Over the past decade, a number of treatment models have been proposed for people with severe mental illness and substance use problems. One of these initiatives, originating in the USA but now taken up in the UK, was to create specifically designed services to tackle this dual diagnosis. In both countries, addiction and generic mental health services have historically functioned separately and have considerable differences in terms of culture, philosophy and treatment strategies. For instance, generic services prefer more assertive approaches to maintain patients in treatment, whereas addiction services encourage individuals to take responsibility in their own treatment. Major cultural shifts are not easy to implement and maintain within services. One of the aims in creating specific dual diagnosis services was to overcome some of these difficulties.
Thus far, three service models have been developed: serial treatment, in which the treatment of psychosis is completed before substance use treatment begins; parallel treatment, in which both problems are treated at the same time, but by different teams; and integrated treatment, in which the same team treats both problems simultaneously. The integrated treatment model has particular benefits for the patient, but it requires well-integrated in-patient care, staff trained to deal with both mental health and substance use problems, assertive community services and supportive living environments. This model can be costly and requires either better use of existing resources or the creation of new ones. All service delivery models aim to provide motivation-based treatments as well as psychosocial methods.
How successful are these models?
It seems only sensible that these treatment models should be fully developed and tested before widespread implementation (Reference Weaver, Renton and StimsonWeaver 1999). However, dual diagnosis services have been set up in the USA and the UK on the basis of models that lack clear evidence even of superiority over standard care. A systematic Cochrane review (Reference Cleary, Hunt and MathesonCleary 2008) to evaluate the efficacy of treatment programmes for people with both severe mental illness and substance misuse identified 25 randomised controlled trials and found no compelling evidence to support any one psychosocial treatment over another in the reduction of substance use or improvement of mental state. It was concluded that existing methodological difficulties hindered pooling and interpreting results because of high drop-out rates, varying outcome measures and comparison groups. The same review, however, concluded that individual studies provided some support in favour of motivational interviewing for substance use reduction and that this intervention is a crucial component in cognitive–behavioural therapy for substance use.
One study mentioned in the Cochrane review, however, reported that a residential programme had a significantly greater chance of retaining people in care than a non-residential approach (Reference Burnam, Morton and McGlynnBurnam 1995).
Despite the lack of unequivocal evidence for an efficient model for managing substance use in severe mental illness, motivational interviewing techniques, community reinforcement approaches, assertive case management, and a blend of standard psychological interventions for substance use with specific pharmacotherapies have each proven successful in extending abstinence (Reference Ziedonis and TrudeauZiedonis 1997; Reference Brunette and MueserBrunette 2006a).
This section is summarised in Key points 1.
Psychological interventions
People with severe mental illness are often denied psychological interventions, even if they ask for them. This is due partly to lack of resources and partly to the belief that they cannot benefit from such treatment, because of their illness and cognitive deficits. Interestingly, a study of potential participants in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) programme found that the negative symptoms of schizophrenia had very little effect and positive symptoms had no effect on decision-making capacity (Reference Stroup, Appelbaum and SwartzStroup 2005). The majority of people with schizophrenia can potentially benefit from psychological interventions and participate in shared decision-making. People who have bipolar disorder show even better cognitive performance than those with schizophrenia (Reference Krabbendam, Arts and van OsKrabbendam 2005).
Motivational interviewing
Over the past two decades several psychological intervention methods have been developed to provide patient-tailored treatment programmes for tackling substance misuse, and some of these have also been used with people with severe mental illness. These methods adapt treatment to the motivational level of the individual. Motivational interviewing therapiesFootnote ‡ can be based on a five-stage scale which indicates the individual's readiness to change (Box 1).
Box 1 The five stages of change in relation to substance misuse
-
1 Pre-contemplation: continuous use with no interest in quitting in past 6 months
-
2 Contemplation: continuous use with ambivalent interest in quitting
-
3 Preparation: continuous use with interest in quitting in the next 30 days
-
4 Action: active attempt to quit
-
5 Maintenance: abstinent for more than 3 months but less than 5 years
The aim of motivational interviewing and motivational enhancement therapy for substance misuse is to strengthen the patient's motivation to change by increasing their awareness of the impact of substance use and encouraging them to take responsibility for their behaviour (Reference Miller and RollnickMiller 2002). The therapist is empathetic, open, non-judgemental and realistic about what can be achieved. Giving advice, reassurance or immediate problem-solving are usually avoided. The interview is strategically directed towards the patient's use of substances and related life events. For some patients the goal of therapy will be harm reduction, for others it will be cessation or maintenance.
The efficacy of motivational interviewing for primary substance use problems has been well established, and several studies have examined its use to treat substance misuse in people with severe mental illness. It seems that some tailoring of the approach is required for people with psychotic disorders. In a large population of (non-psychotic) university students who had been using substances, 3-month follow-up revealed that a single session of motivational interviewing had reduced their use of cigarettes, alcohol and cannabis, albeit mainly through moderation rather than cessation (Reference McCambridge and StrangMcCambridge 2004). However, studies indicate that a single standard session would not be sufficient for people with severe mental illness. One such study, involving individuals with psychotic disorders and co-occurring substance use problems, recommends modifications to standard motivational interviewing to take into account patients' cognitive deficits by simplifying open-ended questions, refining reflective thinking skills, heightening emphasis on affirmations and integrating psychiatric issues into personalised feedback (Reference Martino, Carroll and KostasMartino 2002). It stresses the importance of considering the patients' clinical state and the events they are experiencing during treatment, as these may need to be tackled in the therapy sessions. As in standard motivational interviewing, there is emphasis on reflective listening, discussing obstacles to treatment and identifying factors that would improve motivation.
Combination therapies
To deal with the ‘dual’ set of problems presented, some psychosocial treatment programmes for co-occurring psychotic disorders and substance misuse combine various therapy models. For instance, for an individual in the action stage of change (Box 1), relapse prevention may involve a hybrid behavioural therapy that integrates substance misuse treatment, relapse prevention and social skills training. It is expected that the needs of the patient will be different at each stage. For an individual moving from the action stage to the maintenance stage, special attention may be given, for example, to restoring relationships, seeking employment or engaging in meaningful activities in social settings that include non-users.
Motivational enhancement plus cognitive–behavioural therapy
Various combination therapies for out-patients have been subjected to trials. One example is a manualised group-based intervention for reducing substance use (Reference James, Preston and KohJames 2004). Based on the principles of motivational enhancement and cognitive–behavioural therapy, the 6-week intervention involved weekly 90-min sessions tailored to the patient's stage of change and motivational level. In this Australian randomised controlled study, 63 participants were allocated to the intervention or to a single educational session. Of these, 92% completed a 3-month follow-up assessment of psychopathology, antipsychotic dose, alcohol and substance use, severity of dependence and hospitalisation. Significant improvements were found on most outcome measures in the treatment group in comparison with the control group. However, a limitation of the study is the short period to follow-up.
A UK-based randomised controlled study evaluated the efficacy of individual and family-oriented cognitive–behavioural therapy for treatment-resistant psychosis, combined with motivational interviewing for substance use problems; the treatment group received about 29 sessions of combined therapy (Reference Barrowclough, Haddock and TarrierBarrowclough 2001). Significant improvements in functioning were found in the treatment group at 18-month follow-up (Reference Haddock, Barrowclough and TarrierHaddock 2003). The cost was similar to that of the control treatment.
Insight–adherence–abstinence in first-episode schizophrenia
A therapy developed specifically for patients with first-episode schizophrenia who are using cannabis focuses on the triad ‘insight–adherence–abstinence’. To prevent psychotic relapses, the first year of treatment targets the unique characteristics of such individuals, focusing on building adherence to medication regimens, abstinence from substance use and insight into schizophrenia. The model provides supportive, cognitive–behavioural, behavioural and motivational therapies, skills building and psychoeducation. At the end of a 12-month treatment trial involving 68 individuals with first-episode schizophrenia or schizoaffective disorder, 30 of whom were using cannabis on diagnosis, nearly half of the cannabis users had stopped using and two were using less frequently. The remainder of the group continued frequent use (Reference Miller, Caponi and SevyMiller 2005).
Literature reviews
Non-psychotic populations
A Cochrane review of psychotherapeutic interventions in non-psychotic cannabis misusers analysed six trials involving 1297 people (Reference Denis, Lavie and FatséasDenis 2006). Both group and individual sessions of cognitive–behavioural therapy showed efficacy for the treatment of cannabis dependence and associated problems, but the effect was greater with individual sessions. Two studies suggested that adding voucher-based incentives to effective psychotherapeutic interventions may enhance treatment. Abstinence rates were relatively small overall, but favoured nine or more sessions of cognitive–behavioural therapy. The reviewers reported that the included studies were too heterogeneous to allow the drawing of clear conclusions. However, the low abstinence rate indicated that cannabis dependence is not easily treated by psychotherapies in out-patient settings.
Populations with psychotic disorders
The most recent comprehensive review of psychosocial interventions for people with co-occurring substance use disorder and severe mental illness assessed 45 controlled studies (Reference Drake, O'Neal and WallachDrake 2008). Consistent positive effects on substance misuse were found with three types of integrated intervention: group counselling, contingency management and long-term residential treatment. Group counselling appears to be especially beneficial for educational, skills building and peer support purposes. Contingency management interventions mainly focus on substance use and appear promising, but further validation is required. Long-term residential treatment appears to be the most effective, especially for those who have failed other out-patient interventions.
Interventions other than group counselling, contingency management and long-term residential treatment had no significant effect on substance use outcomes, but often led to improvements in other areas of adjustment. The reviewers emphasised the limitations of the studies in their review, mainly lack of standardisation and diversity of chosen outcomes. They called for further experimental research using interventions that are standardised in terms of programme and duration of treatment, outcome, fidelity and adherence measures, staffing patterns, training approaches and other critical parameters.
Summary
Most studies and reviews of psychosocial interventions for co-occurring severe mental illness and substance misuse indicate that treatment response is more likely to be a short-term reduction in use, rather than short- or long -term abstinence. It can therefore be said that cannabis dependence cannot easily be treated by psychological interventions and that maintaining non-use is particularly difficult. However, most patients will benefit even from reducing their cannabis consumption.
Only a limited number of studies targeted in-patients, even though substance use may continue in acute care settings, exacerbating treatment and management problems. I have noticed that some patients, especially at the beginning of their admission, can admit to the deleterious effect of their substance use. Perhaps it is still fresh in their memory that it was cannabis that made them suspicious and led to their admission to hospital. If a valid treatment model were available, this level of motivation might be harnessed to encourage reduction or cessation. Clearly, more studies examining effective treatment models for in-patients are also needed.
This section is summarised in Key points 2.
Pharmacotherapy
Pharmacotherapy for co-occurring severe mental illness and substance misuse is a recently developing area. It involves study of the pharmacokinetics both of psychotropic medications and substances of misuse and of the interactions between them. It has been suggested that individuals with psychotic disorders may use substances to ‘self-treat’ the negative symptoms or to reduce the side-effects of antipsychotics. Even though the self-medication hypothesis is disputed, patients have reported that cannabis helps them relax, become more energised and sociable, and that it reduces extrapyramidal side-effects (Reference Dixon, Haas and WeidenDixon 1991; Reference Schofield, Tennant and NashSchofield 2006). Hence, targeting the treatment of negative and affective symptoms of schizophrenia or bipolar disorder may have beneficial effects on reducing the drive or the need to use substances. However, in a recent longitudinal study involving patients with first-episode schizophrenia, persistence with substance use was associated with more severe positive and depressive symptoms, but not with negative symptoms, and the latter were noted as a potential target for specific treatment (Reference Harrison, Joyce and MutsatsaHarrison 2007).
Reducing the drive to take substances
An ideal medicine for an individual with severe mental illness who is using cannabis should reduce not only negative, positive and affective symptoms but also the drive to take stimulants. Typical antipsychotics are thought to worsen substance use, mostly because of their unpleasant side-effects and competing metabolism (Reference McEvoy, Freudenreich and LevinMcEvoy 1995). However, a recent comprehensive review has revealed a growing body of evidence that some atypicals may have benefits over the typicals in terms of reducing substance misuse in people with schizophrenia (Reference Green, Noordsy and BrunetteGreen 2008).
Clozapine
Clozapine is the most studied of the atypical anti-psychotics in patients with dual diagnosis, and the evidence so far points towards its beneficial effects, especially in alleviating negative and depressive symptoms. A review of substance misuse by people with treatment-resistant schizophrenia revealed that clozapine produced similar improvements in symptoms and psychosocial functioning levels of both users and non-users, and that a history of substance misuse did not appear to have a negative influence on response to clozapine (Reference Buckley, Thompson and WayBuckley 1994). In a prospective study involving alcohol- and cannabis-using patients with schizophrenia and schizoaffective disorder, those receiving clozapine had significantly better remission rates for substance use than those on typical antipsychotics (Reference Drake, Xie and McHugoDrake 2000). A 10-year follow-up report from the same group showed that individuals taking clozapine were less likely to relapse into substance use over the subsequent year than those treated with other antipsychotics (Reference Brunette, Drake and XieBrunette 2006b). In a retrospective survey of 58 clozapine clinic patients with schizophrenia or schizoaffective disorder and substance use, a significant reduction in cannabis, alcohol and cocaine use was recorded in those who continued to take clozapine, compared with those who discontinued it (Reference Zimmet, Strous and BurgessZimmet 2000). The majority of the patients who carried on taking clozapine also achieved abstinence over the period of the survey, as well as showing significant clinical improvements. However, the authors did not use any measurement scales to study the changes in substance use. They emphasised the need for prospective, controlled studies to shed further light on the effects of clozapine on substance use in this population.
It has been hypothesised that clozapine's positive effect in the pharmacological treatment of schizophrenia and co-occurring substance misuse may be related to its ability to decrease brain reward circuit dysfunction (Reference GreenGreen 2006). It has also been suggested that the weekly professional contact that clozapine patients experience helps sustain a stronger therapeutic alliance (Reference BuckleyBuckley, 1998). However, in the absence of any randomised studies, claims that clozapine has a beneficial effect on substance misuse in severe metal illness remain preliminary.
Clozapine v. risperidone
A retrospective study comparing clozapine with risperidone involving 41 individuals with schizophrenia and comorbid alcohol and/or cannabis misuse found that patients treated with clozapine were more likely to abstain from substance use than those treated with risperidone (Reference Green, Burgess and DawsonGreen 2003).
Other atypicals
Preliminary reports are available on the use of all other atypical antipsychotics in people with dual diagnosis except for ziprasidone. However, most of these studies are retrospective, non-randomised and include only small numbers of participants. Furthermore, the study measures vary widely, making comparisons impossible.
Quetiapine has raised interest because it shares crucial pharmacological properties with clozapine. In a small study, 8 in-patients treated with quetiapine for comorbid substance dependence and non-psychotic anxiety remained abstinent during the 28-day trial and showed reduced anxiety (Reference Sattar, Bhatai and PettySattar 2004). A 12-week, open-label uncontrolled trial involving 24 people with schizophrenia and substance use recorded significant improvements in substance misuse, psychiatric symptoms, extrapyramidal symptoms and cognition during quetiapine therapy (Reference Potvin, Stip and LippPotvin 2006).
Olanzapine and haloperidol were compared in the treatment of 262 individuals with first-episode schizophrenia-related psychosis, 97 (37%) of whom had a lifetime diagnosis of substance use disorder (Reference Green, Tohen and HamerGreen 2004). The 12-week response data indicated that 27% of the participants with substance use disorder responded to either medication, compared with 35% of those without. Individuals with alcohol use disorder were less likely to respond to olanzapine than those without. The authors conclude that the use of substances or alcohol in first-episode psychosis may negatively affect the response to both typical and atypical antipsychotics.
Caveat: smoking cessation and drug plasma levels
Blood plasma levels of antipsychotics such as clozapine and olanzapine are lower in smokers than in non-smokers, mainly because of induction of cytochrome P450 enzymes 1A2 (CYP1A2) by constituents of tobacco smoke (Reference Faber and FuhrFaber 2004). When the patient stops smoking, plasma levels of antipsychotics may increase, leading to adverse drug effects, including antipsychotic intoxication. Reporting this in two patients, Reference Zullino, Delessert and EapZullino and colleagues (2002) warn that antipsychotic plasma levels should be monitored regularly in patients who smoke tobacco or cannabis, to adjust the dose according to a reduction or increase in smoking.
This section is summarised in Key points 3.
Conclusions
Cannabis use clearly has serious implications for severe mental illness. The problem is further complicated by poor assessment, lack of successful educational campaigns and, most significantly, lack of successful models of intervention. However, encouraging research is emerging to inform management approaches. Patients should receive the same message from all clinicians, that the treatment of both their mental health problems and cannabis or substance use is equally important.
There have been positive results from the hybrid use of various psychological interventions for substance misuse in severe mental illness, even though these need fine tuning. Pharmacotherapy for this group of patients is also in its early stages, but there is overall agreement that atypical antipsychotics may prove to be better than the typicals. However, it must be remembered that substance misuse behaviour cannot be explained by pharmaco-kinetics alone. Adding contingency management, psychoeducation and social skills training may enhance the efficacy of pharmacotherapy. Furthermore, most agree that programmes that coordinate pharmacotherapy and psychological interventions are more likely to have beneficial treatment outcomes. It is also important that some meaningful activity replaces the drug-misusing behaviour, as maintaining cannabis or substance use cessation is often the most challenging aspect. More standardised, prospective, randomised and substance-specific research is required to establish an efficient model to help people with severe mental illness to reduce or stop their cannabis use and maintain abstinence.
MCQs
-
1 Regarding psychological interventions for cannabis use in people with severe mental Illness:
-
a motivational interviewing is sufficient to help the person
-
b cognitive–behavioural therapy is not suitable
-
c psychological interventions have shown long-term benefits
-
d most patients respond to interventions by reducing their cannabis use, rather than stopping use altogether
-
e they have no significant effect over standard care.
-
-
2 Regarding pharmacological treatment of cannabis use in people with severe mental illness:
-
a typical antipsychotics may worsen cannabis use
-
b all atypical antipsychotics have been shown to reduce cannabis use
-
c clozapine is not superior to any other antipsychotic
-
d there is good evidence that most atypicals can reduce cannabis use
-
e cannabis smoking cessation reduces the possibility of intoxication with clozapine or olanzapine.
-
-
3 For people with severe mental illness who use cannabis:
-
a combining various psychological intervention models is always beneficial
-
b programmes that coordinate pharmacotherapy and psychological interventions are less likely to have beneficial treatment outcomes
-
c self-medication theory is valid
-
d early use of cannabis has been shown to be associated with development of a psychotic illness
-
e assessment of cannabis use should include its effect on physical health.
-
-
4 Regarding people with severe mental illness:
-
a for those who also use substances integrated treatment is superior to other models
-
b models for dual diagnosis intervention require development and testing before widespread implementation
-
c most such individuals cannot benefit from psychological interventions, owing to their cognitive deficits
-
d reducing cannabis use is of no benefit: complete cessation is necessary
-
e psychoeducation regarding cannabis use is not effective.
-
-
5 The following statements are true:
-
a motivational interviewing is informed by the following stages: pre-contemplation, contemplation, preparation, action, maintenance
-
b knowing why a patient uses cannabis is not necessarily important in designing a treatment plan
-
c following treatment for substance use, establishing the patient's level of substance consumption is a sufficient measure of outcome
-
d during the maintenance phase of the treatment, regular follow-up is sufficient to ensure success
-
e substance use research need not be substance specific, as all substances have similar negative impact on the patient's course of illness.
-
MCQ answers
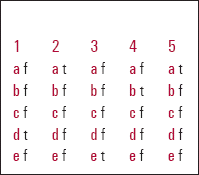
| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | f | a | t | a | f | a | f | a | t |
| b | f | b | f | b | f | b | t | b | f |
| c | f | c | f | c | f | c | f | c | f |
| d | t | d | f | d | f | d | f | d | f |
| e | f | e | f | e | t | e | f | e | f |
KEY POINTS 1 Three service models for people with dual diagnosis

|
KEY POINTS 2 Psychological interventions
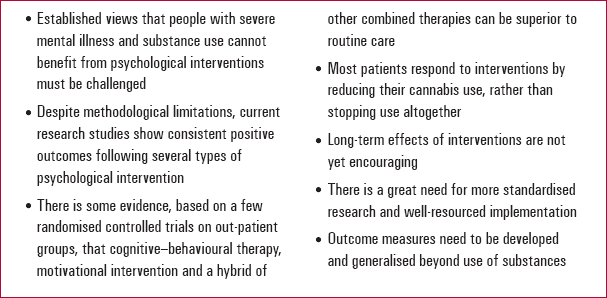
|
KEY POINTS 3 Psychopharmacological interventions

|






eLetters
No eLetters have been published for this article.