Green plants sequester solar energy as starch granules in seeds, fruits, roots, tubers, and other storage organs. The granules are composed of covalently bonded glucose polymers that are insoluble in water and can persist in the archaeological record for long periods of time. They have been recovered from soil sediments, pottery fragments, dental calculus, basketry, and stone tools (Torrence and Barton Reference Torrence and Barton2006; Copeland and Hardy Reference Copeland and Hardy2018). The morphological characteristics of starch granules can distinguish among source plant taxa and provide valuable clues for interpreting human diets in the ancient past. For example, granules preserved on ancient ground stone tools can reveal which plant species were processed and consumed and can even infer tool function (Fullagar Reference Fullagar, Torrence and Barton2006; Liu et al Reference Liu, Ma and Cui2014). Although starch residues on buried artifacts have revealed patterns of past lifeways and human diet, very limited starch research has been conducted on open-air bedrock mortars and metates.
Bedrock metates (Figure 1) are among the most common features in the archaeological record and have the potential to provide evidence for past human lifeways, including foods collected and processed, social gatherings, settlement patterns, land investment, and territorial behavior (Fulkerson and Tushingham Reference Fulkerson and Tushingham2021; Henry Reference Henry and Henry2020; Lynch Reference Lynch2021; O'Connell et al. Reference O'Connell, Hawkes, Blurton-Jones, Kroll and Price1991; Stevens et al. Reference Stevens, Whitaker and Rosenthal2019; Tinsley et al. Reference Tinsley, Louderback, Pavlik, Baker, Townsend, Tucker and Wilks2021; Wisely Reference Wisely2016). Some of the earliest documented bedrock metates are at Natufian sites in the Levant region of the Mediterranean between about 15,500 and 11,500 cal BP (Nadel et al. Reference Nadel, Piperno, Holst, Snir and Weiss2012). Those features come in a variety of types and probably served different functions, including food processing, storage containers, pounding surfaces, and ceremonial or ritual totems (Nadel et al. Reference Nadel, Piperno, Holst, Snir and Weiss2012; Piperno et al Reference Piperno, Weiss, Holst and Nadel2004).

Figure 1. (a) Mono women prepare acorns on a bedrock mortar station (Nellie T. McGraw Hedgpeth, 1904–1905; courtesy of Phoebe Hurst Museum); (b) San Ygnacio woman grinding acorns with a metate (Edward O. Davis Collection, 1911; courtesy of San Diego History Center).
Bedrock metates are commonly associated with the processing of localized seasonal resources (Adams Reference Adams2014; Buonasera Reference Buonasera2013). For example, bedrock mortars and pestles are often linked to acorn processing in California (Kroeber Reference Kroeber1925). In central Nevada, flat, oval slicks or depressions on boulders and rockshelter ledges were used to process pine nuts from nearby pinyon woodlands (Tinsley et al. Reference Tinsley, Louderback, Pavlik, Baker, Townsend, Tucker and Wilks2021). Bedrock metates are found in a variety of locations and settings in western North America, yet they remain little understood in archaeological contexts (Figure 2). We believe, however, that they hold much potential to illuminate past behavior between humans and their environment.

Figure 2. Bedrock milling surfaces in (a) southern California (Burton et al. Reference Burton, Adams, Willis and Nadel2017), (b) south-central Nevada (Tinsley et al. Reference Tinsley, Louderback, Pavlik, Baker, Townsend, Tucker and Wilks2021), (c) southeastern Utah (Pavlik et al. Reference Pavlik, Louderback, Codding, Vernon, Simper, McCool and Wilks2022), and (d) southern Oregon (Louderback et al. Reference Louderback, Wilks and Simper2022). (Color online)
In contrast to ground stone artifacts preserved in a buried context, bedrock mortars and metates are features exposed to erosional and depositional processes. Wind and rain may potentially alter bedrock surfaces both physically and chemically, which can affect the depositional sequence and preservation of starch granules within the milling stone surface. Postdepositional taphonomy and environmental contamination may therefore confound interpretations and must be addressed and controlled for in such archaeological studies (Copeland and Hardy Reference Copeland and Hardy2018; García-Granero Reference García-Granero2020). This project examines the potential for environmental contamination on the surfaces of bedrock metates from three sites in southern Oregon. We compare starch granule yield of extraneous material cleaned from the grinding surface to granule yield extracted from interstitials deeper within the bedrock.
Methods
Bedrock Metates
A total of 58 bedrock surfaces from Warner Valley in southern Oregon were sampled for surface and interstitial starch; 28 from Barry Spring, 24 from Long Lake, and 6 from Corral Lake (Figure 3; Supplemental Table 1). These bedrock features occur on basalt (fine-grained volcanic) rims situated around the edges of dried sink-lake beds. These basalt rims contain petroglyph panels with thousands of individual design elements (Cannon and Ricks Reference Cannon, Ricks and Quinlan2007). Milling surfaces and the associated rock art show varying degrees of patina, a characteristic that researchers believe reflects the long-lasting use of the site for seasonal plant gathering and processing over the last 14,000 years (Cannon and Ricks Reference Cannon and Ricks1999, Reference Cannon, Ricks and Quinlan2007, Reference Cannon and Ricks2014; Middleton et al. Reference Middleton, Smith, Cannon and Ricks2014; Ricks Reference Ricks1995).
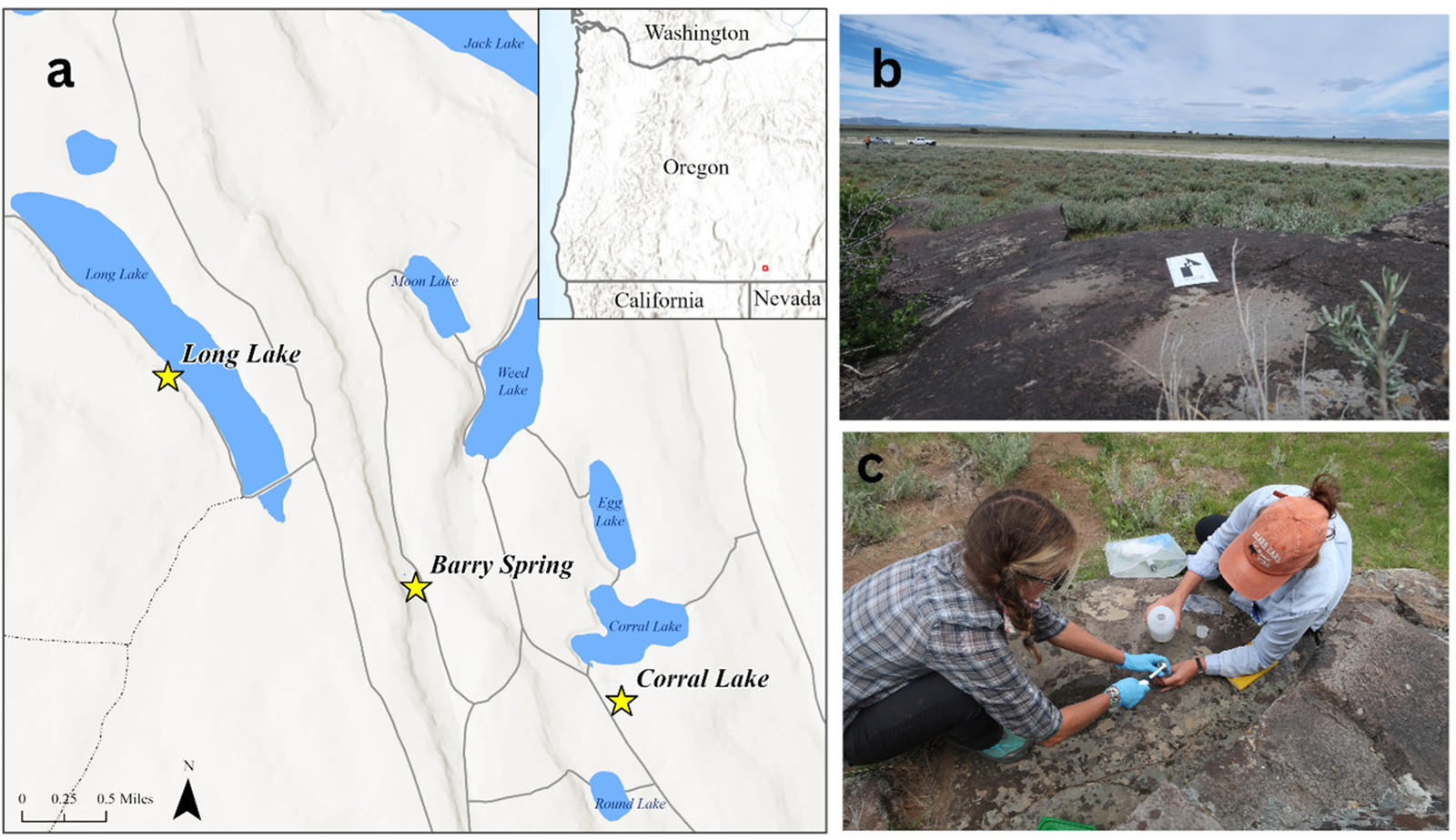
Figure 3. (a) Samples from three Late Pleistocene/Early Holocene sites in Warner Valley in southern Oregon were used in this study, (b) exposed bedrock metates near Long Lake Playa (photo courtesy of Stefania L. Wilks), (c) samples were extracted by authors Wilks and Louderback according to field-collection protocol (photo courtesy of Carolyn Temple). (Color online)
Starch Granule Analysis
Surface and Interstitial Samples
The bedrock metates sampled in this project are located in open-air settings. All loose sediment found on the features is assumed to be comprised of material that came into contact with the milling surface after final use. These surface samples were collected on the same surface prior to and separately from the interstitial plant residues (e.g., starch, pollen, phytoliths) lodged deep in the cracks and crevices of the metate surfaces.
Starch Sample Collection in the Field
Consumable supplies, such as disposable nitrile gloves, ultrasonicating brush heads, syringes, and test tubes, were only used once to prevent cross-contamination during sampling in the field and processing in the laboratory. First, any loose sediment on bedrock metate surfaces was cleaned with a sterile brush before sampling to remove any obvious debris. Next, 50 ml of distilled water (DH20) was applied while using a sonicating toothbrush for approximately five minutes to clean loose material from around the surface (Figure 4a). The DH20 solution was transferred to a 50 ml test tube labeled as “surface extract” with the associated site and feature numbers (Figure 4b). This was repeated until the sampled material was clear. Once the surface was cleaned of extraneous material, about 50 ml of a 2.5% solution of sodium hexametaphosphate (Na-Hex) was added to the bedrock surface to deflocculate residue that was embedded more deeply in the stone's interstitial matrix. The solution was left to soak for approximately one hour. The area was again thoroughly sonicated with about 50 ml of DH20 for five minutes. The residual serum was transferred with a syringe to a 50 ml tube and labeled as “interstitial extract”; presumably it contained archaeological residue (Figure 4d).

Figure 4. (a) Separate sterile-tipped ultrasonic toothbrush heads and DH20 were used to collect all starch residue samples; (b) the first sample collected was labeled as surface extract; (c) a deflocculant was applied and the area was again sonicated; (d) the archaeological sample was labeled as interstitial extract (photographs [a] and [b] courtesy of Stefania L. Wilks; [c] and [d] courtesy of Carolyn Temple). (Color online)
Control Samples
Thirteen control samples (one from Corral Lake, six from Barry Spring, and six from Long Lake) were taken from a noncultural surface of bedrock stone approximately 10 m from the sampled bedrock metate and thus presumably away from human processing activity. These control samples were collected and processed in the same way as the milling surface and interstitial samples. The purpose is to compare the control sample residue to the surface and archaeological residues extracted from the bedrock metate. Although noncultural stone surfaces in the vicinity of bedrock metates may have been contaminated with starch from associated plant materials, this source would have insignificant levels of starch granules when compared to starch granules pressed into the cracks and crevices on the surfaces of features and artifacts during cultural activities. Control samples were labeled in the field as they related to features and numbered consecutively as they were processed in the laboratory.
Laboratory Analysis
Samples extracted in the field were transported to the Archaeobotany Lab at the Natural History Museum of Utah and processed for starch analysis according to lab protocol (Louderback et al. Reference Louderback, Field and Janetski2015). Each sample was sieved through a 125 μm mesh Endecott sieve into a beaker using deionized water (DiH20). Sample solution more than 125 μm was discarded, while the rinsed sample solution <125 μm was retained, transferred to a 50 ml test tube, and centrifuged for three minutes at 3,000 RPM. The supernatant was discarded, and the sample pellet was transferred to a 15 ml test tube and resuspended with DiH20, mixed with a vortex, and centrifuged for three minutes at 3,000 RPM.
After the supernatant was discarded, approximately 7 ml of Lithium heteropolytungstate (LST: specific gravity 2.35) was added to the sample and then centrifuged for 15 minutes at 1,000 RPM. Using a pipette, starch residues were removed from the top 1–2 mm layer of the heavy liquid and then transferred to a 15 ml test tube. To remove residual heavy liquid, each sample was rinsed two to three times with about 10 ml of DiH20 and centrifuged for three minutes at 3,000 RPM. The sample was then decanted and resuspended with around 7 ml of Acetone, mixed with a vortex, and centrifuged for three minutes at 3,000 RPM. All the samples were then decanted for a final time and left to dry overnight, uncapped but covered by a paper towel. Samples were rehydrated with 50% DiH20 and 50% glycerol and mounted on a glass slide for microscopic observation. Each slide was scanned in its entirety with a Zeiss Axioscope 2 microscope. Starch granules were counted and photographed with a Zeiss AxioCam MRc5 (60N-C 1" 1,0×) under 400× magnification using Zen software (version 3.1). In this study, we compared the yields of starch granules between surface and interstitial samples; therefore, no taxonomic identifications were reported (see Wilks et al. Reference Wilks, Louderback, Simper and Cannon2024).
Results
Starch Granule Analysis
Surface and Interstitial Samples. Two (3%) of the 58 surface samples yielded a total of two starch granules, whereas 44 (76%) of the 58 interstitial samples yielded between 1 and 115 granules, for a total of 644 granules (Table 1). A significantly greater number of starch granules were recovered from deep within the cracks and crevices than from the surface.
Table 1. Total Number of Starch Granules Observed in Metate Surface, Metate Interstitial, and Control Samples from the Three Archaeological Sites.
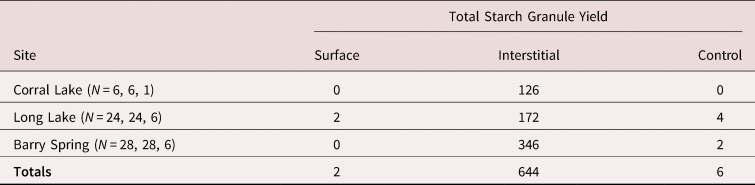
Note: Sample sizes for each datum are indicated in parentheses.
Control Samples. Control samples associated with each site yielded significantly fewer starch granules than the interstitial samples overall (Table 1). Two of six control samples from Barry Spring yielded only two starch granules, and two of six control samples from Long Lake yielded a total of four starch granules. Control samples were almost always taken on a noncultural rock surface that was adjacent to (but not on the same surface as) the cultural bedrock metates. There was one exception, however: one of the control samples from Long Lake was collected on an unworked bedrock surface adjacent to five milling surfaces. This sample produced three starch granules of the same plant taxon represented in the interstitial and surface samples (Wilks et al. Reference Wilks, Louderback, Simper and Cannon2024). Given that we have evidence for the processing of the same plant taxon on those specific bedrock metates, it is not surprising that surfaces in the vicinity may have been contaminated with starch from associated plant materials.
Discussion
This study presents a novel method for collecting starch samples from open-air bedrock metate features. Unlike wind-dispersed pollen, starch granules are released from plant parts by mechanical forces during grinding and pounding on rock surfaces. Collecting surface samples separately from interstitial samples ensures that starch granules recovered from metates have been embedded deep in cracks and crevices by human processing. Collecting control samples from noncultural stone surfaces adjacent to bedrock metates provides a measure of environmental deposition for comparison to granules from archaeological samples. Our study suggests that environmental contamination can be managed if separate surface and control samples are collected and analyzed in conjunction with the interstitial samples. Such practices increase confidence in starch residue results and therefore improve our understanding of past human dietary behavior.
This study also found that open-air metate surfaces contained lichen tissues that subsequently introduced fungi and bacteria into the samples. As a result, we suggest that such contamination caused enzymatic damage to the starch samples in the form of surficial pitting, loss of birefringence due to hydrolyzation of amylose by amylase, and other kinds of damage (see Figure 5; Blazek and Gilbert Reference Blazek and Gilbert2010; Haslam Reference Haslam2004; Hutschenreuther et al. Reference Hutschenreuther, Watzke, Schmidt, Bűdel and Henry2017). Damage to starch samples could be remediated by processing samples immediately on return from the field or by refrigeration.

Figure 5. Evidence of potential enzymatic damage to starch residues caused by fungal contamination includes surficial pitting and loss of birefringence in polarized lighting: (a) undamaged Lomatium spp. starch granule; (b) damaged Lomatium spp.; (c) undamaged Triticeae (wild rye); (d) damaged Triticeae starch granule. (Color online)
Starch contamination can occur in a variety of contexts, including field collection (Dozier Reference Dozier2016; Fullagar Reference Fullagar, Torrence and Barton2006; Hart Reference Hart2011; Laurence et al. Reference Laurence, Thoms, Bryant and McDonough2011; Ma et al. Reference Ma, Zhang, Li, Perry and Yang2017; Mercader et al. Reference Mercader, Abtosway, Baquedano, Bird, Dıez-Martın, Domınguez-Rodrigo and Favreau2017; Washburn et al. Reference Washburn, Shipkova and Pelleymounter2014) and laboratory processing (Barton Reference Barton2007; Crowther et al. Reference Crowther, Haslam, Oakden, Walde and Mercader2014; Louderback et al. Reference Louderback, Field and Janetski2015; Torrence and Barton Reference Torrence and Barton2015). Current research provides information and protocols on how best to minimize starch contamination both in the laboratory (Crowther et al. Reference Crowther, Haslam, Oakden, Walde and Mercader2014; Torrence and Barton Reference Torrence and Barton2015) and in the field (Fullagar Reference Fullagar, Torrence and Barton2006; Mercader et al., Reference Mercader, Abtosway, Baquedano, Bird, Dıez-Martın, Domınguez-Rodrigo and Favreau2017). However, most of those studies deal with artifacts in a buried context (Hart Reference Hart2011; Mercader et al. Reference Mercader, Abtosway, Baquedano, Bird, Dıez-Martın, Domınguez-Rodrigo and Favreau2017) or in museum collections (Barton Reference Barton2007; Louderback et al. Reference Louderback, Field and Janetski2015). For example, Hart (Reference Hart2011) examined ceramic artifacts exhumed from plowed fields and compared plant microfossil (starch and phytoliths) yields from sediments adhering to the artifacts to those from the surrounding soil. He concluded that environmental contamination is low because plant microfossils from the surrounding environment do not transfer into the interstices of ceramic artifacts.
Ground stone artifacts and bedrock milling features exposed to open-air environments are subject to erosion and deposition that could affect starch grain yields (Dozier Reference Dozier2016; Laurence et al. Reference Laurence, Thoms, Bryant and McDonough2011; Mercader et al. Reference Mercader, Abtosway, Baquedano, Bird, Dıez-Martın, Domınguez-Rodrigo and Favreau2017). But as in Hart (Reference Hart2011), we find little evidence for this because deeply embedded starch appears to be intransient unless vigorously extracted and atmospheric transfer is essentially negligible. Examining the depositional sequence of starch granules on open-air metate surfaces and on noncultural surfaces is crucial to understanding starch taphonomy and increasing overall confidence in archaeological starch research.
Acknowledgments
The authors would like to thank Bill Cannon and Carolyn Temple for generously giving their time and expert guidance in the field. In addition, this paper benefited greatly from the detailed critique of Juan Jose Garcia-Granero and an anonymous reviewer, thank you.
Funding Statement
This work was supported by the Lakeview Bureau of Land Management under Cooperative Agreement L20AS00005, and a University of Utah Undergraduate Research Opportunity Grant awarded to Samantha A. Paredes and Stefania L. Wilks.
Data Availability Statement
Starch granule measurements, descriptions, and images are available on Dryad digital repository (https://doi.org/10.5061/dryad.tqjq2bw52).
Competing Interests
The authors declare none.








