In January 2002, as part of a review of electroconvulsive therapy (ECT) undertaken by the UK’s Department of Health, the Service User Research Enterprise (SURE) published the first-ever systematic review of patients’ views on ECT (Service User Research Institute, 2002). The review encompassed several large-scale surveys by or of people who had received ECT in the UK (United Kingdom Advocacy Network, 1996; ECT Anonymous, 1999; Pedler, 2000). In April 2003, the National Institute for Clinical Excellence (now the National Institute for Health and Clinical Excellence, NICE) issued guidance on the use of ECT, and at the same time, the UK ECT Review Group published a review of its safety and efficacy (National Institute for Health and Clinical Excellence, 2003; UK ECT Review Group, 2003). The Royal College of Psychiatrists has established the ECT Accreditation Service and revised its guidelines for practitioners to take into account the NICE advice (Royal College of Psychiatrists, 2005).
Some of the conclusions to come out of the new work – in particular, that at least one-third of patients experience permanent amnesia (Service User Research Institute, 2002; Reference Rose, Fleischmann and WykesRose et al, 2003; Reference ScottScott, 2005), that half of patients had not received an adequate explanation prior to treatment (Reference Rose, Fleischmann and WykesRose et al, 2003, Reference Rose, Wykes and Bindman2005; Reference Philpot, Collins and TrivediPhilpot et al, 2004) and that newer methods of ECT have not resulted in an appreciable decrease in adverse effects (UK ECT Review Group, 2003) – suggest that changes are overdue in both practice and policy.
The new evidence presents opportunities for improving clinical care in several areas: delineating the nature of ECT’s permanent adverse effects; developing adequate and relevant tools to assess patients; and providing consent that is fully informed.
Defining deficits
It is evident from a close reading of patient reports such as those documented by SURE that ‘memory’ is too simple a term to encompass the range of ECT’s permanent adverse effects, yet there has been almost no work done on improving terminology (Box 1). The confusion goes back to the first instrument specifically designed to assess people given ECT, the Squire Memory Questionnaire (SMQ; Reference Squire, Wetzel and SlaterSquire et al, 1979). The SMQ was developed to distinguish between the cognitive impairments associated with depression and those caused by ECT. Although Squire and his colleagues believed that they had done this (Reference Squire and SlaterSquire & Slater, 1983; Reference Squire and ZouzounisSquire & Zouzounis, 1988), others have not used the test for its intended purpose, and it is hard to say whether the SMQ has muddied the water more than it has cleared it. Although often spoken of as if it measured a unitary entity ‘memory’, the SMQ actually encompasses multiple dimensions of cognition: attention, alertness, concentration, learning. It does not at all address the most common effect of ECT, which is variously called amnesia, retrograde amnesia or memory loss. By these terms is generally understood the obliteration of a specific time period in a person’s life.
Box 1 Terminology for ECT’s adverse effects
Memory
-
• Autobiographical memory: one’s store of knowledge of past experiences and learning
-
• Amnesia: the loss of autobiographical memory, i.e. the erasure of all that was thought, done and learned during a particular period. Amnesia is different from ‘forgetting’ in that the memories and knowledge are obliterated and cannot be accessed by effort or reminders
-
• Retrograde amnesia: amnesia for the period prior to ECT
-
• Anterograde amnesia: amnesia for the period after ECT (see also memory disability below)
-
• Working memory: one’s ability to store and access information in everyday life
-
• Memory disability (often called anterograde amnesia): the loss of working memory
Cognition
-
• Higher mental functions, including attention, concentration, flexibility, problem-solving, analytical and abstract thinking, reasoning, executive function, intelligence
-
• Cognitive disability: the loss of any of the higher mental functions of cognition
It is when ‘memory’ is used as a shorthand term for both retrograde amnesia and ongoing difficulties with memory function in the present that confusion ensues, a confusion that intensifies when the latter is sometimes called ‘anterograde amnesia’. Although most will understand anterograde amnesia to mean ongoing memory disability, this is not always the case. The US National Institute of Mental Health has defined anterograde amnesia as the inability to remember events that happened after ECT (National Institutes of Health, 1985) and the College’s new handbook (Royal College of Psychiatrists, 2005) appears to use it in the same way.
To confuse matters further, the term ‘short-term memory loss’ is sometimes used as a synonym for anterograde amnesia. Short term to some will mean temporary, to others it will be seen as a description of the type of memory that is affected – the ability to retain information for a short period, or working memory (Reference Baddeley, Hitch and BowerBaddeley & Hitch, 1974) – which says nothing about its longevity. The term ‘temporary’ rather than ‘short-term’ should always be used to refer to effects that resolve, but even then it should be used with caution because neuropsychology recognises that transient impairment of cognitive function may have residual permanent effects. ‘Dysfunction’ or ‘disability’ should be used rather than ‘loss’ (which implies a one-time event) to refer to ongoing difficulties with memory ability and cognition.
If the term anterograde amnesia must be used, it should be clearly defined as difficulties with memory in daily life, and examples given (Box 2).
Box 2 Notes describing an individual’s memory disability (anterograde amnesia)
-
• Can’t hold onto information
-
• Memory not as good as before or as good as that of others the same age
-
• Forgets where she put items such as keys
-
• Has to make lists all the time
-
• Can’t remember faces and names
-
• Forgets what she was about to say or do
(after Reference Freeman, Weeks and KendellFreeman et al, 1980)
Inevitably, memory overlaps with and subsumes other cognitive functions, such as learning and attention as well as overall intelligence. When individuals who have had ECT report ongoing memory disability, it is necessary for a clinician trained in neuropsychological evaluation to tease out the roles played by attention, concentration, overall slowed mental processing and deficits of executive function such as inability to shift mental set. The ECT psychiatrist and treatment team may not be trained in neuropsychological evaluation, since outside of research settings it is not routinely performed on people who have had ECT. When it is, it is usually initiated by the patient, not the doctor. Because of this, the treating psychiatrist may fear personal liability and thus be unwilling to attribute deficits to ECT.
It has long been known that ECT can produce deficits in non-memory-related cognitive function (Reference CalevCalev, 1994). However, long-term studies comparing controls and people who have had ECT to determine when and if non-memory cognitive function normalises after ECT have not been done. A comprehensive battery of neuropsychological tests carried out on individuals who had had ECT between 9 months and 30 years previously revealed impairment on a range of measures, even after controlling for the effects of illness and medication (Reference Freeman, Weeks and KendellFreeman et al, 1980).
Despite recommendations that psychiatrists inform patients of non-memory cognitive after-effects (Reference CalevCalev, 1994) and warn them that ‘they are not going to function well on more tasks than they anticipate’ (Reference Calev, Gaudino and SquiresCalev et al, 1995), patients are still routinely not informed about these effects; there is no mention of them in the recommended consent forms of the American Psychiatric Association (APA; 2001), the Royal College of Psychiatrists (2005: Appendix IV) or the manufacturers of ECT equipment. This may contribute to the consistent findings (Reference Rose, Fleischmann and WykesRose et al, 2003, Reference Rose, Wykes and Bindman2005; Reference Philpot, Collins and TrivediPhilpot et al, 2004) that half of people given ECT say they did not receive an adequate explanation of the treatment.
The current APA consent forms not only contain no warnings about adverse effects on cognition, but advise that ‘Most patients report that memory is actually improved by ECT’ (American Psychiatric Association, 2001). This statement is contradicted by all service-user research as well as the findings of SURE (2002) and NICE (2003); indeed, Reference ScottScott (2005) remarked that NICE took ‘special note of the evidence from users that cognitive impairment after ECT often outweighed their perception of any benefit from it’.
Is it depression?
If the task of assessing amnesia, memory disability and cognitive deficits is left to a patient’s treating psychiatrist, there may be a tendency to attribute all deficits, without evaluation, to depression, even when the patient has fully recovered. The APA guidelines state that
‘Patients with the greatest symptomatic benefit from ECT typically report the greatest improvement in subjective evaluations of memory. Thus, when patients report subjective memory impairment after ECT, their mood as well as their cognition should be assessed’ (American Psychiatric Association, 2001: p. 72).
It seems that this statement is based on SMQ scores from six studies: Reference Pettinati and RosenbergPettinati & Rosenberg, 1984; Reference Weiner, Rogers and DavidsonWeiner et al, 1986; Reference Mattes, Pettinati and StephensMattes et al, 1990; Reference Sackeim, Devanand and PrudicSackeim et al, 1993 and Reference Coleman, Sackeim and PrudicColeman et al, 1996, which involved the same patients; and Reference Sackeim, Prudic and DevanandSackeim et al, 2000. On average, patients reported improvement in cognitive functions assessed by the SMQ within 1 week of ECT. However, the following should be taken into account: first, the improvement was relative only to immediate pre-ECT status, not baseline, thus in fact reflecting a net impairment; and second, objective testing revealed that the patients were in fact cognitively impaired post-ECT. There are other studies in which patients reported impairment post-ECT on the SMQ (Reference Squire, Wetzel and SlaterSquire et al, 1979; Reference Squire and SlaterSquire & Slater, 1983; Reference Squire and ZouzounisSquire & Zouzounis, 1988). To the extent that a handful of studies support a claim of correlation between memory and cognitive self-rating and mood during or immediately after ECT, there might be a correlation between relatively improved memory self-rating and improved mood. There is no evidence of a correlation between impaired memory/cognition after ECT and impaired mood, much less a causal relationship.
The problem of premature assessment
There are many reasons why hospitalised patients who have received ECT might overestimate their abilities. After each treatment they experience acute organic brain syndrome (Reference SackeimSackeim, 1986). In hospital, they are not exposed to even minimally taxing actions such as shopping and driving. There are no environmental cues as to what they are expected to know and remember in their roles outside the hospital. In a few days or even weeks, patients cannot gain enough experience of using their minds and memories to accurately assess their altered capacities (Reference Weiner, Rogers and DavidsonWeiner et al, 1986; Reference Coleman, Sackeim and PrudicColeman et al, 1996; Reference DonahueDonahue, 2000). In the longer term, i.e. 2–6 months, patients who initially rated their memory and cognition as improved, experience and accurately report impairment (Reference Weiner, Rogers and DavidsonWeiner et al, 1986; Reference Coleman, Sackeim and PrudicColeman et al, 1996).
More recent work using the SMQ suggests that, in the short term as well, patient ratings of memory function are negative and are correlated with the results of objective tests, even when controlling for the level of depression. These researchers say that patient reports of memory impairment ‘must not be dismissed as being depressive complaints only’ (Reference Schulze-Rauschenbach, Harms and SchlaepferSchulze-Rauschenbach et al, 2005).
Differentiating the effects of ECT
Although terms such as memory loss are often used interchangeably by clinicians to describe the temporary effects of depression on cognition (especially attention) and the long-lasting effects of ECT on a range of cognitive functions, this confusion is unnecessary and could be avoided. The effects of ECT are quantitatively and qualitatively different from those of depression (Reference Squire, Wetzel and SlaterSquire et al, 1979) and researchers have consistently distinguished between them (Reference Cronholm and OttosonCronholm & Ottoson, 1963; Reference Squire, Wetzel and SlaterSquire et al, 1979; Reference Squire and SlaterSquire & Slater, 1983; Reference Pettinati and RosenbergPettinati & Rosenberg, 1984; Reference Squire and ZouzounisSquire & Zouzounis, 1988). Numerous controlled studies show that individuals who are depressed but have not had ECT do not suffer amnesia (Reference JanisJanis, 1950; Reference Weiner, Rogers and DavidsonWeiner et al, 1986). People who have experienced the effects of both depression and ECT rarely mistake one for the other (Food and Drug Administration, 1982; Reference DonahueDonahue, 2000): ECT’s effects are different and worse, they occur only after ECT and they persist in the absence of depression and drugs.
Possible mechanisms of action
How might ECT cause permanent amnesia and memory and cognitive disability? There are several theories (Box 3). One is that memory is affected because the applied electrical current is densest in the medial temporal area structures associated with memory, including the hippocampus; these areas have low seizure thresholds. However, this has not been studied directly (Reference CalevCalev, 1994).
Box 3 Possible causes of ECT’s adverse effects
-
• Direct effects of electricity on the hippocampus
-
• Breach of the blood–brain barrier
-
• Increase in cerebral blood pressure
-
• Regional increases in T2 relaxation times
-
• Disturbance of the long-term potentiation mechanism
-
• Excessive release of excitatory amino acids and activation of their receptors
-
• Decreased cholinergic transmission
Other theories focus on ECT’s effects on brain metabolism and neurochemistry: breach of the blood–brain barrier and increased cerebral blood pressure (Reference Bolwig, Hertz and PaulsonBolwig et al, 1977; Reference Taylor, Kuhlengel and DeanTaylor et al, 1985); regional increases in T2 relaxation times (Reference Diehl, Keshavan and KanalDiehl et al, 1994); disturbance of the long-term potentiation mechanism (Reference SackeimSackeim, 2000; Reference Rami-Gonzalez, Bernardo and BogetRami-Gonzalez et al, 2001); excessive release of excitatory amino acids and activation of their receptors (Reference Chamberlin and TsaiChamberlin & Tsai, 1998; Reference Rami-Gonzalez, Bernardo and BogetRami-Gonzalez et al, 2001), and decreased cholinergic transmission (Reference Khan, Mirolo and MiroloKhan et al, 1993; Reference Rami-Gonzalez, Bernardo and BogetRami-Gonzalez et al, 2001). Even temporary alterations in any of these may have permanent effects on the brain.
Since ECT affects both temporal and frontal lobes, it is logical that its effects would not be limited to amnesia, but would involve both memory and non-memory neuropsychological functions (Reference Calev, Gaudino and SquiresCalev et al, 1995). Reference SackeimSackeim (2000) hypothesises that the traditional view that amnesia results from damage to medial temporal lobe structures alone may be wrong, since it is known both that frontal lobe damage can result in amnesia as extensive as that seen after ECT and that ECT exerts its most profound effects on the prefrontal cortex.
If this hypothesis holds, then frontal functions must be affected as well as memory. Simply because there has been very little investigation of ECT’s effects on these functions, doctors should not be sanguine as to lack of permanent effects. Absence of evidence is not evidence of absence. In particular, Reference SackeimSackeim (2000) points to the lack of formal research on ECT’s effects on the executive functions of the prefrontal cortex: working memory (holding onto information in the service of a range of cognitive functions), logical reasoning and abstraction, shifting of mental set, problem-solving, planning and organising. These are ‘fundamental to organising one’s life and controlling behavior, yet there has been little investigation of the impact of ECT’ (Reference SackeimSackeim, 2000).
Three trials, two controlled and one small and uncontrolled, support the theory of frontal lobe involvement in functional impairment, although assessments were carried out only during or immediately after ECT (Reference Neylan, Canick and HallNeylan et al, 2001; Reference Rami-Gonzalez, Salamero and BogetRami-Gonzalez et al, 2003; Reference Schulze-Rauschenbach, Harms and SchlaepferSchulze-Rauschenbach et al, 2005).
A generation ago, one researcher, reviewing the literature on ECT experimentation, wrote that the ease of its administration has resulted in its widespread use
‘without the usual background information customarily thought appropriate for most treatment modalities … this is undoubtedly the case because of the clinically observed changes in affect and behavior that result from such treatment. While such behavioral observations are certainly fruitful, such a model should be reversed to allow behavioral inferences to the possible effects on neocortical structures of such a procedure’ (Reference Goldstein, Filskov and WeaverGoldstein et al, 1977).
The evidence base
In the absence of long-term follow-up studies over the past two decades, the best available evidence for the permanent effects of ECT on memory ability and cognition has been generated by former patients. This has most often taken the form of patient-designed survey instruments, which ask specifically about cognition. Of the groups whose findings were incorporated into the SURE systematic review, one found that 65% of people who had had ECT reported impaired organisational skills (ECT Anonymous, 1999). Another found that one-third had difficulty concentrating, and 15% reported loss of reasoning ability (Reference PedlerPedler, 2001). A third asked people whether they had experienced a loss of intelligence ‘soon after the treatment’, and about 40% answered affirmatively (they were not asked whether the loss persisted) (Reference Philpot, Collins and TrivediPhilpot et al, 2004). However, former patients have publicly testified that ECT can result in a very significant (>30 point) permanent decrement in IQ score (Food and Drug Administration, 1982; Reference AndreAndre, 2001; Reference CottCott, 2005: p. 5) and have documented the claims by extensive neuropsychological evaluation.
Although surveys and case reports are not rigorous controlled trials, in the absence of such trials conducted months or years after ECT, they provide a basis for inferences as to the treatment’s permanent adverse effects and possible mechanisms of action.
What’s wrong with the way patients have been assessed?
Claims that ECT does not have permanent adverse effects on memory and cognitive ability have been based on extremely gross measures of mental function such as the Mini-Mental State Examination (MMSE; Reference Folstein, Folstein and McHughFolstein et al, 1975) and other dementia screening scales (Reference Stoudemire, Hill and MorrisStoudemire et al, 1993; Reference SackeimSackeim, 2000; Reference McCall, Dunn and RosenquistMcCall et al, 2004). But if ECT had produced wholesale dementia on a scale gross enough to be detected by these tests, it would have been abandoned decades ago.
Researchers have used very simple, brief measures to assess patients – typically, highly structured tests of verbal learning involving familiar material. Examples include the Auditory Verbal Learning Test (AVLT; Reference ReyRey, 1964) and various forms of paired associates, with very short retention intervals. But there is no evidence that ECT interferes with well-established skills such as vocabulary or with short recall periods (Reference Squire and ChaceSquire & Chace, 1975; Reference Zervas and JandorfZervas & Jandorf, 1993).
Even people with severe brain injury or lobotomy can perform well on simple tests of overlearned verbal material that require culturally common information, for example the Wechsler Memory Scale. Highly motivated and concerned ECT patients are even more likely to do well on these tests. However, clinicians who conclude from this that there is ‘no memory loss’ have not measured memory loss at all, and certainly not the type of memory and cognitive disability that people can experience after ECT (National Insitute for Clinical Excellence, 2003).
Collectively, the comments of people who have had ECT indicate loss of complex skills that underlie real-world roles such as student, professor, nurse or physicist, and often inability to return to those roles post-ECT (Box 4).
Box 4 A representative sample of patient reports of permanent amnesia and disability
‘I’ve got 13 GCEs, top grade, but no professional qualifications since ECT. I’ve sat only one exam, and despite its being 70% project work and continual assessment, I’ve struggled to just pass, well bottom – my memory and impaired concentration can’t cope.’
‘After ECT I could no longer play my guitar. I could not remember chord sequences/ patterns, words or songs that I had performed hundreds of times before ECT. The ability to play or learn new music has never returned.’
‘In addition to destruction of entire blocks of pre-ECT memories, I have continued to have considerable difficulty in memory recall with regard to academic pursuits. I have been forced to tape-record all education materials that require memorisation. I was forced to “re-take” accounting. Now I am again forced to “re-take” a basic one semester course in computerised word processing.’
‘I can’t remember new information with the ease that I could before ECT. Distractions and interruptions seriously interfere with information retention, and any new bit of information may “cancel out” the bit that preceded it.’
‘I have trouble with my memory today. My IQ was 120 before the treatments and it is not anywhere near that now. I have trouble just trying to cook a meal. I do not work. I make lists so that I can try to recall what I need to do.’
‘I had to drop out of school when I realised I could not remember what I had studied before entering the hospital, and I was totally unable to absorb new material. I continue to have difficulty concentrating for extended periods of time.’
‘Before ECT, I studied math up through calculus. After ECT, I can just barely make change in a store. ECT gives a person a different brain from the one a person had.’
(Food and Drug Administration, 1982; Reference PedlerPedler, 2001; Service User Research Enterprise, 2002)
The sensitivity of the tests used after ECT depends largely on whether and how well they reflect actual cognitive demands of the type placed on ex-patients. Researchers have assured patients that ECT has no permanent adverse effects on the basis of the assumption that these demands will be minimal (Reference McCall, Dunn and RosenquistMcCall et al, 2004). But this assumption has never been tested, and patient reports warn against it. The ECT patient population includes people who are in the prime of life, highly educated and involved in demanding professions, and who can be very articulate in describing their deficits. If simple standardised tests cannot detect these deficits, the challenge is not to dismiss their comments but to find or devise more appropriate tests.
The Autobiographical Memory Interview
Reference Weiner, Rogers and DavidsonWeiner et al(1986) and Sackeim and his colleagues (Reference Coleman, Sackeim and PrudicColeman et al, 1996; Reference Sackeim, Prudic and DevanandSackeim et al, 2000) have attempted to measure amnesia with an unvalidated instrument of their own design, known as the (Columbia University) Autobiographical Memory Interview. This test is insensitive to ECT-induced amnesia in two related ways: it measures very old information whereas ECT amnesia is known to be densest for more recent memory; and as many as 60% of the 200–300 test items involve overlearned and highly rehearsed facts – grandparents’ names, telephone numbers of close relatives, etc. – which are not likely to be erased by ECT. The overlearned and the old information may overlap (as in questions such as ‘What is your address’) or it may not, but in either case confounding the testing with these factors will unnecessarily result in an underestimate of the extent of retrograde amnesia.
Furthermore, the Autobiographical Memory Interview assumes that amnesia is limited to events that took place within the 12 months prior to ECT and does not attempt to assess amnesia that is not limited to that time period. However, only about 20% of the questions ask specifically about that year; the rest ask about overlearned personal information (What are your parents’ names? What are the rooms in your house?) or about events that have ‘ever’ happened to patients or their families.
Thus, it is remarkable that even as insensitive an instrument as this has shown extensive permanent retrograde amnesia measured at 2 months (Reference Coleman, Sackeim and PrudicColeman et al, 1996) and 6 months (Reference Weiner, Rogers and DavidsonWeiner et al, 1986) after ECT.
Assessment of amnesia
Routine neuropsychological tests are unhelpful in attempting to assess retrograde amnesia (Reference Rose, Fleischmann and WykesRose et al, 2003). Reference Squire and SlaterSquire & Slater (1983) attempted to measure amnesia by asking people who had had ECT to make a time line showing the amount of life lost. The accuracy of this depends, of course, on the patients’ ability to assess the extent of their amnesia, which can take many years, as they can only discover what they have forgotten when prompted by others to remember it. If asked soon after ECT, they are very likely to underestimate the extent of retrograde amnesia.
Because the information stored in memory is unique to each individual, standardised questionnaires or checklists may prove insensitive to amnesia even when the patient can describe or demonstrate it. Reference JanisJanis (1950) interviewed patients before and 1 month after ECT. He suggested general topics but let the patients speak at length. After ECT, he attempted to elicit the same information, but could not. For each individual he could count 10–20 significant life experiences that had been erased that were not limited to the period immediately before the treatment. Even when he prompted patients to recount events they had described in great detail before treatment, they could neither recall nor recognise them. One year after ECT, the amnesias remained stable. The same interviews were given to controls matched in all ways: age, gender, education, duration of hospitalisation, type and duration of mental disorder; the controls, who had not had ECT, had no amnesia.
The Janis test (Reference JanisJanis, 1950) can be done easily and cost-effectively even by those with no special research training. SURE (2002) in particular calls for the replication of this study, as have others over the years. But it has not yet been done.
Assessment of memory and cognitive ability
Tests of memory and cognitive ability must assess a range of functions, because ECT impairment may vary not only between individuals (Reference Goldstein, Filskov and WeaverGoldstein et al, 1977) but within individuals when they undergo more than one course of treatment. Simple tests of rote verbal learning or the memory sub-tests of the Wechsler are not sufficient, since ECT (if the amnesia is not catastrophic) spares vocabulary, overall wealth of knowledge and overlearned verbal skills. Patients who have had ECT should be evaluated with the type of neuropsychological batteries that would be used for patients with a known or suspected history of brain injury. These should include tests of non-verbal and visuospatial memory and reasoning such as those listed in Box 5.
Box 5 Neuropsychological batteries suitable for use after ECT
Non-verbal and visuospatial memory and reasoning
-
• Benton Visual Retention Test (Reference SivanSivan, 1992)
-
• Bender Gestalt (Reference BenderBender, 1938)
-
• Test of Non-verbal Intelligence (Reference Brown, Sherbenou and JohnsenBrown et al, 1982)
Working memory
-
• Digits Backwards (Reference WechslerWechsler, 1997)
-
• Speaking Span Test (Reference Daneman and GreenDaneman & Green, 1986)
Executive function
-
• Wisconsin Card Sort (Reference HeatonHeaton, 1981)
-
• Halstead Category (Reference Reitan and WolfsonReitan & Wolfson, 1993)
-
• Booklet Category (Reference de Filippis, McCampbell and Rogersde Filippis et al, 1979)
Reasoning and working memory
-
• Sub-tests of the Reference WechslerWechsler (1997) such as Arithmetic and Picture arrangement
If there are constraints of time and finances, tests should be tailored to the individual’s deficits, which can be identified by narrative self-report and by rating scales such as the Cognitive Failures Questionnaire (Reference Broadbent, Cooper and FitzGeraldBroadbent et al, 1982).
When should patients be tested?
If there is to be baseline testing, compensation must be made to account for the difference between the patient’s true memory and cognitive capacity and the performance when preoccupied by depression, medicated or hospitalised. If such compensation is not made, all a return to ‘baseline’ function after ECT would show would be that ECT’s effects are roughly equal to – although not necessarily the same as – those of depression (Reference Calev, Gaudino and SquiresCalev et al, 1995).
A better estimation of pre-ECT capacity would be the patient’s history and normal functioning at school, work or in some other capacity. Many patients, at least in the USA, will have had an IQ test, which can be used for comparison with post-ECT scores.
Patients cannot be meaningfully evaluated in hospital during or soon after ECT. Neither self-reports nor crude memory tests may be reliable (Reference Cronholm and OttosonCronholm & Ottoson, 1963). A patient may do well on the MMSE or counting serial sevens but may not know that her friend visited her the day before – and will not know she doesn’t know. Having had no reason theretofore not to trust her memory, and not having been warned to expect severe dysfunction, she will adamantly insist that her memory cannot be faulty. It is not the psychological defence mechanism of denial, nor is it only the acute organic brain syndrome which occurs with ECT, that causes this genuine unawareness. Most patients have never before experienced a day in their life when they did not know what they ate for dinner or who they had seen or what they had read the day before. They do not even know that this is possible, let alone that it is happening to them.
The ECT Accreditation Service (2005) recommends that patients should be interviewed 3 and 6 months after ECT. But at 3 months, they may not have recovered the ability to hold on to day-to-day memories (they may still be within the period of anterograde amnesia, estimated by the US National Institutes of Health (1985) to average 2 months). We propose that follow-up should be no sooner than 6 months. One year allows for optimal stabilisation of permanent cognitive deficits and better assessment of retrograde amnesia.
The Service User Research Enterprise (2002) has called for a research study with ‘long followup because losses of memory prior to ECT may only become apparent after a long interval’, as have Reference Greenhalgh, Knight and HindGreenhalgh et al (2005: p. 78).
What should patients be told?
Amnesia
The clinician who tells her patients that there is a lack of research on the permanent adverse effects of ECT will certainly be on solid ground; however, this is unlikely to help patients in making a potentially life-altering decision. The best she can do is present her patients with what is known (and not known) and encourage them to assess the risk in light of their personal situation.
Thus, patients can be told that permanent amnesia is one of the ‘common’ (Reference SackeimSackeim, 2000) or ‘serious/ frequently occurring’ (Royal College of Psychiatrists, 2005: p. 207) effects of ECT and that it affects at least one-third of patients (Service User Research Institute, 2002; Reference Rose, Fleischmann and WykesRose et al, 2003). Such amnesia may be presented as having multiple dimensions: the amount of life lost, the temporal gradient, the nature of what is lost, and the effect of the memory erasure on the individual’s life.
The amount of life lost to amnesia cannot be predicted; patients should be warned that it has been known to extend to 10–20 years (Reference PedlerPedler, 2001; Service User Research Enterprise, 2002). It should be made clear that amnesia is not limited to information about discrete events or to facts that are easily regained, such as dates and telephone numbers, but that it encompasses all thoughts, feelings, personal interactions and relationships, learning and skills associated with the erased time period, and thus there is no simple or easy way to recapture what is lost. Since the temporal gradient of ECT amnesia is the opposite of normal forgetting, patients should be warned that the most recent months or years will be most affected.
When amnesia is permanent it has profound, rarely positive, effects on all aspects of the patient’s subsequent life. For many people the effects of permanent amnesia and/or memory and cognitive disability negate any benefit sustained from ECT (National Institute for Clinical Excellence, 2003).
The College now advises psychiatrists to discuss the topic of retrograde amnesia carefully (Royal College of Psychiatrists, 2005: p. 7). But profound and sudden retrograde amnesia has no parallel in ordinary human experience. Doctors cannot be expected to understand the myriad ways in which permanent amnesia can disrupt one’s life. For this reason, prospective patients should be encouraged to speak with, or read accounts written by, people who have experienced amnesia. Such accounts are contained within the above-mentioned reports of SURE, NICE and MIND (Reference PedlerPedler, 2001), and are widely available in print (e.g. Reference DonahueDonahue, 2000) and through online forums, e.g. http://www.ect.org) where prospective patients and families can sometimes ask questions directly of former patients.
Cognitive impairment
The Royal College of Psychiatrists (2005: p. 19) and NICE (2003) advise that the potential for cognitive impairment be highlighted during the consent process. Patients should be clearly told that ECT may have serious and permanent effects on both memory ability and non-memory cognition. These are best described in everyday terms: ‘the ability to plan and organise and get things done’ rather than ‘executive function’.
Intact memory and intelligence are highly prized in our culture. The more valuable a possession, the more important it is to know about even a small chance that it might be permanently lost. Even if the answer to how often IQ is permanently lowered is ‘We don’t know’, that is a material fact to be weighed by the patient. As individuals, patients vary greatly in the demands placed on their intellect and the potential consequences of permanent impairment. The decision to agree to ECT is theirs; the duty to inform, their physician’s.
Conclusions
Evaluation and re-evaluation of ECT’s risks and benefits by SURE, NICE and the Royal College of Psychiatrists, and the growing recognition of the extent and importance of research by and involving people who have experienced ECT, as well as increased interest in qualitative data, should lead to improvement in both patient care and research. In light of alarming findings that 50% of patients report receiving inadequate warnings of the potential side-effects of ECT, informed consent practices need to be revised. In particular, prospective patients should be warned of the significant risk of permanent amnesia and the possibility of permanent memory and cognitive disability. Research to adequately assess the nature and longevity of these effects should be undertaken, ‘incorporating patients’ perspectives on the impact of ECT into future RCTs’ (Reference Greenhalgh, Knight and HindGreenhalgh et al, 2005: p. 78). By all accounts this is long overdue.
Declaration of interest
None.
MCQs
-
1 The proportion of patients who will experience permanent retrograde amnesia after ECT is:
-
a a few outliers
-
b a small minority
-
c less than one-third
-
d at least one-third
-
e zero.
-
-
2 Permanent retrograde amnesia can best be assessed by:
-
a the Squire Memory Questionnaire
-
b the Columbia Autobiographical Memory Inventory
-
c the MMSE
-
d the memory scales of the Wechsler
-
e the Janis test.
-
-
3 Which of the following statements accurately describe retrograde amnesia?
-
a studies have proven that it reverses itself within 6 months
-
b it may be severe, erasing 10 years or more of the patient’s life
-
c it erases only bad memories
-
d it is limited to dates and details that can be easily relearned
-
e most patients consider it a small price to pay for ECT’s benefits.
-
-
4 Difficulty or disability with memory function on an ongoing basis is best described as:
-
a retrograde amnesia
-
b anterograde amnesia
-
c depression
-
d memory disability
-
e memory loss
-
-
5 Tests that may be useful in assessing non-memory cognition include:
-
a Wisconsin Card Sort Test
-
b paired associates
-
c the MMSE
-
d the Benton Visual Retention Test
-
e the Speaking Span Test.
-
MCQ answers
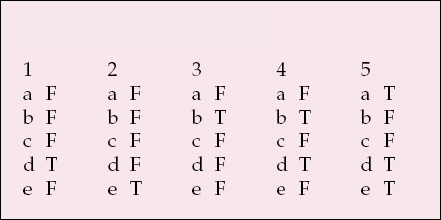
| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | F | a | F | a | F | a | F | a | T |
| b | F | b | F | b | T | b | T | b | F |
| c | F | c | F | c | F | c | F | c | F |
| d | T | d | F | d | F | d | T | d | T |
| e | F | e | T | e | F | e | F | e | T |



eLetters
No eLetters have been published for this article.