mach, Lepage, Levenez, Denis, Lemonnier, Leplat, Billon, Berri, Dore, Rogel-Gaillard and Estelle2016). The modulation of the gut microbiota offers a potential therapeutic target for both promotion of newborns growth and prevention of growth impariment (Blanton et al., Reference Blanton, Charbonneau, Salih, Barratt, Venkatesh, Ilkaveya, Subramanian, Manary, Trehan, Jorgensen, Fan, Henrissat, Leyn, Rodionov, Osterman, Maleta, Newgard, Ashorn, Dewey and Brown2014; Dror et al., Reference Dror, Dickstein, Dubourg and Paul2017). Thus, strategies resulting in the establishment of an effective gut ecosystem should be implemented in early life.
Faecal microbiota transplantation (FMT) involves administration of the whole commensal microbial community from healthy donor stool into the recipient’s intestinal tract to modify intestinal microbiota and is gaining research concerns. Faecal microbiota transplantation has now become widely accepted as a highly successful rescue treatment for recurrent infections (i.e. Clostridium difficile) (Bafeta et al., Reference Bafeta, Yavchitz, Riveros, Batista and Ravaud2017). The mechanisms by which FMT exerts its therapeutic effect inculde increasing gut microbial diversity, introducing ‘healthy’ microbes essential for maintaining epithelial integrity, and decreasing gut permeability (Brown, 2014). Faecal microbiota transplantation has also been associated with improved growth in children (Walia et al., Reference Walia, Garg, Song, Girotra, Cuffari, Fricke and Dutta2014). However, it is not known whether maternal faecal microbiota (including a large number of mature and commensal microbiota) administered to neonatal piglets after birth can improve performance and regulate gut ecosystem and immunity. Taken together, we hypothesised that early intervention with maternal faecal microbiota might improve growth performance, decrease intestinal permeability, and modulate microbial composition and metabolism of suckling piglets. Therefore, the aim of this study was to investigate the effects of early intervention with maternal faecal microbiota on growth performance, microbial populations, and gut permeability and immune system in suckling piglets.
Materials and methods
Animals and housing
A total of 12 multiparous Landrace sows with an average parity of 4.95±1.00 were selected to this study. Sows did not have diarrhea or other digestive disorders, never received antibiotics before the study. The sows were artificially inseminated by one Duroc boar to minimise the genetic variation among their off-spring. Sows were moved from the gestation stalls to the farrowing rooms on day 107 of gestation. The sows and piglets were individually housed in farrowing pens (2.2×1.7 m) with crates, slatted floors and heat pads for piglets. The farrowing room temperature was maintained at 20°C. The maize–soyabean meal diets were formulated according to National Research Council (2012) requirements for gestating and lactating sows. The diets did not contain any probiotics, antibiotics or other medicine. The sows were individually fed and had ad libitum access to water and diet throughout the experimental period.
At parturition, each piglet was individually weighed and the number of live-born piglets recorded. The litter size was then standardised to 10 piglets per sow within 8 h after birth. The average initial BW of all selected piglets (n=120 piglets) was 1.61±0.20 kg. All surplus piglets were fostered off to the non-experimental sows. Piglets were given iron supplementation and had their teeth and tails cut within the first 3 days life, but had no access to creep feed during the experiment and therefore relied on sow’s milk as their sole source of nutrients. The piglets had no access to probiotics and antibiotics.
Preparation of faecal microbiota suspension of donor sows
On the basis of the standard for donor identification described by Hamilton et al. (Reference Hamilton, Weingarden, Sadowsky and Khoruts2012), faecal donor sows did not have diarrhoea, never received antibiotics before the study, and were fed a diet without antibiotics and probiotics for at least 2 month before faeces collection. On day 109 of gestation, the fresh faeces of all the sows in present study were collected separately after 12-h fasting. The faecal suspension was prepared as described by Pang et al. (Reference Pang, Hua, Qian, Ding, Che, Li, Wei, Bucheli and Zhao2007). In brief, fresh faeces were diluted 20-fold and homogenised in sterile pre-reduced potassium phosphate buffer (0.1 mol/l, pH 7.2) containing 10% glycerol (vol/vol). The homogenates were filtered through a 0.5mm pore mesh to remove larger particles, dispensed into 20-ml centrifuge tubes, and then stored at −20°C until faecal transplantation. The microbiota population in stool suspension of sows was assessed by 16S ribosomal RNA (rRNA) gene sequencing. The bacterial composition of maternal stool suspension on phylum and genus level is shown in Supplementary Table S1.
Transplantation of faecal microbiota experiment and treatments
In order to study the effects of postnatal maternal faecal microbiota intervention on newborn piglets, 10 piglets in each litter were randomly allotted to one of two treatments (the FMT group and the control group) on day 1. Thus, each treatment consisted of 12 replicates with five newborn piglets per replicate. Newborn piglets in the FMT group were first infused by an injector without the needle with faecal suspension of their nursing sow at 12 h after their birth. At the same time, piglets in the control group received by oral inoculation a placebo (0.1M potassium phosphate buffer containing 10% glycerol (vol/vol)) inoculant. The dosage of inoculant was 2 ml/piglet once daily in the first 3 days. The three time points for oral administration were 12, 36 and 60 h after birth, respectively. Piglets were gently put on the heat pads of farrowing pen immediately after the swallowing to avoid stress caused by the operation. In the first 3 days after delivery, the faeces of nursing sows were removed promptly to prevent piglets from contacting and ingesting faecal material.
Determination of growth performance and diarrhea rate
Body weights of piglets were individually measured on days 1, 7, 14 and 21 to determine average daily gain (ADG). The health status and mortality of each piglet was recorded, and the occurrence of diarrhoea was visually assessed and evaluated by individual scoring the consistency of the faeces at 0900 and 1600 h each day by trained observers blinds to the treatments according to the method of Marquardt et al. (Reference Marquardt, Jin, Kim, Fang, Frohlich and Baidoo1999). In brief, scores were 0, firm faeces, normal; 1, pasty faeces, slight diarrhea; 2, semi-liquid faeces, moderate diarrhoea; or 3, liquid and unformed faeces, severe diarrhoea. The occurrence of diarrhoea was defined as maintaining a score of two or three for 1 day. The diarrhea rate (%) was calculated as [(the total number of piglets with diarrhoea within a treatment)/(total number of experimental piglets within a treatment × total observational days)] ×100, in which ‘total observational days’ was the whole suckling period (21 days). Diarrhoea index was calculated as sum of faeces score/(total number of experimental piglets × total observational days).
Sample collection
On days 7, 14 and 21, two median-weight piglets (one from the FMT group and the other from the control group) from each litter (total of 12 piglets/treatment) were randomly selected to collect blood and faecal samples. Blood samples were collected from piglets by vena jugularis with a minimum amount of stress into heparinised tubes (5ml). Plasma samples were then obtained by centrifuging the blood samples at 3000×g for 10min at 4°C and were stored at −80°C until analysis. Fresh faecal samples were individually collected using sterile 20 ml centrifuge tubes (without any treatment) from the piglets. Samples were transported (the tubes frozen on dry ice) immediately to the laboratory and then stored at −80°C until analysis.
Quantification of selected faecal bacteria
Total microbial DNA was extracted and purified from faeces samples on days 7, 14 and 21 using a QIAamp DNA stool kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Genomic DNA from faeces was pooled and amplified through routine PCR using species and genus-specific primers (Table 1). After PCR amplification with a Taq DNA polymerase kit (Promega, WI, USA) and electrophoresis on a 1.5% agarose gel, PCR products were purified according to the manufacturer’s protocol (Omega, Norcross, GA, USA).
Table 1 Absolute quantitative real-time PCR Primers for detecting microbial populations in faeces of suckling piglets

The purified PCR products were linked to the pMD 18-T vector system (Takara, Japan) and then transferred to Escherichia coli DH5α (Qiagen, Hilden, Germany) for cloning. After checking the size of the cloned inserts with PCR amplification, the extracted plasmids of the positive clones were sequenced commercially to obtain the positive plasmids (Shanghai Sangon Biological Engineering Technology Service Co. Ltd, Shanghai, China).The plasmids were extracted and purified using the Qiagen Plasmid Midi kit (Qiagen, Hilden, Germany), and its concentration was quantified using a spectrophotometer (Thermo Co., Vantaa, Finland). Serial dilutions of these positive plasmids served to generate standard curves using quantitative real-time PCR (Bio-Rad, CA, USA), permitting estimations of absolute quantification based on respective gene copies. Absolute quantitative real-time PCR was performed according to our previous study (Wei et al., Reference Wei, Xue, Zhou and Peng2017). The PCR amplification efficiencies for total bacteria, Lactobacillus spp., Escherichia coli, Enterococcus faecalis and Faecalibacterium prausnitzii were 99.95%, 99.75%, 99.90%, 99.98%, 99.80%, respectively. The mean threshold cycle values from the triplicate of each sample were used for the calculations. The data are presented as gene copy numbers per gram of wet faeces and presented as Log10 cfu/g faeces for data analysis.
Faecal and plasma short-chain fatty acids analysis
The SCFAs concentrations of faeces and plasma in day 21 were analysed by a gas chromatographic method. Specifically, approximately 1.50±0.11 g of faeces were first homogenised in 1.5ml of deionised water. The entire sample was centrifuged at 15 000×g at 4°C for 10 min. Thereafter, supernatant of faeces and plasma samples were then acidified with 25% metaphosphoric acid at a 1 : 5 ratio (1 volume of acid for 5 volumes of sample) for 30min on ice. The sample was injected into a gas chromatograph (GC-2010, Shimadzu, Japan) equipped with a CP-Wax 52 CB column 30.0m×0.53mm i.d. (Chrompack, Middelburg, The Netherlands). The injector and detector temperatures were 75°C and 280°C, respectively. Total SCFAs in faeces were determined as the sum of analysed acetate, propionate, butyrate, valerate, isobutyrate and isovalerate. In addition, branch-chain fatty acids were determined as the sum of isobutyrate and isovalerate. All procedures were performed in duplicate.
Determination of hormones, zonulin, endotoxin, diamine oxidase, cytokines in plasma, and lipocalin-2 and secretory immunoglobulin A in faeces
Plasma and stool samples were collected at days 7, 14, and 21. Faecal homogenates were prepared by solubilising faeces in phosphate buffered saline (10% wt : vol) and stored at −80°C. The plasma growth hormone (GH), IGF-1, interleukin 6 (IL-6), interleukin 10 (IL-10), tumour necrosis factor α (TNF-α), transforming growth factor β (TGF-β), zonulin, endotoxin and immunoglobulin G (IgG) concentrations and the faecal total Lipocalin-2 and secretory immunoglobulin A (sIgA) concentrations were determined by using porcine ELISA kits (Bio-Swamp Life Science, Wuhan, China) according to the manufacturer’s instructions. The plasma diamine oxidase activity was performed using enzymatic kinetic spectrophotometry method with the diamine oxidase assay kit (Nanjing Jiancheng Bioengineering institute, Nanjing, China). Values were obtained by measuring the OD450 using a Labsystems Multiskan MS microplate reader (Thermo Co.). All procedures were performed in duplicate.
Statistical analysis
The data were analysed using the GLM procedure of the Statistical Analysis System (SAS 8.1 SAS Institute Inc., Cary, NC, USA). The normal distribution of data were verified by a Kolmogorov–Smirnov test. The litter was the experimental unit for growth performance and diarrhoea scores data (BW, ADG, diarrhoea rate and diarrhoea index), and the piglet was the experimental unit for other indices. All data excepting diarrhoea rate were analysed by one-way ANOVA where treatment (control and FMT) was the main factor. The χ 2 test was used to test for diarrhoea rate. Data were given as means±SEM. A P<0.05 was considered to indicate significance for all analyses.
Results
Growth performance and diarrhoea rate of piglets
Four piglets from the control group were excluded because of sudden death due to unknown causes at the beginning of week 1. The ADG during week 3 and the whole experiment period was higher (P<0.01) for piglets in the FMT treatment than that in the control group (Table 2). The diarrhoea rates during the whole suckling period were lower (P<0.05) in FMT piglets compared with the control piglets. During the whole experiment period, diarrhoea index were significantly lower (P<0.01) in FMT piglets compared with the control piglets (Table 2).
Table 2 Effect of faecal microbial transplantation on the growth performance and diarrhea rate of suckling pigletsFootnote 1

FMT=faecal microbial transplantation group; ADG=average daily gain; Wk=Week.
1 All results are presented as mean±SEM (n=12).
a,bValues within a row with different superscripts differ significantly at P<0.05.
Plasma concentrations of hormones
At day 7 of lactation, the concentrations of plasma IGF-1 in the FMT group was increased (P<0.01) compared with that in the control group (Table 3). Compared with the control group, the FMT group had significantly increased (P<0.01) concentrations of plasma GH and IGF-1 on days 14 and 21 and plasma IgG on day 21 (Table 3).
Table 3 Effect of faecal microbial transplantation on plasma parameters of suckling pigletsFootnote 1

FMT=faecal microbial transplantation group; GH=growth hormone.
1 All values are presented as means±SEM (n=12).
a,bValues within a row with different superscripts differ significantly at P<0.05.
Selected microbial populations in faeces
The effects of faecal microbial transplantation on selected microbial populations in faeces are shown in Figure 1. There was no difference (P>0.05) in populations of the total bacteria, E. faecalis, and E. coli between the two treatments. Compared with piglets in control group, the suckling piglets orally administered with FMT had a significantly higher (P<0.01) population of Lactobacillus spp. and F. prausnitzii on days 14 and 21 of lactation.
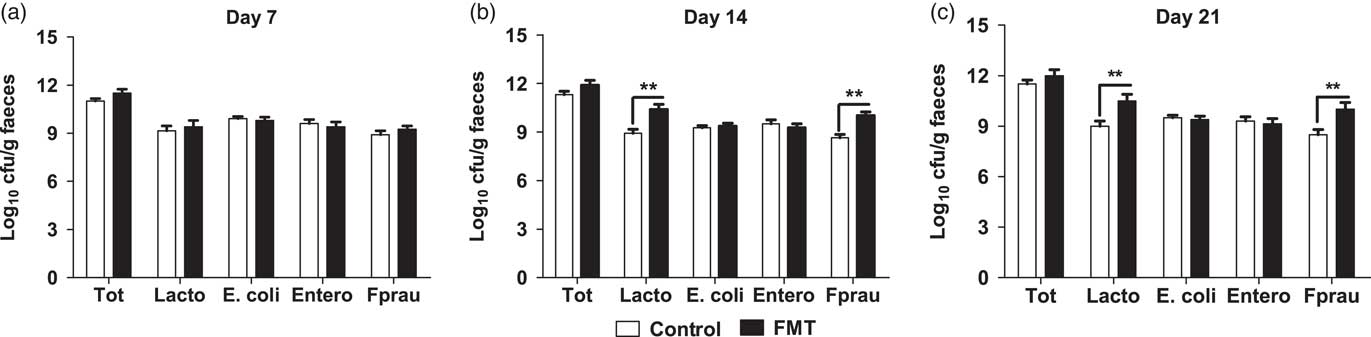
Figure 1 Effect of faecal microbial transplantation (FMT) on seletcted microbial populations in faecal samples of 7-day-old piglets (a), 14-day-old piglets (b) or 21-day-old piglets (c) Tot=total bacteria; Lacto=Lactobacillus spp.; Entero=Enterococcus faecalis; E. coli= Escherichia coli; Fprau=Faecalibacterium prausnitzii; Log10=16S ribosomal RNA gene copies/g faeces. All values are presented as means±SEM (n=12). **Effect of treatment (P<0.01).
Faecal and plasma short-chain fatty acids concentrations
Figure 2 shows the concentrations of SCFAs determined in the faeces and plasma of piglets on day 21. There was no significant difference (P>0.05) in the faecal concentrations of propionate, isobutyrate, and isovalerate between the two treatments. However, the FMT group had higher (P<0.05) concentrations of the acetate, butyrate, and total SCFAs in faeces and plasma than the control group. In addition, the FMT piglets had higher (P<0.01) concentrations of faecal valerate than the control piglets.
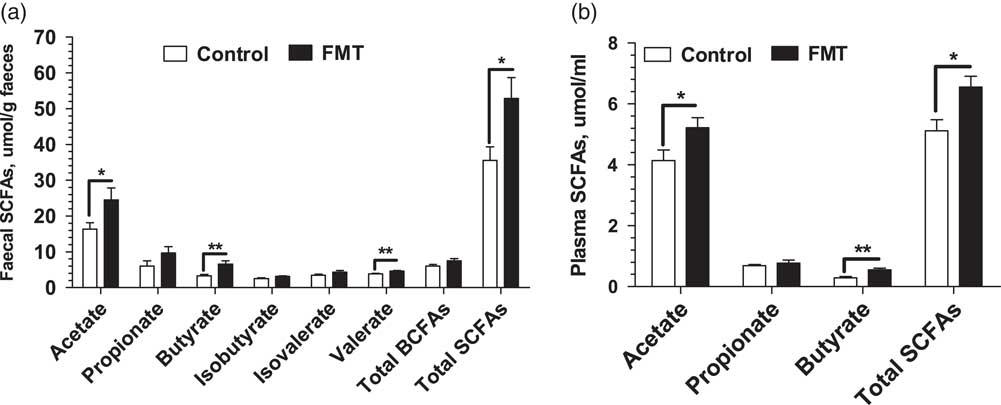
Figure 2 Effect of faecal microbial transplantation (FMT) on short-chain fatty acids (SCFAs) in faecal (a) and plasma (b) samples of suckling piglets on day 21. Total SCFAs in faeces is the sum of acetate, propionate, butyrate, valerate, isobutyrate and isovalerate; branch-chain fatty acids (BCFAs) is the sum of isobutyrate and isovalerate. Total SCFAs in plasma is the sum of acetate, propionate and butyrate. All values are presented as means±SEM (n=12). *Effect of treatment (P<0.05); **effect of treatment (P<0.01).
Plasma zonulin, endotoxin, diamine oxidase, and inflammatory cytokines and faecal lipocalin-2 measurement
The plasma zonulin, endotoxin, and diamine oxidase concentrations on different days (days 7, 14, and 21) of lactation are presented in Figure 3. On days 7 and 14, the FMT piglets had lower (P<0.05) concentrations of plasma zonulin, endotoxin and diamine oxidase activity than the control piglets. On day 7, the FMT piglets had lower (P<0.01) concentrations of faecal lipocalin-2 concentrations than the control piglets. There was no difference (P>0.05) in plasma zonulin, endotoxin and diamine oxidase concentrations of piglets on day 21 between the control and FMT groups.

Figure 3 Effect of faecal microbial transplantation (FMT) on plasma zonulin (a), endotoxin (b) and diamine oxidase (c) and faecal lipocalin-2 (d) of suckling piglets. All values are presented as means±SEM (n=12). *Effect of treatment (P<0.05); **effect of treatment (P<0.01).
Plasma immunoglobulins and faecal secretory immunoglobulin A concentrations
The plasma IgG and faecal total sIgA concentrations on different days (days 7, 14 and 21) of lactation are presented in Figure 4. Compared with the control group, the FMT group had significantly increased (P<0.01) concentrations of plasma IgG on day 21. On day 21, the FMT piglets tend to have higher (P=0.054) concentrations of faecal sIgA than the control piglets.

Figure 4 Effect of faecal microbial transplantation (FMT) on plasma immunoglobulin G (IgG) (a) and faecal secretory immunoglobulin A (sIgA) (b) of suckling piglets. All values are presented as means±SEM (n=12). **Effect of treatment (P<0.01); #effect of treatment (P<0.1).
Plasma inflammatory cytokines measurement
Table 4 shows the concentrations of inflammatory cytokines determined in plasma of piglets on days 7, 14 and 21. There was no significant difference (P>0.05) in the plasma concentrations of IL-10 and TNF-α between the two treatments. However, the FMT group had lower (P<0.05) concentrations of plasma IL-6 on day 7 and higher (P<0.05) concentrations of plasma TGF-β on days 14 and 21 than the control group.
Table 4 Effect of faecal microbial transplantation on plasma concentrations of cytokines in pigletsFootnote 1

FMT=faecal microbial transplantation group; IL-6=interleukin 6; IL-10=interleukin 10; TNF-α=tumour necrosis factor α; TGF-β=transforming growth factor β.
1 All values are presented as means±SEM (n=12).
a,bValues within a row with different superscripts differ significantly at P<0.05.
Discussion
Several studies have suggested that early postnatal birth is a critical window for gut microbiota manipulation to optimise the gut health and immunity (Nguyen et al., Reference Nguyen, Himes, Martinez and Permar2016; Torow and Hornef, Reference Torow and Hornef2017). Faecal microbiota transplantation involves administration of the whole commensal microbial community from healthy donor stool into the recipient’s intestinal tract to normalise or modify intestinal microbiota composition and function and has now become widely accepted as a highly successful rescue treatment for recurrent infections (i.e. C. difficile) (Brown, 2014). However, there is little information about maternal faecal microbiota use as the source of microbiota to affect growth performance, intestinal microbial colonisation and intestinal mucosal immune system development in neonatal piglets during the critical window for gut microbiota colonisation. In the present study, oral administration of 2ml of maternal faecal microbiota daily for three days after birth could significantly increase ADG in suckling pigs. Moreover, plasma concentrations of GH and IGF-1 were also significantly increased by oral faecal microbiota administration. In mammals, postnatal growth is controlled by the activity of the somatotropic axis, which GH instructs the liver and peripheral tissues to produce IGF-1, to promote organ and systemic growth (Butler and Roith, Reference Butler and Roith2001). Schwarzer et al. (Reference Schwarzer, Makki, Storelli, Machuca-Gayet, Srutkova, Hermanova, Martino, Balmand, Hudcovic, Heddi, Rieusset, Kozakova, Vidal and Leulier2016) reported that the gut microbiota is necessary to boost postnatal growth by enhancing GH sensitivity and thereby facilitating IGF-1 production and activity. Accumulated evidence has demonstrated that early exposure with desirable microbiota followed by an appropriate colonisation process in early life could alter the pattern of microbial succession (Rauch and Lynch, Reference Rauch and Lynch2010). Moreover, the gut microbiota within the gastrointestinal tract has been suggested to be crucial in the shaping of piglet growth traits (Ramayo-Caldas et al., Reference Ramayo-Caldas, Mach, Lepage, Levenez, Denis, Lemonnier, Leplat, Billon, Berri, Dore, Rogel-Gaillard and Estelle2016). Thus, our results indicated that oral maternal faecal microbiota administration may positively regulate the pattern of microbial colonisation, which promoted growth by facilitating IGF-1 production and activity in suckling piglets.
To detect the effect of maternal faecal microbiota on the pattern of microbial colonisation in newborn piglets, four selected bacteria of piglet faeces were quantified by real-time quantitative PCR method. Our results showed that early FMT intervention improved the population of Lactobacillus spp. and F. prausnitzii in piglets on days 14 and 21. Lactobacillus was one of the most dominant genera, accounting for approximately 15% of 16S rRNA gene sequences from porcine intestinal samples, regardless of age (Niu et al., Reference Niu, Li, Hao, Zhang, Kim, Li, Ma, Gao, He and Wu2015). The positive correlation between Lactobacillus spp. and growth performance has been confirmed (Hou et al., Reference Hou, Zeng, Yang, Liu and Qiao2016). Moreover, Lactobacillus species are common probiotics, and they play important roles in pathogen defense, and improve intestinal barrier function and immunity in piglets (Yang et al., Reference Yang, Qian, Wang and Wu2017). Faecalibacterium prausnitzii is one of the most abundant butyrate-producing bacterium in the intestinal tract and has been demonstrated as a microbial biomarker of intestinal homeostasis (Miquel et al., Reference Miquel, Martín, Rossi, Bermúdez-Humarán, Chatel, Sokol, Thomas, Wells and Langella2013). Therefore, early FMT intervention may play a positive role in modulating gut microbial composition of suckling piglets.
Commonly, changes in microbial communities are coupled with the measurement of selected byproducts of fermentation. Short-chain fatty acids are the major end products from the fermentation processes of gut microbiota and play an important role in the maintenance of colonic function such as improving barrier function, inhibiting adherence of pathogens, and contributing to energy utilisation (Kelly et al., Reference Kelly, Zheng, Campbell, Saeedi, Scholz, Bayless, Wilson, Glover, Kominsky, Magnuson, Weir, Ehrentraut, Pickel, Kuhn, Lanis, Nguyen, Taylor and Colgan2015; Corrêa-Oliveira et al., Reference Corrêa-oliveira, Fachi, Vieira, Sato and Vinolo2016). Our results showed that oral administration of maternal faecal microbiota increased the concentration of acetate, butyrate, and the total SCFAs in faeces and plasma of piglets. Gall et al. (Reference Gall, Gallois, Sève, Louveau, Holst, Oswald, Lallès and Guilloteau2009) suggested that higher production of SCFAs in intestine contributes to good growth performance. Moreover, butyrate has been proved associated with positive effects on pathogen control and gut barrier function, especially colonic energy utilisation, cell proliferation in the gastrointestinal tract and stabilisation of the luminal pH in animals (Guilloteau et al., Reference Guilloteau, Martin, Eeckhaut, Ducatelle, Zabielski and Immerseel2010; Kelly et al., Reference Kelly, Zheng, Campbell, Saeedi, Scholz, Bayless, Wilson, Glover, Kominsky, Magnuson, Weir, Ehrentraut, Pickel, Kuhn, Lanis, Nguyen, Taylor and Colgan2015). Our results agreed with the findings of Lu et al. (Reference Lu, Su and Ajuwon2012) who reported that butyrate supplementation to suckling piglets enhanced preweaning growth performance. Thus, it is reasonable to assume that the growth performance and intestinal health were also improved via impact on intestinal metabolism.
As a protein that participates in tight junctions between cells of the wall in the digestive tract, zonulin was found to reflect intestinal permeability (Tripathi et al., Reference Tripathi, Lammers, Goldblum, Shea-Donohue, Netzel-Arnett, Buzza, Antalis, Vogel, Zhao, Yang, Arrietta, Meddings and Fasano2009). Simultaneously, endotoxin and diamine oxidase in plasma are also important indicators for intestinal epithelial permeability and integrity (Luk et al., Reference Luk, Bayless and Baylin1983). The impaired intestinal mucosal integrity may enhance concentrations of zonulin and diamine oxidase and increase contents of endotoxin in blood. Results of our current study revealed that maternal FMT significantly decreased plasma contents of endotoxin and diamine oxidase activity as compared with control group on days 7 and 14, which indicated that early intervention with maternal faecal microbiota may promote the maturation of the intestinal barrier function. Indeed, the protection of intestinal barrier function in the FMT group was confirmed by lower diarrhoea rates during the whole experiment period and faecal lipocalin-2 concentrations on day 7. It is well documented that the occurrence of diarrhoea is commonly accompanied by a pathological increase in intestinal permeability (Yu et al., Reference Yu, Wang, Wei and Ni2012). Moreover, an increased intestinal permeability that precedes the development of metabolic endotoxaemia, which increases and triggers inflammation and metabolic disorders (Cani et al., Reference Cani, Bibiloni, Knauf, Waget, Neyrinck, Delzenne and Burcelin2008). Lipocalin-2, originally appreciated as being highly abundant in neutrophils, has since been shown to be elevated in intestinal inflammation (Chassaing et al., Reference Chassaing, Srinivasan, Delgado, Young, Gewirtz and Vijaykumar2012). Therefore, a decrease of lipocalin-2 in faeces on day 7 in present study indicated that FMT may alleviate the occurrence of intestinal inflammation.
Recently published data have proven that early colonisation of gut microbiota plays a fundamentally important role in the maturation of the immune system (Gensollen et al., Reference Gensollen, Iyer, Kasper and Blumberg2016). In the current study, oral administration of maternal faecal microbiota increased the concentrations of plasma IgG and faecal sIgA on day 21. The greater plasma IgG concentrations in FMT piglets indicated an enhanced systemic immune function. Meanwhile, sIgA is the most abundant antibody molecule on mucosal surfaces of humans and most other mammals (Mathias et al., Reference Mathias, Pais, Favre, Benyacoub and Corthésy2014). Production of IgA at mucosal surfaces contributes to host defense against intestinal pathogens (Mathias et al., Reference Mathias, Pais, Favre, Benyacoub and Corthésy2014). In addition, FMT treatments with high sIgA exhibited higher acetate content in faeces which agree with the findings of Wu et al. (Reference Wu, Sun, Chen, Yao, Liu and Cong2016) who reported that acetate promoted intestinal IgA response. Interleukin 6, which is usually used as a marker for pro-inflammatory responses (Fonseca et al., Reference Fonseca, Santos, Canhão and Choy2009), was observed to be reduced in FMT treatment than piglets in control group on day 7. In contrast, TGF-β, which has commonly been regarded as mediating immune tolerance and preventing excessive immune responses (Yang et al., Reference Yang, Pang and Moses2010), had an increased concentrations in faeces of the FMT piglets. Therefore, piglets orally administered with FMT may prevent the over-activation of immune responses thus allowing a greater proportion of the nutrients contained in milk to be directed towards growth rather than the immune response.
Probiotics are defined as living microorganisms that beneficially affect the host animal by improving its intestinal microbial composition and activity. Several recent studies with lactic acid bacteria such as Lactobacillus, run in neonatal piglets, noted an improvement of microbiota and intestinal barrier function (Yang et al., Reference Yang, Hou, Zeng and Qiao2015). However, inter-individual variation in microbiome composition, as well as strain-level differences, result in differential responsiveness to probiotics intervention, limiting the efficacy of a ‘one strain fits all’ therapeutic approach (Andersen et al., Reference Andersen, Cilieborg, Lauridsen, Mørkbak and Sangild2017). Our findings supported that FMT might also be an efficient way to improve neonatal animal’s growth performance, intestinal barrier function and immune function via modulating the composition of the gut microbiota. Since this study was carried out on neonatal animals with immature intestinal development, the physiological characters of animals need to be considered in future clinical applications. Therefore, additional larger, controlled and prospective studies are needed to clarify both the safety and efficacy of FMT in animals with different physiological characters.
In conclusion, the results of this study suggested that early postnatal period is a critical window for gut microbiota manipulation to optimise the immunity and growth traits of newborn piglets. Moreover, early intervention with maternal faecal microbiota, which contains a large number of mature and commensal microbiota, may be a potentially effective means to improve growth performance, decrease intestinal permeability, and modulate microbial composition and metabolism of neonatal piglets. In addition, further prospective studies are needed to clarify both the safety and efficacy of FMT in animals with different physiological characters.
Acknowledgements
This research was supported by the National Key Research and Development Project of China (NO. 2017YFD0500503), China Agriculture Research System (CARS-36), Hubei Provincial Creative TeamProject of Agricultural Science and Technology (2007-620), Fundamental Research Funds for the Central Universities of China (2662017PY017). All authors have no conflicts of interest. The authors acknowledge the YangXiang Joint Stock Company for providing sow feeding facilities. S-W J and JP designed the experiments; C-SC performed the experiments and wrote the manuscript; H-KW and PW analysed the data; and H-CY and X-MZ tested the parameters in plasma. All authors finally approved the version to be published. All authors approved the final manuscript.
Declaration of interest
The authors declare that there is no conflict of interest.
Ethics statement
The experimental protocol had been approved by the Institutional Animal Care and Use Committee of Huazhong Agricultural University (permit number: HZAUSW-2016-139).
Software and data repository resources
None of the data were deposited in an official repository
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1751731118001611










