Acute agitation is common in psychotic illness, as schizophrenia, schizoaffective disorder and bipolar I disorder all present at some time with a high incidence of agitation. However, agitation is a term poorly defined and often misused by healthcare professionals. As a result of the lack of consensus, agitation is often inappropriately used interchangeably with the terms anxiety, aggression, hyperactivity, problem or disruptive behaviour, and non-purposeful behaviour. Attempts to formalise the meaning of agitation in psychiatry and avoid subjectivity in usage resulted in definitions such as:
-
• excess motor or verbal activity (Reference CitromeCitrome 2004a);
-
• inappropriate verbal, vocal or motor activity not judged to be directly related to apparent needs (Reference Cohen-MansfieldCohen-Mansfield 1986);
-
• a transnosological syndrome that describes a state of poorly organised and aimless psycho-motor activity stemming from physical or mental unrest, with motor restlessness and heightened responsivity to stimuli hallmark features (Reference LindenmayerLindenmayer 2000).
However, despite the multitude of formal definitions, the vagary and subjectivity of terms such as ‘judged’ (used in the second definition above) persisted, and a uniform definition could not be reached.
Assessment scales
Several scales have been developed to provide standardisation of agitation assessment, including, but not limited to, the Overt Agitation Severity Scale (Reference Yudofsky, Kopecky and KunikYudofsky 1997) and the Agitated Behavior Scale (Reference CorriganCorrigan 1989), which are used in the context of traumatic brain injury, and the Positive and Negative Syndrome Scale, Excited Component (PANSS-EC; Reference Kay, Fiszbein and OplerKay 1987), used in the context of psychotic illness. These scales tend to conceptualise agitation on a spectrum, ranging from anxiety to aggression, and reflecting the three major components present in most definitions of agitation: strong emotion, excessive motor/vocal behaviour, and inappropriate or non-purposeful motor/vocal behaviour (Reference CitromeCitrome 2004a). Anxiety, at one end of the spectrum, as defined by DSM-IV (American Psychiatric Association 1994), entails subjective symptoms manifested through a variety of somatic complaints, including distractibility, nervousness and difficulty concentrating. At the other end of the spectrum, aggression is more consistent with verbal abuse, verbal and physical threats, and threats of violence (Reference Ness, House and NessNess 2000). In some cases, agitation may even manifest as explicit violent behaviour directed at others (Reference Kopecky, Kopecky and YudofskyKopecky 1998).
Evolution of management methods
In the USA, as in other countries, early efforts at managing agitation were focused on physically restraining and/or sedating the patient, a form of ‘chemical restraint’ – generating a stuporous or even unconscious state in an agitated patient (Reference WiseWise 2000). Rather than addressing the source of agitation, sedation merely masked it. This masking effect hindered not only agitation management but also diagnostic efforts. Sedation may be mistaken for negative symptoms and/or cognitive deficits (Reference MillerMiller 2004), compromising assessment and diagnosis, and may also contribute to non-adherence to medication because of patients’ negative feelings about taking it (Reference CitromeCitrome 2004a).
Agitation management has evolved significantly, with a strong move away from physical and ‘chemical’ restraints that risk physically injuring (Reference Buckley, Noffsinger and SmithBuckley 2003) or over-sedating patients (Reference Downey, Zun and GonzalesDowney 2007). Nevertheless, these restraint methods continue to be used in about 60% of emergency departments throughout the USA (Reference Downey, Zun and GonzalesDowney 2007).
The introduction of antipsychotics and rapid tranquillisation
The use of typical antipsychotics in agitation management brought a respite from the overly sedating benzodiazepines and barbiturates that effectively kept patients asleep, obviating the need to deal with their agitated behaviour directly. With typical antipsychotics, the sources of agitation could be directly targeted rather than masked. Antipsychotics provided a mechanism of treating the underlying cause of agitation, mainly psychosis, thus calming the patient while reducing unnecessary and unwanted sedation (Reference WiseWise 2000).
As in the UK, rapid tranquillisation was introduced. Described as ‘the use of psychotropic medication to control agitated, threatening or destructive psychotic behaviour’ (Reference Pereira, Paton and WalkertPereira 2005), such tranquillisation offered calming without sedation (Reference McAllister-Williams and FerrierMcAllister-Williams 2002).
Unfortunately, the typical antipsychotics brought with them significant adverse effects such as movement disorders and cardiac complications, including the risk of sudden cardiac death (Reference Abdelmawla and MitchellAbdelmawla 2006).
In 2002, with the licensing in the USA of the first fast-acting intramuscular atypical antipsychotic (ziprasidone), it became possible to calm an agitated patient rapidly by targeting the underlying source of agitation, with less sedation and without the unpleasant, unnecessary and potentially fatal adverse effects more commonly seen with typical antipsychotics. The atypicals have been proven to be at least as efficacious as the typicals in reducing agitation and other symptoms of psychosis, but with a much reduced risk of medication-induced movement disorders (Reference CanasCanas 2007). Consequently, atypical antipsychotics have been promoted to first-line choices in the treatment of agitation in acute psychotic disorders (Reference Mohr, Pecenák and SvestkaMohr 2005).
Modern management of acute agitation
Consensus guidelines (Expert Consensus Panel for Behavioral Emergencies 2005) now indicate that clinicians should aim to calm rather than sedate acutely agitated patients (Reference Battaglia, Lindborg and AlakaBattaglia 2003). The ultimate goal of management is more than just tranquillisation: it is to defuse the state of hyperarousal as rapidly and safely as possible and to restore the patient to ‘optimal self-regulatory functioning’ (Reference CitromeCitrome 2004a).
Non-pharmacological interventions
In the UK, the National Institute for Health and Clinical Excellence (NICE) emphasises the importance of non-pharmacological interventions in managing agitation, recommending that rapid tranquillisation (also known as urgent sedation) be used only ‘in situations requiring the rapid control of agitation, aggression or excitement … when other less coercive techniques of calming … such as verbal de-escalation … have failed’ (National Collaborating Centre for Nursing and Supportive Care 2005: p. 27).
In both the USA and the UK, the primary concern when first approaching an agitated patient is the safety of the patient and those nearby. For this reason, isolation of the patient from others while the crisis is contained is crucial (Reference WiseWise 2000). Stimulation from radio, television and so on may also contribute to an agitated state and should be minimised. Verbal one-on-one interaction (de-escalation) has been demonstrated to reduce anxiety and help patients regain control and should be enacted early in management (Reference FisherFisher 1994).
In the USA, the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) mandated in 2000 that restraint and/or seclusion can only be used in an emergency, when other attempts to manage agitation have failed and there is imminent risk of harm to a patient or others (Reference WiseWise 2000). Therefore, in the USA seclusion and restraints are considered treatments of last resort and should be used as such (Reference Buckley, Noffsinger and SmithBuckley 2003). In the UK, however, the NICE guidelines recommend seclusion if rapid tranquillisation is contraindicated but they note that it should not be considered a therapeutic intervention; rather, it should be seen as allowing a period of calming for the patient.
The UK is in agreement with the USA in recommending that physical restraint (which NICE calls physical intervention) be used only as a last resort, when there is a real possibility of significant harm if it is not used. In most situations, the restraint should be limited to manual holding: mechanical devices such as belts or handcuffs should not be used, except in exceptional circumstances, usually within high-security settings (National Collaborating Centre for Nursing and Supportive Care 2005: p. 26).
Pharmacological interventions (rapid tranquillisation)
Pharmacological management of acute agitation has been typically limited to four classes of medication: barbiturates, benzodiazepines, typical antipsychotics and, more recently, atypical antipsychotics. For years, barbiturates and then typical antipsychotics with or without benzodiazepines (most commonly, intramuscular administration of 5 mg haloperidol and 2 mg lorazepam) have been the mainstay of treatment (Reference BellnierBellnier 2002). However, 93% of patients prefer oral formulations during a behavioural emergency, perceiving parenteral administration as coercive and abusive (Reference Altamura, Sassella and SantiniAltamura 2003). Recent literature has shown that, for patients willing to take them, oral atypical antipsychotics are at least as effective as intramuscular typical antipsychotics in acute psychotic agitation (Reference Currier, Trenton and WalshCurrier 2006a). They should therefore be the first-line method of pharmacological administration (Reference Mohr, Pecenák and SvestkaMohr 2005).
In 2005, the US Journal of Psychiatric Practice published a supplement on the treatment of behavioural emergencies as part of its Expert Consensus Guidelines Series (Expert Consensus Panel for Behavioral Emergencies 2005). It was generated from responses to a survey by 48 of 50 invited leading American experts in psychiatric emergency medicine. The publication received financial support in the form of grants from AstraZeneca, Janssen and Pfizer but, to reduce any possible bias, the experts contacted were not aware of this. The survey resulted in the following recommendation for first-line treatment of acute schizophrenia or mania: oral olanzapine, oral risperidone, or haloperidol plus a benzodiazepine (Expert Consensus Panel for Behavioral Emergencies 2005).
Oral disintegrating (orodispersible) formulations that dissolve in seconds prevent patients from hiding tablets in their mouths and subsequently spitting them out; they are bioequivalent to regular tablets (Reference Van Schaick, Lechat and RemmerieVan Schaick 2003). However, the efficacy of oral formulations is limited by patients’ cooperation in taking them, which is problematic in many severely agitated patients. One literature review found that severe agitation could be managed with oral formulations alone in only 55% of cases (Reference De FruytDe Fruyt 2004).
The NICE guidelines recommend that vital signs be monitored after rapid tranquillisation and that pulse oximeters be available. Blood pressure, pulse, temperature, respiratory rate and hydration should be recorded regularly (National Collaborating Centre for Nursing and Supportive Care 2005: p. 102).
The most commonly used and recommended pharmacological agents for the management of agitation in psychosis are reviewed in the following sections.
Benzodiazepines
Benzodiazepines (Table 1) produce anti-agitation effects via modulation of γ-aminobutyric acid (GABA) neurotransmission and evidence suggests that they are at least as effective as – and at higher doses superior to – typical antipsychotics in agitation treatment (Reference Foster, Kessel and BermanFoster 1997; Reference AllenAllen 2000; Reference BattagliaBattaglia 2005). Benzodiazepines have significantly fewer extrapyramidal side-effects than typical antipsychotics, but they can cause respiratory depression, ataxia and excessive sedation (Reference AllenAllen 2000; Reference BattagliaBattaglia 2005). The sedative effect of benzodiazepines is the major limiting factor in their use for managing agitation: as discussed above, sedation should not be the endpoint of management and may hinder diagnosis.
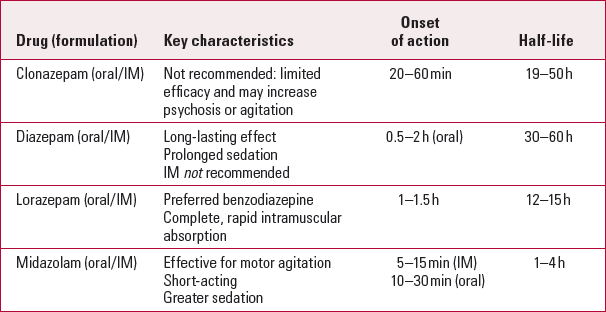
TabLE 1 Benzodiazepines for acute agitation
| Drug (formulation) | Key characteristics | Onset of action | Half-life |
|---|---|---|---|
| Clonazepam (oral/IM) | Not recommended: limited efficacy and may increase psychosis or agitation | 20–60 min | 19–50 h |
| Diazepam (oral/IM) | Long-lasting effect Prolonged sedation IM not recommended |
0.5–2 h (oral) | 30–60 h |
| Lorazepam (oral/IM) | Preferred benzodiazepine Complete, rapid intramuscular absorption |
1–1.5h | 12–15 h |
| Midazolam (oral/IM) | Effective for motor agitation Short-acting Greater sedation |
5–15 min (IM) 10–30 min (oral) |
1–4 h |
Lorazepam is the most popular benzodiazepine for agitation, owing to its complete and rapid intramuscular absorption, onset within 60–90 minutes, half-life of 12–15 hours, and 8–10 hour duration of action (Reference BattagliaBattaglia 2005; Reference Greenblatt, Blaskovich and NuwayserGreenblatt 2005). Diazepam is frequently used for its long-lasting effect, but prolonged sedation of a patient is often not desirable, making shorter-acting benzodiazepines such as lorazepam more attractive. Evidence for the superiority of midazolam over haloperidol for managing motor agitation is encouraging, but it is overly sedating and the majority of patients treated with midazolam fell asleep after intramuscular administration (Reference Mendoza, Djenderedjian and AdamsMendoza 1987). Clonazepam has limited efficacy in treating agitation. It may in fact increase psychosis or agitation in some individuals and is therefore not recommended (Reference BattagliaBattaglia 2005).
Typical antipsychotics
Typical antipsychotics, especially haloperidol, have been a mainstay of agitation treatment. They are thought to produce anti-agitation effects by inhibition of dopaminergic transmission, along with histamine and noradrenaline (norepinephrine) blockade, although the blockade mechanism has been less clearly established (Reference LeonardLeonard 1992; Reference Altamura, Sassella and SantiniAltamura 2003).
Adverse effects more commonly seen in typical antipsychotic usage include extrapyramidal symptoms of dystonia, akathisia and Parkinsonism. In addition, antipsychotics (typical and atypical) carry the risk of neuroleptic malignant syndrome. Another critical concern for antipsychotics is the increased risk of cardiac arrhythmias associated with prolongation of the QT interval.
Haloperidol is the most frequently used typical antipsychotic for the management of acute agitation and has an onset of action from 15 to 60 minutes (Reference BattagliaBattaglia 2005; National Collaborating Centre for Nursing and Supportive Care 2005). Chlorpromazine (both oral and intramuscular) is not recommended because of the hypotensive and QTc interval effects associated with its use at the doses suitable for rapid tranquillisation. Intramuscular haloperidol at 6.5 mg yields an NNTFootnote a of 5 (95% CI 3–11) and at 7.5 mg an NNT of 3 (95% CI 2–5) (Reference CitromeCitrome 2007).
The most commonly problematic adverse effects with haloperidol are extrapyramidal symptoms, for which anticholinergics may be given as needed or prophylactically (Reference BattagliaBattaglia 2005).
Owing to its pervasive use and established efficacy in managing agitation in psychosis, haloperidol has become the gold standard against which all anti-agitation medications, including the atypical antipsychotics, are measured.
Atypical antipsychotics
Atypical antipsychotics (Table 2) are a newer development in the management of agitation. Owing to their action on serotonergic as well as dopaminergic receptors they carry an adverse risk profile distinct from that of typical antipsychotics, with less dysphoria, akathisia and extrapyramidal side-effects (Reference BattagliaBattaglia 2005). Interestingly, some patients have even been reported to request an additional dose of an atypical antipsychotic when being treated for an agitated state in the emergency room (Reference Preval, Klotz and SouthardPreval 2005).
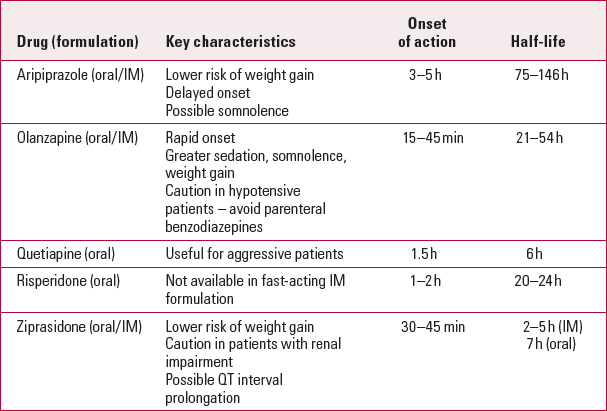
TabLE 2 Atypical antipsychotics for acute agitation
| Drug (formulation) | Key characteristics | Onset of action | Half-life |
|---|---|---|---|
| Aripiprazole (oral/IM) | Lower risk of weight gain Delayed onset Possible somnolence |
3–5 h | 75–146h |
| Olanzapine (oral/IM) | Rapid onset Greater sedation, somnolence, weight gain Caution in hypotensive patients – avoid parenteral benzodiazepines |
15–45 min | 21–54 h |
| Quetiapine (oral) | Useful for aggressive patients | 1.5 h | 6 h |
| Risperidone (oral) | Not available in fast-acting IM formulation | 1–2 h | 20–24h |
| Ziprasidone (oral/IM) | Lower risk of weight gain Caution in patients with renal impairment Possible QT interval prolongation |
30–45 min | 2–5 h (IM) 7 h (oral) |
Atypicals are available in both oral and intramuscular formulations. Intramuscular ziprasidone was made available in the USA in 2002, with indications for agitation associated with schizophrenia. Intramuscular olanzapine followed in 2004 for agitation associated with schizophrenia and bipolar mania. Finally, in 2006, intramuscular aripiprazole became available for agitation associated with schizophrenia and bipolar mania (Reference CitromeCitrome 2007).
The consensus guidelines mentioned above (Expert Consensus Panel for Behavioral Emergencies 2005) currently recommend atypical anti-psychotics as first-line agents for acute agitation in schizophrenia, although evidence of atypical antipsychotic efficacy in managing agitation in bipolar I disorder has also been established (Reference Meehan, Zhang and DavidMeehan 2001; Reference Zimbroff, Marcus and ManosZimbroff 2007). Olanzapine and risperidone are both available in disintegrating oral tablets and are therefore optimal as first-line interventions for patients who will take them.
Ziprasidone
Ziprasidone primarily acts as a dopamine/ serotonin antagonist. It has low potential for causing extrapyramidal symptoms and was the first atypical antipsychotic made available in rapid-acting intramuscular formulation, with an onset of action of 30–45 minutes (Reference BattagliaBattaglia 2005).
In the USA, the drug was approved for acute agitation in schizophrenia on the basis of two double-blind trials (Reference Daniel, Potkin and ReevesDaniel 2001; Reference Lesem, Zajecka and SwiftLesem 2001). Ziprasidone (10–20 mg) yields an NNT of 3 (95% CI 2–4) with the response criterion defined as at least a 2-point reduction in the Behavioural Activity Rating Scale, 2 hours after injection (Reference CitromeCitrome 2007). Incidence of movement disorders, including akathisia, dystonia and extrapyramidal symptoms, is < 4% compared with 12–38% for haloperidol (Reference MendelowitzMendelowitz 2004).
Safety concerns with intramuscular ziprasidone include caution when treating patients with renal impairment, as the cyclodextrin excipient is cleared by renal filtration. Ziprasidone is also known to cause greater dose-related QT interval prolongation than haloperidol, olanzapine and risperidone. Owing to fatal arrhythmias associated with QT prolongation caused by other drugs, ziprasidone is contraindicated in patients with a known history of QT prolongation, recent myocardial infarction or uncompensated heart failure (Reference GlassmanGlassman 2001; Pfizer 2009).
Olanzapine
Olanzapine, which acts on multiple receptors, has an onset of action of 15–45 minutes (Reference BattagliaBattaglia 2004). Three placebo-controlled trials that showed the superiority of 2.5–10 mg of intramuscular olanzapine over placebo and its non-inferiority to 7.5 mg intramuscular haloperidol provided the basis for olanzapine use in acute agitation associated with schizophrenia (Reference Wright, Birkett and DavidWright 2001; Reference CitromeCitrome 2007). However, placebo-controlled trials have indicated a higher incidence of sedation compared with placebo, with somnolence present in 29% of patients compared with 13% in the placebo arm (Eli Lilly 2006). Olanzapine can be as sedating as haloperidol or lorazepam (Reference Currier, Allen and BunneyCurrier 2004a). In a trial involving patients with schizophrenia, the NNT for 10 mg olanzapine was 3 (95% CI 2–3). In bipolar I mania, 10 mg olanzapine also yielded an NNT of 3 (95% CI 2–5) (Reference Meehan, Zhang and DavidMeehan 2001). Intramuscular olanzapine is as efficacious as haloperidol and has significant advantages in terms of extrapyramidal symptoms: Parkinsonism was avoided in 1 in 7 patients, acute dystonia in 1 in 14, and anticholinergic prescriptions were avoided in 1 in 7 patients (Reference Tulloch and ZedTulloch 2004; Reference CitromeCitrome 2007).
Safety concerns related to olanzapine include hypotension, bradycardia with or without hypotension, tachycardia and syncope. Consequently, parenteral benzodiazepines should be avoided in patients simultaneously receiving intramuscular olanzapine (Eli Lilly 2008). The number needed to harm (NNH) was significant for hypotension, at 50 (95% CI 30–154) compared with placebo (Reference CitromeCitrome 2007).
If possible, an oral formulation should be given at a loading dose of 5 to 20 mg, as this has been shown to be safe and effective at quickly calming acutely agitated psychotic patients (Reference Karagianis, Dawe and ThakurKaragianis 2001). Oral olanzapine can be used alone or with oral lorazepam, which has been shown to be as effective as oral haloperidol plus lorazepam in reducing acute agitation (Reference Escobar, San and PerezEscobar 2008). Oral olanzapine is uniquely advantageous in its efficacy as a monotherapy in both emergency rooms and psychiatric units, which not only contributes to medication adherence and a positive doctor–patient relationship, but also avoids the adverse effects often seen in transition from intramuscular to oral medications (Reference Escobar, San and PerezEscobar 2008).
Risperidone
Risperidone is a potent dopamine antagonist with high affinity for D2 receptors and action on multiple serotonergic receptors. Despite the fact that risperidone is not currently available in a fast-acting intramuscular formulation, it has been found to be effective in managing agitation in patients willing to take oral medication. Earlier studies found that oral risperidone plus oral lorazepam was tolerable and comparable to intramuscular haloperidol plus lorazepam for short-term treatment of agitated psychosis in patients who accept oral medications (Reference Currier and AllenCurrier 2000, Reference Currier and Simpson2001, Reference Currier, Chou and Feifel2004b). A later study found that oral risperidone plus oral lorazepam was more successful at managing acute psychotic agitation 2 hours after administration when compared with intramuscular conventional antipsychotics such as haloperidol and zuclopenthixol (Reference Lejeune, Larmo and ChrzanowskiLejeune 2004). A meta-analysis found that risperidone yielded superior efficacy to haloperidol in managing hostility and aggression in patients with schizophrenia (Reference Aleman and KahnAleman 2001).
Aripiprazole
Aripiprazole differs pharmacologically from the other atypical antipsychotics in its partial agonism at dopamine D2 and serotonin 5-HT1A receptors and antagonism at 5-HT2a receptors (Reference Burris, Molski and XuBurris 2002). In at least three randomised, double-blind, placebo-controlled studies it was found to be efficacious, safe and tolerable in treating agitation in patients with bipolar I disorder, schizophrenia or schizoaffective disorder (Reference Andrezina, Josiassen and MarcusAndrezina 2006; Reference Currier, Citrome and ZimbroffCurrier 2007; Reference Tran-Johnson, Sack and MarcusTran-Johnson 2007). In one of these studies (Reference Andrezina, Josiassen and MarcusAndrezina 2006) the efficacy of 9.75 mg intramuscular aripiprazole was established v. 6.5 mg intramuscular haloperidol. Aripiprazole demonstrated rapid and effective control of agitation in schizophrenia and schizoaffective disorder with a significant reduction in events involving extrapyramidal side-effects (1.7% v. 12.6%) (Reference CitromeCitrome 2007). A dose of 9.75 mg intramuscular aripiprazole was shown to be the most effective and best tolerated from a range of 1, 5.25, 9.75 and 15 mg (Reference GlassmanGlassman 2001). In schizophrenia, 9.75 mg aripiprazole has an NNT of 6 (95% CI 4–16); in bipolar mania, 10 mg and 15 mg aripiprazole have an NNT of 4 (95% CI 3–10) (Reference CitromeCitrome 2007). Aripiprazole has been associated with reduced sedation compared with placebo (Reference CitromeCitrome 2007), but somnolence has been reported to be marginally higher than with placebo (Bristol-Myers Squibb 2003). Compared with those on haloperidol, patients on aripiprazole had a 10% lower risk of extrapyramidal side-effects (Reference CitromeCitrome 2007).
Quetiapine
In emergency room psychiatric settings, quetiapine at doses ranging from 300 to 800 mg/day has been shown to be as effective as olanzapine and risperidone, and better tolerated than haloperidol (Reference Villari and RoccaVillari 2008), despite preliminary data that suggested it to be less efficacious than olanzapine and risperidone (Reference Raja and AzzoniRaja 2003). Relative to haloperidol, quetiapine at doses ranging from 150 to 750 mg has direct calming effects on agitation independent of its effect in improving psychosis. It demonstrated a reduction in both hostility and agitation among patients experiencing an acute exacerbation of schizophrenia (Reference Chengappa, Goldstein and GreenwoodChengappa 2003). In patients with aggressive psychosis, it has been reported to reduce overall aggression within the first 24 hours of treatment, aggression towards others by 83% within 2 days of treatment, and sustained reduction in overall aggression over 5 days of treatment (Reference Ganesan, Bilsker and KhanbhaiGanesan 2005).
The transition from intramuscular to oral formulations
Consensus guidelines recommend switching from intramuscular medication to oral formulations when managing long-term agitation subsequent to acute episodes (Expert Consensus Panel for Behavioral Emergencies 2005). This transition, although recommended, can engender adverse effects. Such effects are more common with typical than with atypical antipsychotics. In a comparison of haloperidol (without concomitant anticholinergic medication) and olanzapine, dystonia affected 4.3% v. 0% (P = 0.026) and akathisia affected 5.2% v. 0% (P = 0.013) of participants on transition from intramuscular to oral formulations of the respective drugs (Reference Wright, Meehan and BirkettWright 2003). The same study reported that the initial alleviation of agitation was sustained in the transition. The absence of spontaneously reported acute dystonia in the olanzapine-treated patients over several days of continued oral treatment demonstrated its superior extrapyramidal side-effect safety profile compared with haloperidol.
Haloperidol has demonstrated increased rates of events involving extrapyramidal side-effects in transition compared with aripiprazole and ziprasidone and it frequently requires concomitant administration of anticholinergic medications (Reference Daniel, Zimbroff and SwiftDaniel 2004; Reference CitromeCitrome 2007). One of the major benefits of using the more expensive atypical anti-psychotics is the obviation of anticholinergic use both prophylactically and acutely (Reference Raja and AzzoniRaja 2001).
Comparison of atypical antipsychotics
Relative to placebo, the atypical antipsychotics demonstrate notable efficacy in the management of acute agitation in the populations discussed above. Additionally, they significantly reduce the incidence of movement disorders and sedation compared with older treatments. However, there are significant differences between the atypical antipsychotics that should help guide the decision-making process regarding their use. Unfortunately, only a few head-to-head comparisons of the atypicals have been conducted (see below). Most of the comparisons of efficacy have been generated relative to respective comparisons with placebo and older treatments, including haloperidol and lorazepam.
Regarding US Food and Drug Administration (FDA) indications (analogous to ‘licenses’ in the UK), ziprasidone is indicated for agitation in schizophrenia only, while olanzapine and aripiprazole are the only atypical antipsychotics discussed with indications for agitation associated with either schizophrenia or bipolar I mania. Neither risperidone nor quetiapine, despite their established efficacy, currently have specific indications for the treatment of agitation in schizophrenia or bipolar I mania. However, the absence of specific indications for agitation should not necessarily dissuade the provider from choosing a particular agent for the management of agitation, as the process of obtaining additional indications for an already approved medication is both lengthy and costly.
Efficacy
Earlier research indicated that olanzapine is the most effective oral atypical antipsychotic as, unlike risperidone, it does not require adjunctive oral lorazepam (Reference Currier and SimpsonCurrier 2001). However, a more recent trial compared oral risperidone solution monotherapy with oral olanzapine tablets and found equal efficacy in successfully reducing acute agitation (Reference Hatta, Kawabata and YoshidaHatta 2008). One ambitious study compared five atypical antipsychotics with each other and demonstrated superior efficacy of olanzapine and risperidone over quetiapine, ziprasidone and aripiprazole for the acute treatment of schizophrenia, schizoaffective disorder or schizophreniform disorder (Reference McCue, Waheed and UrcuyoMcCue 2006). Another study, conducted in an emergency room, found olanzapine and risperidone to be superior in efficacy to quetiapine in treating acute psychosis (Reference Raja and AzzoniRaja 2003). In a head-to-head trial involving acutely ill patients with schizophrenia, olanzapine and aripiprazole produced similar improvement in agitation and positive symptoms during the first 5 days of in-patient treatment (Reference Kinon, Stauffer and Kollack-WalkerKinon 2008).
The literature thus suggests that the five atypical antipsychotics discussed here have all demonstrated efficacy in managing agitation in psychosis. However, since olanzapine and risperidone have demonstrated superiority in monotherapy and are both available in oral formulations, there may be an advantage in their use at the onset of treatment.
Safety
Ziprasidone should be used with caution in patients with renal impairment and is contraindicated in patients with cardiac impairment, as mentioned above, and in patients taking medications that also prolong the QT interval. Olanzapine has a significant risk of hypotension and should be used with caution in patients with conditions that predispose to hypotension. Parenteral benzodiazepines with intramuscular olanzapine or aripiprazole should be used only if essential and with caution because of the increased risk of excessive sedation and cardiorespiratory depression. Since aripiprazole and olanzapine have been shown to be effective in monotherapy this should not be a frequent problem. In a small open-label study of quetiapine, orthostasis was present in 40% of patients at 100–200 mg doses (Reference Currier, Trenton and WalshCurrier 2006a ).
Antipsychotics and the risk of sudden cardiac death
Cardiovascular causes account for 5% of sudden and unexpected deaths in patients with schizophrenia. Prospective studies have indicated that patients with QT interval prolongation beyond 500 ms are at an increased risk of arrhythmias (e.g. ventricular tachycardia) and torsades de pointes (Reference Abdelmawla and MitchellAbdelmawla 2006). Ziprasidone has been shown to increase the QT interval by 15–35 ms, and haloperidol, quetiapine and olanzapine do so by 5–15 ms (Reference Abdelmawla and MitchellAbdelmawla 2006). Antipsychotics affect the QT interval via blockade of various cardiac ion channels. In particular, blocking of potassium channels is responsible for slowing repolarisation, which leads to prolongation of the QRS interval and ultimately extension of the QT interval (Reference DuBuskeDuBuske 1999). Prospective cohort trials of antipsychotic use reported relative risks of sudden death of 2.06 over 4 years (Reference Montout, Casadebaig and LagnaouiMontout 2002) and 2.39 over 5 years (Reference Ray, Meredith and ThapaRay 2001). These effects tend to be dose-dependent and are of greatest concern in patients who take large overdoses (Reference Abdelmawla and MitchellAbdelmawla 2006). However, in cases of emergency use for the management of agitation (rapid tranquillisation), several large trials involving over 1500 patients given haloperidol, ziprasidone, olanzapine, midazolam or a haloperidol–promethazine mix reported no deaths or serious cardiovascular events (TREC Collaborative Group 2003; Reference Citrome, Brook and WarringtonCitrome 2004b). Despite the relatively low risk related to antipsychotics in emergency (rapid tranquillisation) settings, the patient's medical history and baseline electrocardiogram can help guide antipsychotic choice; if a patient has risk factors for sudden cardiac death or QT prolongation, haloperidol or olanzapine would be preferred to quetiapine and ziprasidone, which are known to cause a greater prolongation (Reference Abdelmawla and MitchellAbdelmawla 2006).
Clinical guidelines
Figure 1 outlines the procedure to follow in managing acute agitation, and medication choices if verbal de-escalation is unsuccessful.

FIG 1 Management of psychotic agitation. Medications are listed in numerical order of preferred use, given demonstrated efficacy while minimising sedation. Medications listed as a, b, c are comparable options with similar efficacy and should be selected on the basis of the patient's medical profile. CNS, central nervous system.
Ensuring safety
The first step should be to maximise safety for all those present: the individual should be isolated from other patients (Reference MarderMarder 2006) and distractions such as TV and radio should be switched off (Reference CitromeCitrome 2004a).
Verbal de-escalation
Staff should present a non-aggressive show of force and demonstrate that they are in control of the situation (Reference CitromeCitrome 2004a). They should convey calm, empathy, concern for the patient's well-being and assurances that the patient is safe (Reference PetitPetit 2005). The patient should be allowed to express their feelings and concerns, and should not be shouted at or threatened (Reference CitromeCitrome 2004a). Simultaneously, the patient should be assessed for changes in medical condition, possible substance intoxication and development of akathisia.
Pharmacotherapy
Medication may be initiated alone or as an adjunct to unsuccessful or only mildly successful verbal de-escalation, and agent(s) must be selected with consideration for aetiology of the agitation and diagnosis (Reference PetitPetit 2005). If the patient is willing and able to take them, oral atypical antipsychotics should be the drug of first choice.
In agitation secondary to substance intoxication, evidence-based guidelines recommend treatment based on underlying aetiology, if known (Reference AllenAllen 2000). This recommendation is very important as certain drugs of misuse have anticholinergic properties and psychotropic drugs with anti-cholinergic effects may potentiate the toxicity of the substance. Therefore, if anticholinergic delirium is suspected in an agitated patient, benzodiazepines are the first-line choice of pharmacotherapy and antipsychotics should be avoided (Reference CitromeCitrome 2004a; Reference PetitPetit 2005).
Seclusion and restraint
Seclusion and physical restraint should be considered only as a last resort, if behavioural modifications and pharmacotherapy fail or are contraindicated, and/or if patients are a danger to themselves or others.
Conclusions
The management of agitation in the context of psychosis presents a challenge that is as multi-faceted as it is prevalent. Over the past decade, there have been significant contributions to the research of agitation which now afford clinicians an extensive assortment of treatment options. However, rather than using the most current, evidence-based practices to tailor customised treatment plans for their agitated patients, clinicians often fall back on ‘what works’ (in their experience). The goal of this article was to provide a thorough and concise review of the literature to enable clinicians to effectively manage agitation with minimal detriment to the diagnostic process, and, most importantly, to the patient.
MCQs
Select the single best option for each question stem
-
1 The pharmacological agent that has demonstrated particular efficacy in managing hostility/aggression in agitation is:
-
a risperidone
-
b midazolam
-
c lorazepam
-
d quetiapine.
-
-
2 The pharmacological agent with quickest onset of action is:
-
a olanzapine
-
b risperidone
-
c lorazepam
-
d midazolam.
-
-
3 The first step in managing acute agitation should be:
-
a prepare intramuscular haloperidol and lorazepam
-
b physically restrain the patient
-
c offer patient oral risperidone
-
d isolate patient from other patients.
-
-
4 Which of the following has the greatest potential to increase psychosis and/or worsen agitation?
-
a lorazepam
-
b midazolam
-
c diazepam
-
d clonazepam.
-
-
5 Which class of pharmacological agent should be avoided in patients who are being given olanzapine?
-
a selective serotonin reuptake inhibitors
-
b typical antipsychotics
-
c benzodiazepines
-
d fluoroquinolones.
-
MCQ answers

| 1 | d | 2 | d | 3 | d | 4 | d | 5 | c |






eLetters
No eLetters have been published for this article.