Major depressive disorder and bipolar disorder can occur in quite young children. However, since they are more common in adolescence than in childhood, and there is a greater evidence base for medication use in this age group, we focus here primarily on adolescents.
Prescribing in depression
Background
The prescribing of ‘new-generation antidepressants’ to children and adolescents with depression has been controversial in the UK since the publication of the Committee on Safety of Medicines (CSM) report in 2003 (Committee on Safety of Medicines 2003a). This report highlighted the important matter of the non-publication of negative trial results, and questioned both the effectiveness and safety of these medications, particularly an increased risk of suicidality. The controversy over effectiveness has now spread to prescription of these drugs to adults (Reference Kirsch, Deacon and Huedo-MedinaKirsch 2008). Concerns have also been expressed regarding the unbalanced media coverage of these issues and the potential detrimental effect of such negative publicity on the treatment of major depressive disorder (Reference Nutt and MaliziaNutt 2008). Before discussing the practicalities of prescribing, we will review the evidence for the CSM findings.
The tricyclic antidepressants will not be discussed here, as the adverse side-effect profile associated with these drugs outweighs the relatively small benefits that may be gained (Reference Varley and McClellanVarley 1997; Reference Hazell, O'Connell and HeathcoteHazell 2002).
Evidence base for effectiveness and suicidality
Placebo-controlled randomised trials of new-generation antidepressants
The CSM report concluded that, of the new-generation antidepressants, only fluoxetine shows a positive risk–benefit ratio in treating depression in young people when compared with a drug placebo (Committee on Safety of Medicines 2003a).
A more recent meta-analysis of new-generation antidepressants for paediatric major depressive disorder, obsessive–compulsive disorder (OCD) and non-OCD anxiety disorders (Reference Bridge, Iyengar and SalaryBridge 2007) examined 15 randomised controlled trials (RCTs). It found a pooled difference for response of 11% between drug and placebo that significantly favoured the antidepressants (number needed to treat, NNT = 10). However, this effect was more modest for major depressive disorder than for OCD or anxiety, and only fluoxetine showed a significant benefit. Fluoxetine also showed a larger effect overall (difference 20%, NNT = 5) consistent with the findings of the CSM. Bridge and colleagues concluded that, overall, new-generation antidepressants are efficacious when compared with placebo, but other authors have been more circumspect (Reference Hetrick, Merry and McKenzieHetrick 2007; Reference Tsapakis, Soldani and TondoTsapakis 2008). Hetrick and colleagues highlighted methodological problems with the trials, which may inflate effect sizes, and also questioned whether the improvement found with fluoxetine is clinically meaningful.
What are we to make of these findings? First, although the overall effects of new-generation antidepressants appear modest, many of the trials excluded suicidal young people and none included the most severe, complex cases that would commonly be treated with antidepressants by child and adolescent mental health services (CAMHS) in the UK. Therefore, we do not know how the most vulnerable depressed children and adolescents would respond. There is some indication from the adult literature that greater effects are seen in more severe depression as a result of a reduced placebo response (Reference Kirsch, Deacon and Huedo-MedinaKirsch 2008), and increased severity of depression has also been associated with reduced placebo effects in children and adolescents (Reference Bridge, Birmaher and IyengarBridge 2009). Therefore it is likely that antidepressant/placebo differences would be greater for severe paediatric depression.
Second, it can be argued that the overall response seen with antidepressants also needs to take into account the sizeable placebo effect (up to 50% in children and adolescents) since placebos cannot be prescribed in clinical practice (Reference Nutt and MaliziaNutt 2008).
Finally, although the effect size of antidepressants compared with placebo appears to be modest, do the psychological treatments fare any better when compared with placebo? Many psychological treatment trials have used non-active comparators such as waiting lists and have, unsurprisingly, found large effects. Thus far, only one psychological treatment trial involving adolescents has used a pill placebo and this compared cognitive–behavioural therapy (CBT) with both fluoxetine and pill placebo (Treatment for Adolescents With Depression Study (TADS) Team 2004). At 12 weeks, the overall effect size for depressive symptoms relative to placebo was 0.68 for fluoxetine (a moderate to large effect) and −0.03 for CBT alone (equivalent to placebo); fluoxetine was significantly superior to CBT (effect size 0.66).
Suicidality
Meta-analyses conducted since the CSM report (Committee on Safety of Medicines 2003a) generally indicate that there appears to be a small, non-significant increased risk of suicidality with the new-generation antidepressants compared with placebo, but no deaths by suicide have been reported (Reference Dubicka, Hadley and RobertsDubicka 2006; Reference Hammad, Laughren and RacoosinHammad 2006; Reference Bridge, Iyengar and SalaryBridge 2007). One analysis, for example, reported a rate of 4.8% for all suicide-related events and self-harm in children and adolescents receiving new-generation antidepressants v. 3.0% for those receiving placebo in the short term (Reference Dubicka, Hadley and RobertsDubicka 2006). Although these findings are concerning, a number of other issues need to be considered.
First, in most trials suicidality was examined in clinical notes retrospectively and there was no predetermined agreed definition of suicidal events or standardised way of data collection, weaknesses that undermine the certainty of their findings (Reference Kutcher and GardnerKutcher 2008). Only TADS examined suicidality prospectively. This four-arm study (fluoxetine; CBT; fluoxetine plus CBT; pill placebo) found a reduction in suicidality in all four groups and no significant differences between groups for suicidal ideation at 12 weeks (Reference Emslie, Kratochvil and VitielloEmslie 2006). However, there did appear to be an increased risk of suicidal events in adolescents receiving fluoxetine alone at both 12 and 36 weeks (Treatment for Adolescents With Depression Study Team (TADS) 2007).
Second, evidence from other sources does not consistently support an increased risk of suicidality. For example, the risk is highest in the month before treatment is started (Reference Simon, Savarino and OperskalskiSimon 2006); longer-term treatment is associated with a reduction in suicide attempts (Reference Valuck, Libby and SillsValuck 2004) and fewer attempts occur if depression has been treated with antidepressants (Reference Gibbons, Brown and HurGibbons 2007a). As regards completed suicide, antidepressants are rarely found in post-mortem examinations of child and adolescent suicides (Reference Leon, Marzuk and TardiffLeon 2006), ecological studies do not support an increased risk of suicide with the general rise in antidepressant prescribing (Reference Baldessarini, Tondo and StrombomBaldessarini 2007), and the decrease in antidepressant prescribing in the USA and The Netherlands following the public health warnings on their use has been associated with an increase in child and adolescent suicide (Reference Gibbons, Brown and HurGibbons 2007b). Although a similar association has not been reported in the UK (Reference Wheeler, Gunnell and MetcalfeWheeler 2008), a UK study confirms that affective disorder remains the most common psychiatric diagnosis in child and adolescent suicides and that new-generation antidepressants had been prescribed to only 8% of those who had taken their lives (Reference Windfuhr, While and HuntWindfuhr 2008).
Third, the antidepressant trials excluded the most suicidal children and adolescents, so the balance of risks and benefits remains speculative in this group.
Last, it is mental illness that remains untreated owing to a reluctance to seek help that has been most strongly implicated in child and adolescent suicides, rather than the adverse effects of receiving treatment (Reference Moskos, Olson and HalbernMoskos 2007).
Overall, therefore, the evidence suggests that anti-depressants are an important therapeutic option for moderate to severe adolescent depression, and although clinicians need to continue to monitor the risk of suicidality, this is likely to be far greater in untreated depression.
Guidelines for antidepressant use
The National Institute for Health and Clinical Excellence (NICE) guidelines on the management of depression in children and adolescents advocate the use of the selective serotonin reuptake inhibitor (SSRI) fluoxetine as the first-line pharmacological treatment, with citalopram and sertraline as the second-line choice (National Collaborating Centre for Mental Health 2005). This is broadly consistent with the guidelines produced by the Texas Consensus Conference Panel on Medication Treatment of Childhood Major Depressive Disorder in the USA (Reference Hughes, Emslie and CrismonHughes 2007), although the Texas guidelines advise the use of any of these three SSRIs as a first-line medication. However, NICE advises starting SSRIs only in combination with a specific psychological treatment, and only if there has been no response to psychological treatment over 4–6 weeks. The Texas guidelines advise a non-specific treatment intervention initially, which concurs with our findings from the UK Adolescent Depression Antidepressant and Psychotherapy Trial (ADAPT) (Reference Goodyer, Dubicka and WilkinsonGoodyer 2007), in which 20% of adolescents with moderate to severe depression responded to our brief ‘specialised treatment as usual’ (STAU).
Key points of pharmacological treatment guidelines and drug licensing are summarised in Table 1.
TABLE 1 Pharmacological treatment for depression in children and adolescents: UK and US guidelines and licensing

| Guideline advice in terms of pharmacological treatment | Minimum age for licensed prescribing, years | |||
|---|---|---|---|---|
| Drug | NICE | Texasa | UK | USA |
| Citalopram | Second-line | First-line | ≥18 | ≥18 |
| Escitalopram | Not discussed | Second-line | ≥18 | ≥12 |
| Fluoxetine | First-line | First-line | ≥8b | ≥8 |
| Paroxetine | Contraindicated by CSM | Second-linec | ≥18 | ≥18 |
| Sertraline | Second-line | First-line | ≥18 | ≥18 |
| Venlafaxine | Contraindicated by CSM | Third-line | ≥18 | ≥18 |
Specialised treatment as usual
In CAMHS, specialised treatment as usual (Box 1) amounts to active routine clinical care and we suggest that it be adopted as the initial intervention for depression, before medication or more specialised psychological treatments are added (Reference Kelvin, Wilkinson, Goodyer, Rey and BirmaherKelvin 2009). The duration of initial STAU should depend on the response to treatment and the severity of the presentation. In more severe depression, with continuing significant impairment, suicidality and/or psychosis, we suggest that antidepressant treatment be started after 2–4 weeks of non-response; for patients at high risk, antidepressants might be prescribed even sooner. Individuals who do not wish or are unable to take medication and those with less severe depression who fail to respond to initial STAU should be offered specialised psychological treatment. As recommended in similar frameworks suggested in other guidelines (Reference Hughes, Emslie and CrismonHughes 2007; American Academy of Child and Adolescent Psychiatry 2007a), SSRIs should not be given without STAU.
BOX 1 Specialised treatment as usual in CAMHS
-
• Engagement
-
• Empathic, reflective framework
-
• Formulation
-
• Instilling hope and managing expectations
-
• Psychoeducation
-
• Mental state monitoring
-
• Risk assessment and management, especially suicidality
-
• Treating comorbid illnesses
-
• Family work
-
• Addressing parents' mental health issues
-
• Context management
-
• Liaison with schools and services
-
• Problem-solving
-
• Addressing alcohol and substance use
-
• Advising on diet, sleep, exercise and activities
-
• Relapse prevention work
The ADAPT study found that CBT plus STAU did not confer any additional advantage in terms of improved clinical outcomes or a protective effect against suicidality over and above an SSRI plus STAU. This questions the need for more specialised specific psychological treatment as an initial adjunct to medication in order to effect remission within 28 weeks of the start of treatment.
Prescribing
Starting doses should be low (e.g. fluoxetine 10 mg) and the dose should be gradually titrated according to response and side-effects. Although there is a widely held belief regarding delayed onset of action of antidepressants, this does not concur with trial data in adults, which suggest that 35% of eventual improvement occurs in the first week (Reference Posternak and ZimmermanPosternak 2005). As regards continuation treatment, current evidence suggests that continuing antidepressants for 6 months after an adequate response at 12 weeks significantly reduces the risk of relapse when compared to placebo (Reference Emslie, Kennard and MayesEmslie 2008). The NICE guidelines on child and adolescent depression recommend continuing treatment for at least 6 months after recovery (full functioning for 8 weeks and no symptoms) (National Collaborating Centre for Mental Health 2005).
Discontinuation should be gradual (Reference Hughes, Emslie and CrismonHughes 2007; Reference Anderson, Ferrier and BaldwinAnderson 2008) as discontinuation symptoms may occur, although fluoxetine is less likely to lead to such symptoms because of its long half-life. These symptoms are usually self-limiting and of short duration. The Texas guidelines suggest tapering the dose by 25% per week over 2–3 months, and NICE suggests tapering over 6–12 weeks. Before stopping medication, consideration needs to be given to any current stresses that may increase the risk of relapse and may make discontinuation unadvisable. In addition, those with a history of chronic depression or a history of recurrence should be considered for longer maintenance treatment of more than a year (American Academy of Child and Adolescent Psychiatry 2007a). Relapse is most likely to occur within the first 6 months following discontinuation, so close monitoring is recommended during this time.
Adverse effects
The SSRIs are associated with side-effects such as sedation, insomnia and gastrointestinal disturbances, although these are uncommon (< 5%) (Reference Emslie, Kratochvil and VitielloEmslie 2006). Even more rarely, SSRIs can induce bleeding, serotonin syndrome, mania and agitation (Reference Cheung, Emslie and MayesCheung 2005; Reference Emslie, Kratochvil and VitielloEmslie 2006; American Academy of Child and Adolescent Psychiatry 2007a; Reference Anderson, Ferrier and BaldwinAnderson 2008).
Predictors of response
Factors associated with better response to treatment include younger age, less severe depression, higher functioning, less comorbidity and suicidality, lower levels of family conflict and fewer adverse life events (Reference Curry, Rodhe and SimonsCurry 2006; Reference Asarnow, Emslie and ClarkeAsarnow 2009; Reference Wilkinson, Dubicka and KelvinWilkinson 2009); however, more severe depression has also been associated with a larger drug v. placebo response (Reference Bridge, Birmaher and IyengarBridge 2009). Better response to SSRIs has also been found in children and adolescents with anxiety and depression who are homozygous for the more functional long allele of the promoter of the serotonin transporter gene (Reference Kronenberg, Apter and BrentKronenberg 2007).
Failure to respond
About one-third of adolescents fail to show an adequate response to antidepressants (Box 2). The rate of improvement in the early weeks of treatment is a good indicator of eventual remission (Reference Rongrong, Emslie and MayesRongrong 2009). Before considering alternative treatment strategies, reassessment is required to review possible reasons for non-response. These can include misdiagnosis, comorbidity, inadequate treatment, non-adherence, side-effects and life events (American Academy of Child and Adolescent Psychiatry 2007a). After reassessment, increasing the dose (up to 40 mg with fluoxetine, depending on the side-effect profile) may be a reasonable first strategy before considering switching, although there is limited evidence for this (Reference Anderson, Ferrier and BaldwinAnderson 2008).
BOX 2 Predictors of poorer outcome with treatment in depression
-
• Older age
-
• Severity of depression
-
• Obsessive–compulsive disorder
-
• Suicidality
-
• Greater impairment
-
• Disappointing life events
-
• Melancholia
-
• Hopelessness
-
• Chronicity of depression
-
• Poor social function
-
• Two or more comorbid disorders
-
• Family conflict
-
• Low expectations
The NICE guidelines on child and adolescent depression recommend using either sertraline or citalopram if there is no response to fluoxetine, and this advice is consistent with the results of the more recent Treatment of SSRI-Resistant Depression In Adolescents (TORDIA) study (Reference Brent, Emslie and ClarkeBrent 2008). This study found that in adolescents who failed to respond to an SSRI, switching to another SSRI was as effective as switching to the serotonin-noradrenaline reuptake inhibitor venlafaxine at 12 weeks, with fewer adverse effects. Nearly half of these treatment-resistant adolescents showed a positive response to a second-line SSRI. Direct switching of SSRIs without a washout period seems to be well tolerated (Reference Anderson, Ferrier and BaldwinAnderson 2008). The Texas guidelines include escitalopram and paroxetine as second-line SSRIs, although the evidence base for escitalopram is limited (Reference Wagner, Jonas and FindlingWagner 2006). Note that in the UK paroxetine is currently contraindicated for patients under 18 years of age because of its adverse risk–benefit profile (Committee on Safety of Medicines 2003b).
With regard to third-line treatment, the Texas guidelines suggest using an antidepressant from a different class. However, there have been no positive RCTs of other classes of antidepressants in children and adolescents. Again, note that venlafaxine is contraindicated in the UK for use in people under 18, owing to its adverse risk–benefit profile (Committee on Safety of Medicines 2003c). The Texas guidelines also suggest augmentation strategies in the event of partial response to an SSRI, but the evidence base for this in children and adolescents is sparse.
Adjunctive psychological treatment
Although the NICE guidelines on child and adolescent depression advise the prescription of antidepressants together with a specialised psychological treatment, the ADAPT study and other treatment guidelines (American Academy of Child and Adolescent Psychiatry 2007a; Reference Hughes, Emslie and CrismonHughes 2007; Reference Anderson, Ferrier and BaldwinAnderson 2008) would suggest that this is not always necessary. TADS reported an advantage of combining fluoxetine with CBT over fluoxetine alone (without STAU), although this advantage was lost in more severe cases (Reference Curry, Rodhe and SimonsCurry 2006). The TORDIA study also reported an advantage of combined treatment (Reference Brent, Emslie and ClarkeBrent 2008), but this effect was not consistent on all measures. Two other studies in addition to ADAPT did not find an advantage (Reference Clarke, Debar and LynchClarke 2005; Reference Melvin, Tonge and KingMelvin 2006). Although TADS indicated that CBT was protective against suicidality when combined with fluoxetine, neither the ADAPT nor the TORDIA studies found this effect and a meta-analysis of these treatment studies found a limited effect of combined treatment (Reference Dubicka, Elvins and RobertsDubicka 2010). We would therefore suggest that an adjunctive specialised psychological treatment should be targeted at children and adolescents who are receiving STAU and do not respond to antidepressants and require either treatment augmentation or a change of treatment. Currently the strongest evidence base for psychological treatments exists for CBT and interpersonal psychotherapy (IPT) (American Academy of Child and Adolescent Psychiatry 2007a).
Adjunctive pharmacological treatment
In psychotic depression the addition of an atypical antipsychotic may need to be considered, although the evidence base for this is limited (Reference Hughes, Emslie and CrismonHughes 2007; Reference Anderson, Ferrier and BaldwinAnderson 2008). The choice of antipsychotic should be determined on an individual basis after a discussion of side-effect profiles with the young person and their family. The dose should be continued for 2–3 months following remission of psychotic symptoms and then slowly discontinued (Reference Hughes, Emslie and CrismonHughes 2007).
There is some evidence that antipsychotics may be effective as an augmentation strategy in treatment-resistant depression in adults (Reference Anderson, Ferrier and BaldwinAnderson 2008), but there remains little evidence to guide clinicians in the management of children and adolescents with treatment resistance. Various strategies have been tried with adults but any extrapolation from the adult literature to children and adolescents needs to be treated with caution (for review, see Reference Anderson, Ferrier and BaldwinAnderson 2008).
Alternative treatment strategies
Interestingly, a small study found a significant effect, compared with placebo, for omega-3 fatty acids in alleviating depression in children (Reference Nemets, Nemets and ApterNemets 2006). There is some evidence that omega-3 fatty acids are effective as an augmentation strategy in treatment-resistant depression in adults (Reference Anderson, Ferrier and BaldwinAnderson 2008).
St John's wort appears to be effective for depression in adults (Reference Szegedi, Kohnen and DienelSzegedi 2005). Although results from an open-label study of St John's wort for juvenile depression are promising (Reference Findling, McNamara and O'RiordanFindling 2003), data relating to its use in children and adolescents remain limited and NICE does not recommend it for children and adolescents.
Prescribing in bipolar disorder
Diagnostic issues
The diagnosis of bipolar disorder and mania in children and adolescents is controversial. Pre-pubertal mania is recognised more commonly in the USA than in the UK (Reference Dubicka, Carlson and VailDubicka 2008) and more individuals, including adults, are being diagnosed with an ‘atypical’ presentation of bipolar disorder (Reference CarlsonCarlson 2005). Rates of diagnosis of bipolar disorder have increased dramatically in recent years in the USA (Reference Moreno, Laje and BlancoMoreno 2007), but it is not clear whether this is due to increased recognition, increased prevalence or overly inclusive diagnoses.
This review will focus on the DSM–IV–TR (American Psychiatric Association 1994) and ICD–10 (World Health Organization 1992) definitions of bipolar disorder (Box 3), which are broadly consistent with existing guidelines regarding diagnosis and treatment (American Academy of Child and Adolescent Psychiatry 2007b). The NICE guidelines on bipolar disorder specify that adult criteria should be used with children and adolescents but that mania must be present (not just depression and a family history of bipolar disorder); that euphoria should be present most of the time for at least a week; and that irritability should not be used as a core criterion, except in older adolescents, since this symptom is non-specific in younger children (National Collaborating Centre for Mental Health 2006). However, controversy still remains regarding the interpretation of core symptoms, namely elation or grandiosity, as it depends on the subjective view of the clinician (American Academy of Child and Adolescent Psychiatry 2007b; Reference Dubicka, Carlson and VailDubicka 2008). The NICE guidelines also state that bipolar II disorder should not be diagnosed in children and younger adolescents, in view of the uncertainties regarding the diagnostic criteria.
BOX 3 DSM–IV–TR and ICD–10 criteria for bipolar affective disorder

| DSM–IV–TR a | ICD–10 b |
| Manic episode | Manic episode |
| Mood elation, expansiveness or irritability Minimum 7 days' duration (less if admitted to hospital) Specify three additional listed symptoms (four if irritability present), e.g. grandiosity, decreased need for sleep, pressured speech, flight of ideas Marked impairment or hospital admission or psychosis Mixed episode excluded |
Mood elation or irritability or suspiciousness Minimum 7 days' duration Several other symptoms should be present in addition to mood change and increased energy (number of symptoms not specified) Significant impairment Psychosis may be present |
| Hypomania | Hypomania |
| Lesser degree of mania (no psychosis) | Lesser degree of mania (no psychosis) |
| Bipolar I | Bipolar affective disorder |
| Presence of manic or mixed episode (therefore can be diagnosed in absence of depression); minimum 7 days' duration (less if admitted to hospital) Significant impairment | At least two episodes where mood (hypomania/mania and depression) and activity levels are significantly disturbed |
| Bipolar II | |
| Periods of major depression and hypomania lasting at least 4 days | |
| Rapid cycling | |
| At least four mood episodes in one year |
The prognostic and treatment implications of subthreshold bipolar disorder not meeting diagnostic criteria are unclear, but there is some evidence to suggest that, although only a minority of affected individuals develop a more classic disorder over 4 years, young people with subthreshold symptoms show lower rates of recovery compared with those with bipolar I or II disorder, and also have similar levels of impairment and suicidality (Reference Birmaher, Axelson and GoldsteinBirmaher 2009). However, it remains unknown how many will progress to more classic presentations in adulthood, and therefore the reliability of early diagnosis in such cases is unclear. A prospective study of children diagnosed with mania using modified criteria for elation and grandiosity demonstrated that their illness had a chronic, impaired course with strong continuity into adulthood (Reference Geller, Tillman and BolhofnerGeller 2008). However, the use of these broader diagnostic criteria in children remains a topic of much debate and is an area that requires further research (Reference GoodwinGoodwin 2009).
Evidence base for pharmacological treatment
There have been few studies of pharmacological treatment of bipolar disorder in children and adolescents (Reference Consoli, Deniau and HuynhConsoli 2007; Reference Smarty and FindlingSmarty 2007; Reference Carlson and DubickaCarlson 2010). The studies that have been published are problematic as they include few RCTs, most of which have been small and in which treatment response rates have varied widely, partly a result of inconsistent definitions of bipolar disorder and differing response criteria.
Lithium has been the most widely studied pharmacological treatment for mania in children and adolescents and in adults. Trials support its use in adults (Reference GoodwinGoodwin 2009) and early trials of lithium in children and adolescents were generally positive, although their conclusions are limited owing to methodological problems (American Academy of Child and Adolescent Psychiatry 2007b). Of the more recent placebo-controlled trials, one study in adolescents with bipolar disorder and substance dependence reported improved functioning with lithium compared with placebo (Reference Geller, Cooper and SunGeller 1998). However, another study in adolescents with mania found no difference in relapse rates between lithium and placebo (Reference Kafantaris, Coletti and DickerKafantaris 2004). Further data are therefore needed on the use of lithium in bipolar disorder and a large RCT is now underway (Reference Findling, Frazier and KafantarisFindling 2008). Interestingly, a recent placebo-controlled trial of lithium in 7- to 17-year-olds with severe mood dysregulation did not provide any support for using lithium (Reference Dickstein, Towbin and Van Der VeenDickstein 2009).
As regards anticonvulsants, a recent RCT comparing valproate semisodium (divalproex) with placebo in children and adolescents with bipolar I disorder, mixed or manic states, did not provide support for the drug (Reference Wagner, Redden and KowatchWagner 2009). An RCT of valproate in children and adolescents with bipolar-spectrum disorder who had a parent with bipolar disorder was also negative (Reference Findling, Frazier and YoungstromFindling 2007), in contrast to adult results for acute mania (Reference Fountoulakis and VietaFountoulakis 2008).
There is very little evidence to support the use of carbamazepine in children and adolescents (Reference Consoli, Deniau and HuynhConsoli 2007; Reference Smarty and FindlingSmarty 2007; Reference Carlson and DubickaCarlson 2010), although there is an evidence base for adults (Reference Fountoulakis and VietaFountoulakis 2008; Reference GoodwinGoodwin 2009).
Lamotrigine is approved for maintenance treatment in adults in the USA and is preferentially effective in depression (Reference Fountoulakis and VietaFountoulakis 2008; Reference GoodwinGoodwin 2009). Open-label studies in adolescents suggest that lamotrigine may be effective in bipolar depression as well as controlling manic symptoms (Reference Chang, Saxena and HoweChang 2006; Reference Pavuluri, Henry and MossPavuluri 2009; Reference Biederman, Joshi and MickBiederman 2010). Overall, therefore, there is little evidence currently supporting the use of anticonvulsants for bipolar disorder in children and adolescents.
There is increasing evidence that the second-generation antipsychotics are effective in adults with bipolar disorder (Reference GoodwinGoodwin 2009) and one placebo-controlled trial has been conducted involving adolescents with mania. This large study demonstrated that olanzapine was efficacious but weight gain and metabolic adverse effects were a problem (Reference Tohen, Kryzhanovskaya and CarlsonTohen 2007). Although the combination of quetiapine with valproate has been shown to be superior to valproate plus placebo in adolescents with mania (Reference DelBello, Schwiers and RosenbergDelBello 2002), this finding needs to be considered in the context of the above negative trial of valproate (Reference Wagner, Redden and KowatchWagner 2009) and, since quetiapine was not studied without adjunctive valproate, the implications of this study are unclear. Open trials suggest that risperidone may be effective (Reference Smarty and FindlingSmarty 2007).
Pharmacological treatment guidelines
Owing to the lack of a strong evidence base for treatment of bipolar disorder in children and adolescents, guidelines are largely extrapolated from the adult literature, although even this evidence base is limited (Reference Fountoulakis and VietaFountoulakis 2008). As this is a rapidly changing area, clinicians need to keep abreast of the emerging literature. Medication remains the first-line treatment for cases of bipolar disorder that meet diagnostic criteria, although caution should be used with early-onset mania as the effectiveness and safety of medication has not yet been established in this age group (American Academy of Child and Adolescent Psychiatry 2007b).
UK and US pharmacological treatment guidelines are discussed separately below and key points are summarised in Table 2.
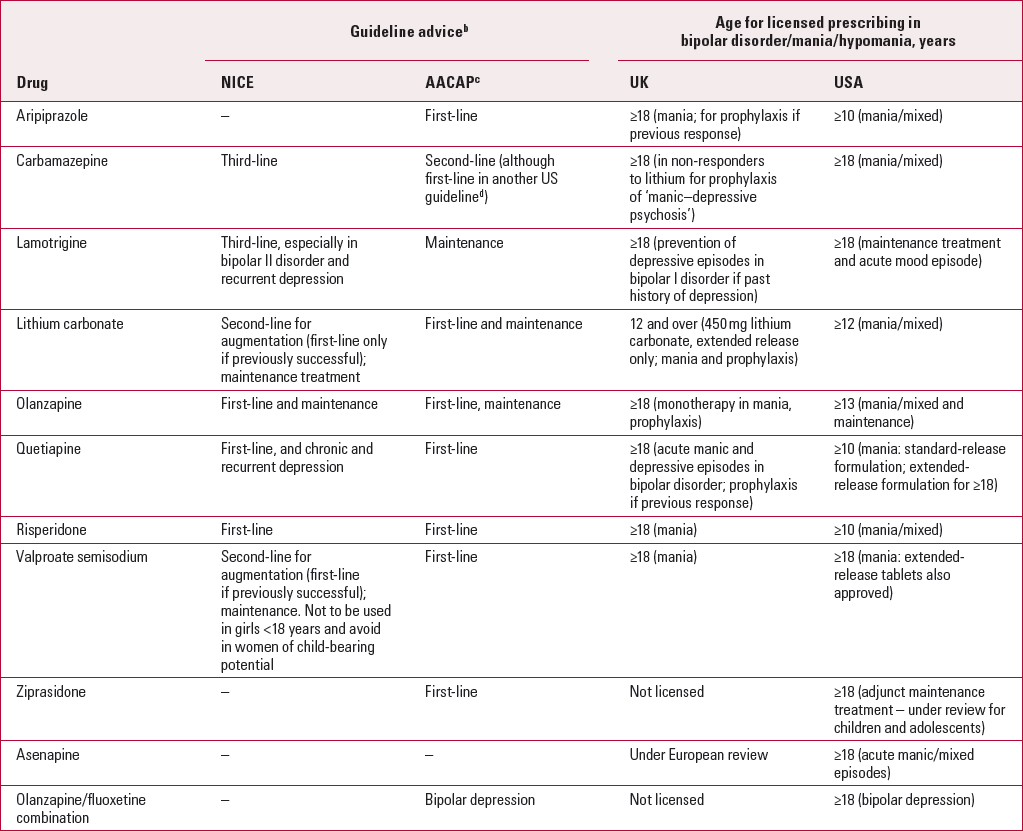
TABLE 2 Pharmacological treatment for mania/hypomania and bipolar disorder in children and adolescents: UK and US guidelines and licensinga
| Guideline adviceb | Age for licensed prescribing in bipolar disorder/mania/hypomania, years | |||
|---|---|---|---|---|
| Drug | NICE | AACAPc | UK | USA |
| Aripiprazole | – | First-line | ≥18 (mania; for prophylaxis if previous response) | ≥10 (mania/mixed) |
| Carbamazepine | Third-line | Second-line (although first-line in another US guidelined) | ≥18 (in non-responders to lithium for prophylaxis of ‘manic–depressive psychosis’) | ≥18 (mania/mixed) |
| Lamotrigine | Third-line, especially in bipolar II disorder and recurrent depression | Maintenance | ≥18 (prevention of depressive episodes in bipolar I disorder if past history of depression) | ≥18 (maintenance treatment and acute mood episode) |
| Lithium carbonate | Second-line for augmentation (first-line only if previously successful); maintenance treatment | First-line and maintenance | 12 and over (450 mg lithium carbonate, extended release only; mania and prophylaxis) | ≥12 (mania/mixed) |
| Olanzapine | First-line and maintenance | First-line, maintenance | ≥18 (monotherapy in mania, prophylaxis) | ≥13 (mania/mixed and maintenance) |
| Quetiapine | First-line, and chronic and recurrent depression | First-line | ≥18 (acute manic and depressive episodes in bipolar disorder; prophylaxis if previous response) | ≥10 (mania: standard-release formulation; extended-release formulation for ≥18) |
| Risperidone | First-line | First-line | ≥18 (mania) | ≥10 (mania/mixed) |
| Valproate semisodium | Second-line for augmentation (first-line if previously successful); maintenance. Not to be used in girls <18 years and avoid in women of child-bearing potential | First-line | ≥18 (mania) | ≥18 (mania: extended-release tablets also approved) |
| Ziprasidone | – | First-line | Not licensed | ≥18 (adjunct maintenance treatment – under review for children and adolescents) |
| Asenapine | – | – | Under European review | ≥18 (acute manic/mixed episodes) |
| Olanzapine/fluoxetine combination | – | Bipolar depression | Not licensed | ≥18 (bipolar depression) |
The NICE guidelines
The use of atypical antipsychotics rather than mood stabilisers as a first-line treatment for acute mania has become increasingly common in clinical practice (Reference GoodwinGoodwin 2009) and is supported by the current NICE guidelines (National Collaborating Centre for Mental Health 2006). The guidelines also suggest concurrent use of a benzodiazepine such as lorazepam to manage agitation if required. If antidepressant medication is being taken, this should be withdrawn abruptly or gradually, depending on clinical need and risk of discontinuation/withdrawal symptoms. In the UK, olanzapine, quetiapine, risperidone and aripiprazole are currently licensed for use in adults (18 years and over) with mania, but lithium carbonate remains the only medication licensed in this country for young people (from 12 years of age) with this disorder (although risperidone, quetiapine and aripiprazole have also been approved in the USA for people from the age of 10, and olanzapine from age 13). Lithium or valproate are suggested as a first-line treatment if either has been previously successful with the patient, although lithium is not recommended for acute mania if symptoms are severe, in view of its slower onset of action.
The guidelines recommend that valproate should not be prescribed to girls, owing to its possible association with polycystic ovarian syndrome.
It is suggested that lithium or valproate can also be used as an augmentation strategy, if an anti-psychotic alone is ineffective. NICE recommends that carbamazepine, gabapentin, lamotrigine and topiramate should not be routinely used for acute mania.
For long-term treatment, NICE advises the use of lithium, olanzapine or valproate, and either switching medications or using a second agent in the event of an inadequate response. Carbamazepine and lamotrigine should be considered if a trial of combined agents proves ineffective, although only carbamazepine is licensed for use in adults in the UK (Table 2).
The US guidelines
The US guidelines differ primarily in the recommendation of carbamazepine as a first-line treatment and the use of valproate in girls. Guidelines from the Child Psychiatric Workgroup on Bipolar Disorder (Reference Kowatch, Fristad and BirmaherKowatch 2005) suggest that carbamazepine can be used as a first-line treatment in bipolar I disorder, manic or mixed states, without psychosis, and the American Academy of Child and Adolescent Psychiatry (2007b) guidelines advise that treatment should begin with an agent that is approved by the Food and Drug Administration for use in adults, which now includes carbamazepine. Valproate is not contraindicated in girls, although clinicians are advised to be aware of the concerns regarding the development of polycystic ovaries (American Academy of Child and Adolescent Psychiatry 2007b).
Adverse effects
All mood stabilisers and second-generation antipsychotics are associated with potentially harmful adverse effects, and should therefore be used judiciously, with careful monitoring.
Antipsychotics
It appears that children and adolescents are at higher risk than adults for antipsychotic-induced hyperprolactinaemia, weight gain and, possibly, associated metabolic abnormalities (Reference Correll and CarlsonCorrell 2006).
Although there is some good evidence that olanzapine is effective in mania, it is also associated with the greatest degree of weight gain and, after risperidone, is most likely to induce hyperprolactinaemia. Therefore, careful consideration needs to be given to its use, particularly in the longer term.
Aripiprazole seems least likely to induce hyperprolactinaemia and weight gain, but there are few data on its use for mania in children and adolescents.
Extrapyramidal side-effects are also associated with antipsychotics and there is some evidence that people with bipolar disorder may be particularly vulnerable to them (Reference Gao, Kemp and GanocyGao 2008). Other important rarer adverse effects are tardive dyskinesia and neuroleptic malignant syndrome.
Mood stabilisers
Valproate has been associated with teratogenicity (Reference Nguyen, Sharma and McIntyreNguyen 2009) and weight gain, as well as polycystic ovarian syndrome, although the latter association is controversial (Reference Joshi, Robb, Geller and DelBelloJoshi 2008).
Lithium can cause a range of adverse effects, including hypothyroidism, renal impairment and lithium toxicity (Reference Fountoulakis and VietaFountoulakis 2008).
Lamotrigine has been associated with a rare but serious rash and subsequent progression to Stevens–Johnson syndrome (generally agreed to be a form of toxic epidermal necrolysis).
Principles of prescribing
In view of the paucity of the evidence base and concerns regarding potentially serious adverse effects, the risks and benefits of medication for bipolar disorder in children and adolescents need careful consideration with the family and young person. If medication is commenced, the principle is to ‘start low, go slow’, with closer monitoring than in adults (National Collaborating Centre for Mental Health 2006). Medication should be prescribed in a psychosocial therapeutic framework with an emphasis on managing overactivity, sleep hygiene, diet and structured activities. Bipolar disorder affects numerous developmental processes, including academic, social and family functioning, therefore treatment needs to be multimodal, including liaising with appropriate agencies and targeting relapse prevention (American Academy of Child and Adolescent Psychiatry 2007b).
Other adjunctive treatment
Functional family therapy (psychoeducation, medication adherence sessions, communication training, problem-solving and relapse prevention) seems to be an effective adjunct to medication for reducing depressive symptoms in adolescents with bipolar disorder over the longer term (Reference Miklowitz, Axelson and BirmaherMiklowitz 2008) and for reducing recurrence and hospital admissions in adults with the disorder (Reference Rea, Tompson and MiklowitzRea 2003).
Fluoxetine is licensed by the Food and Drugs Administration in the USA in combination with olanzapine for depression in adult bipolar disorder, but the usefulness of antidepressants for the disorder remains controversial (Reference Fountoulakis and VietaFountoulakis 2008). NICE does not recommended antidepressants for rapid-cycling or mixed affective states (National Collaborating Centre for Mental Health 2006).
Conclusions
Major depression and bipolar disorder are serious, disabling conditions in young people and medication has an important role in the treatment of both disorders. However, the risks and benefits of pharmacological treatment require careful consideration before prescribing and medication should always be given within a psychosocial treatment framework, with recourse to careful, regular review and addition of specific psychological therapies as indicated.
MCQs
Select the single best option for each question stem
-
1 The first-line antidepressant of choice for major depression in children and adolescents is:
-
a sertraline
-
b citalopram
-
c venlafaxine
-
d fluoxetine
-
e amitriptyline.
-
-
2 Components of ‘specialised treatment as usual’ in depression include:
-
a Socratic questioning
-
b working in the transference
-
c psychoeducation
-
d exploring defence mechanisms
-
e cognitive restructuring.
-
-
3 Predictors of poor treatment outcome in depression include:
-
a obsessive–compulsive disorder
-
b younger age
-
c low impairment
-
d high expectation
-
e no comorbidity.
-
-
4 According to the NICE guidelines, first-line pharmacological treatment for a first presentation of acute mania is:
-
a lithium
-
b valproate
-
c lamotrigine
-
d olanzapine
-
e fluoxetine.
-
-
5 The following medications are licensed for use in bipolar disorder in adolescents in the UK:
-
a valproate
-
b olanzapine
-
c lithium carbonate
-
d carbamazepine
-
e quetiapine.
-
MCQ answers

| 1 | d | 2 | c | 3 | a | 4 | d | 5 | c |




eLetters
No eLetters have been published for this article.