There have been several developments of relevance to practising addictions professionals since the publication of my previous articles in Advances (Reference LutyLuty 2003, Reference Luty2006). In this article I review new challenges in the addictions field. In the next issue I will discuss developments in pharmacological treatments (Reference LutyLuty 2015, in press), after which I will address psychosocial treatment.
Worldwide, smoking is the second leading risk factor contributing to the total burden of disease, alcohol is third and drug misuse is nineteenth (**Reference Lim, Vox and FlaxmanLim 2012) (Box 1). To put this into context, smoking is now the leading cause of preventable deaths (435 000 deaths per year) in the USA, alcohol is the third (85 000) and drug misuse is tenth (17 000 deaths) (**Reference Mokdad, Marks and StroupMokdad 2004). Smoking cessation treatment tends to be provided by services separate from conventional drug and alcohol teams, so these will not be considered here.
BOX 1 Worldwide ranking of causes of preventable death
-
1 High blood pressure
-
2 Tobacco smoking
-
3 Alcohol use
-
4 Household air pollutants from solid fuels
-
5 Diet low in fruit
-
6 High body mass index (obesity)
-
7 High fasting plasma glucose (diabetes)
-
8 Childhood underweight (malnutrition)
-
9 Ambient particulate matter pollution (air pollution)
-
10 Physical inactivity
-
11 Diet high in sodium
-
12 Diet low in nuts and seeds
-
13 Iron deficiency
-
14 Suboptimal breastfeeding
-
15 High total cholesterol
-
16 Diet low in grains
-
17 Diet low in vegetables
-
18 Diet low in seafood omega-3 fatty acids
-
19 Drug use
-
20 Occupational injuries
There are significant political controversies relating to alcohol and drug addiction. For example, minimum pricing for alcohol has been postponed in Scotland and abandoned in the rest of the UK, despite overwhelming evidence for its effectiveness (**Reference Stockwell, Auld and ZhaoStockewell 2012; Reference GornallGornall 2014; **Reference Sheron, Chilcoot and MatthewsSheron 2014). This inaction is presumably due to legal challenge and lobbying from the drinks industry (**Reference Babor, Caetano and CasswellBabor 2003). In August 2013, Uruguay became the first country in modern times to legalise the production and sale of cannabis (marijuana). In February 2013, 18 states of the USA had passed laws allowing cannabis to be used for a variety of medical conditions, while in 2012, voters in two states, Colorado and Washington State, approved policies legalising the sale and recreational use of marijuana.
Principal changes to the addictions field, especially in the UK, include the development of non-statutory services and non-medical prescribers, the rise and fall of coercive treatment (such as the UK drug treatment and testing orders and drug rehabilitation requirements) and the increasing move away from residential treatment and brief admissions for detoxification. These political controversies will not be explored further here other than consideration of abstinence v. methadone maintenance treatment.
It is notable that the new diagnostic criteria in DSM-5 (American Psychiatric Association 2013) have removed the distinction between substance misuse and dependence by creating a continuum on which severity is determined by the number of clinical features from 2 (mild) to 6 or more (severe).
It is also salutary to commemorate the passing in 2012 of Professor Griffiths Edwards and Professor Hamid Ghodse. In a seminal report from 1976, Professor Edwards created the classification of alcohol dependence based on criteria including craving, withdrawal and tolerance which is still in use. Professor Ghodse founded the International Centre for Drug Policy at St George’s, University of London.
Abstinence v. maintenance
Abstinence-based treatments comprise the majority of treatment options for substance misuse, with the prominent exception of opioid dependence. Detoxification is the process of reducing and stopping the use of addictive substances such as alcohol, often with some form of medical assistance (e.g. a prescription for benzodiazepines over 7–10 days). Stimulants (such as cocaine and amphetamine) do not require any prescribed medication to assist detoxification although, in practice, hypnotics or other sedative medication are often provided for a short time.
Subsequent editions of the UK Department of Health’s clinical guidelines for substance misuse have oscillated between abstinence and maintenance treatment, with the prevailing emphasis (HM Government 2010) currently in favour of abstinence. This illustrates the cyclical nature of the debate and possibly the preference of incumbent governments.
Maintenance treatment is only recommended for opioid dependence. Methadone (or buprenorphine or lofexidine) may be used in short-term opioid detoxification (over 7–28 days). Alternatively, a maintenance prescription may be used whereby provision of a substitute drug (historically this has been methadone) continues over months or years with no requirement stop. (The term ‘methadone maintenance’ is often subsumed under the title ‘harm reduction’, which also includes other measures such as needle exchanges.)
There is overwhelming evidence that long-term maintenance treatment is consistently superior to detoxification (and other ‘abstinence-based treatments’) in the treatment of opioid dependence. For example, a report from British Columbia of over 25 000 methadone treatment episodes showed that in only 1 in 40 episodes did the individual achieve a successful ‘recovery’ – abstinence from prescribed methadone with no treatment reentry within 18 months. The authors concluded that, ‘the [vast] majority of patients attempting to taper from methadone maintenance treatment will not succeed’ (**Reference Nosyk, Sun and EvansNosyk 2012). There are multiple comparable research reports (**Reference Amato, Minozzi and DavoliAmato 2011). Fewer than 10% of patients dependent on prescribed opioids were able to achieve abstinence for 3 months after a 4-week taper using buprenorphine–naloxone (**Reference Weiss, Potter and FiellinWeiss 2011). Almost identical results were sensationally reported from the UK in the press 6 years ago (Reference EastonEaston 2008). By contrast, around half of patients who are maintained on methadone can almost completely abstain from heroin (**Reference Gossop, Marsden and StewartGossop 2001; **Reference Hubbard, Craddock and AndersonHubbard 2003).
Regardless of the scientific evidence, the political battle between abstinence and harm reduction continues to rage. For example, in 2010 the newly elected UK government announced that substance misuse services should follow a ‘recovery’ model (HM Government 2010). Patients on opioid substitution therapy are ‘encouraged’ to reduce and stop all addictive drugs, including prescribed methadone, although there is no time limit suggested for this process. This illustrates the barriers to adoption of evidence-based practice.
Misuse of prescribed opioids
Misuse of opioids that were prescribed for pain (Table 1) was not a recognised problem 20 years ago. Indeed, the prevailing view was that ‘strong’ opioids such as morphine should be used more liberally, especially in acute settings. In practice, these agents were used primarily for severe acute pain in hospitals or for cancer pain in the community (**Reference Freynhagen, Geisslinger and SchugFreynhagen 2013). There has been a significant increase in prescribing of high-potency opioids for non-cancer pain in recent decades, driven partly by aggressive marketing of these products (**Reference Freynhagen, Geisslinger and SchugFreynhagen 2013). Illicit prescription opioids have become the second most common illicit substance of misuse after cannabis (**Reference Weiss, Potter and FiellinWeiss 2011). In the USA in 2009, the use of a prescription opioid for non-medical reasons was 20 times more common than the use of heroin (**Reference Weiss, Potter and FiellinWeiss 2011). There are now more deaths in the USA from prescription opioids than from heroin, and deaths from overdose following diversion and misuse of these products is the second leading cause of accidental death. In 2010, 5.1 million people over 12 years of age in the USA reported that they had used prescription opioids non-medically in the previous month (1.7% of the entire population) (Substance Abuse and Mental Health Services Administration 2011).
TABLE 1 Relative potency (compared with morphine) and half-life of common prescribed opioids that are misused (oral formulation unless otherwise stated)
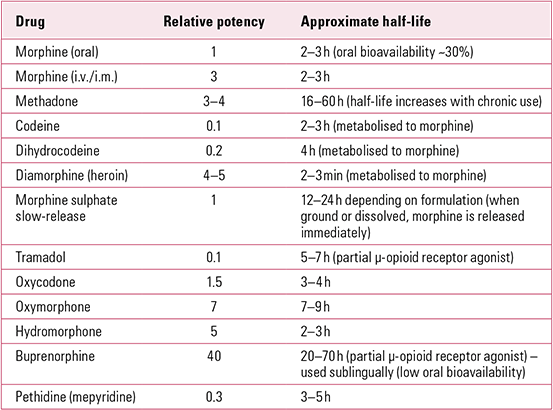
Estimates suggest that 4% of all opioid doses prescribed in the USA are resold on the black market (**Reference Katz, Birnbaum and CastorKatz 2010). The overall societal cost of prescribed opioid misuse in the USA in 2007 was estimated at almost $56 billion (US$ in 2009) (**Reference Birnbaum, White and SchillerBirnbaum 2011). Furthermore, there has been an 8% increase in patients seeking help for prescribed analgesic dependency in the UK over the past few years, with 154 deaths from tramadol (compared with 486 for methadone) in 2010–2011 (**Reference Ghodse, Corkery and SchifanoGhodse 2011; Reference StannardStannard 2013).
Expert reviews do not support the long-term prescription of opioids, especially at high doses, for many forms of non-cancer pain (**Reference Noble, Treadwell and TregearNoble 2010). For example, a Cochrane review in 2009 of 10 randomised trials of opioids for osteoarthritis showed that most opioids conferred minimal benefit (**Reference Nüesch, Rutjes and HusniNüesch 2009). Up to 1 in 3 patients who are prescribed opioids for chronic pain show signs of misuse or dependence, especially those with a history of substance use disorder (**Reference Boscarino, Rukstalis and HoffmanBoscarino 2011).
An instrument has been developed (**Reference Webster and WebsterWebster 2005) that might help minimise the misuse of prescribed opioids. Called the Opioid Risk Tool, it involves simple precautions such as drug testing, prescribing contracts specifying the duration of prescription and non-response criteria, objective measures of improvement, regular review, frequent dispensing of small quantities, use of a single prescriber and a single, named pharmacy. However, the principal advice from published guidelines is that opioids should be stopped if they do not help at reasonable doses.
A randomised trial has been reported involving 653 treatment-seeking out-patients dependent on prescription opioids who were prescribed a buprenorphine–naloxone combination to achieve abstinence. Only 6.6% of patients became abstinent from prescription opioids in phase 1 of the trial, a 1-month buprenorphine–naloxone taper plus 2-month follow-up. Those who relapsed were admitted to phase 2. These patients received 3 months of buprenorphine–naloxone stabilisation, followed by a 1-month taper and 2-month follow-up. Almost 50% achieved a successful outcome 2 months after the gradual taper (patients received a substitute prescription for 4 months). (For information, patients were randomised to medication only or medication plus counselling sessions, although the results showed the counselling strategy provided no additional benefit). The trial showed excellent results using a substitute prescription for a modest time rather than rapid detoxification. However, these patients were all in receipt of prescribed opioids rather than using illicit drugs. They were also required to express a desire to come off their prescription in order to take part in the trial. They were therefore likely to have better social functioning and greater motivation than many patients dependent on illicit heroin (**Reference Weiss, Potter and FiellinWeiss 2011).
Cardiac arrhythmias and methadone
Methadone is postulated to produce QT prolongation at high doses and in those with pre-existing cardiac disease. This is elegantly demonstrated in a large study of antidepressants in which methadone was included to demonstrate assay sensitivity. QT prolongation was dose-dependent even at modest doses of methadone (10–50 mg daily; **Reference Castro, Clements and MurphyCastro 2013). QT prolongation (typically over 450 ms in men and 470 ms in women) is thought to predispose to more serious, life-threatening arrhythmias such as torsades de pointes and ventricular fibrillation. However, the rate of serious cardiac events is uncertain in opioid-dependent patients. It is estimated that serious electrocardiogram (ECG) abnormalities affect 2–5% of these patients, while less serious abnormalities affect 10–30%. Patients in methadone treatment are at risk of sudden death as a result of other cardiovascular risk factors, especially smoking, endocarditis and stimulant misuse. Other risk factors for ECG abnormalities include the patient’s age, duration of methadone treatment, previous cardiovascular disease (including hypertension), male gender and concurrent medication (especially drugs that alter potassium balance). Hence, it is not possible to estimate the number, if any, of cardiac deaths that may be caused by methadone.
An expert panel of cardiologists recommended routinely asking patients about symptoms of syncope (such as faints and blackouts). The same panel also recommended that ECGs should be performed annually on all patients who are prescribed methadone, especially those on higher doses (perhaps arbitrarily taken to be doses more than 100 mg) (**Reference Krantz, Martin and StimmelKrantz 2009). Unfortunately, a significant proportion of these ECGs are abnormal (estimated at between 25 and 50%). Furthermore, an independent Cochrane review failed to find support for routine screening for cardiac arrhythmias in all patients on methadone (**Reference Pani, Trogu and MaremmaniPani 2013). Hence, it remains debatable whether annual ECG screening is justified for all patients on methadone or just those who are at high cardiac risk (such as those on daily methadone doses exceeding 100 mg; Department of Health 2007).
Deaths from diverted methadone
Deaths from methadone have been a recurrent problem – around half are caused by diverted prescribed methadone bought on the black market. In England in the 1990s, methadone was responsible for more deaths than illicit heroin. Subsequently, strict recommendations were introduced concerning supervised consumption of methadone, where patients take the drug under direct observation, usually by a pharmacist (Department of Health (England) 2007).
Despite variation in the practice of supervised consumption (**Reference Holland, Maskrey and SwiftHolland 2014), it has reduced the number of deaths by about fourfold (**Reference Strang, Hall and HickmanStrang 2010). In 2011, the National Programme on Substance Abuse Deaths at St George’s, University of London analysed the 1883 drug-related deaths in the UK (**Reference Ghodse, Corkery and ClaridgeGhodse 2012). It reported that 455 (37%) involved heroin/morphine (including 136 (11%) where no other substance was implicated). By contrast, there were 308 deaths (25%) involving methadone (including 87 (7%) with no other substance). Only 131 of the 308 people whose deaths were methadone-related were in receipt of a methadone prescription at the time of death; 177 (58%) presumably obtained methadone from illicit sources. In Scotland methadone was implicated in more than 237 deaths, compared with 221 deaths from heroin/morphine (National Records of Scotland 2013).
A well-known double-blind trial involving 193 intravenous opioid addicts revealed that 53% of the urine samples after 30 weeks were opioid-positive in those randomised to 80–100 mg methadone, compared with 62% of those receiving 40–50 mg (number needed to treat NNT = 11) (**Reference Strain, Bigelow and LiebsonStrain 1999). However, this trial was performed in a research population who were starting treatment and it involved supervised consumption for all participants over the 30 weeks. A more recent US trial involving 1267 opioid-dependent patients supports many other reports by showing increased retention in treatment over 6 months for patients on methadone doses over 60 mg/day (80% v. 74% for doses ≤60 mg; NNT = 16), while doses over 120 mg had 91% retention (NNT ~6) (**Reference Hser, Saxon and HuangHser 2013). However, it was unclear whether retention in treatment necessarily indicated a better outcome in terms of reduced opioid use, as the trial compared methadone with buprenorphine–naloxone and those that dropped out were not followed up. Unfortunately, in practice many patients will receive take-home doses of methadone after 3–6 months. Dispensing higher doses of methadone for patients to take home is likely to greatly increase the risk to other drug users.
Legal highs
‘Legal highs’ are newly available, synthetic psychoactive substances that are not regulated under current legislation such as the UK Misuse of Drugs Act 1971 (Table 2) (Faculty of Addictions Psychiatry 2014). Briefly, legal highs are taken for their temporary stimulant and euphoric effects in a similar manner to cannabis, ecstasy or ketamine. Side-effects and overdose produce symptoms similar to those of illegal stimulants. A Europe-wide survey in 2011 suggested that 5–10% of young people had taken a legal high in the previous year (compared with 25–50% for cannabis, depending on age) (Gallup Organization 2011). Legal highs are usually bought on the internet or sold by the same dealers as cannabis and ecstasy. There are many hundreds of compounds: examples include ‘meow meow’ (mephedrone), GBL (gammabutyrolactone, an industrial solvent), BZP (benzylpiperazine) and salvia (an extract from the sage plant). The exact content of the illicit tablets or powder is extremely variable and some supplies may have completely different active agents when purchased at different times.
TABLE 2 Examples of legal highs (description and action) and related drugs of misuse
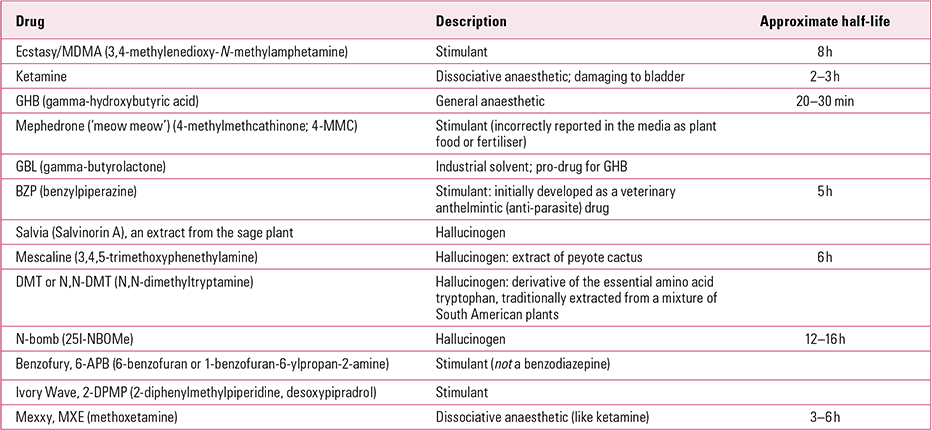
Testing a single psychoactive product to determine whether it is likely to cause harm typically costs around £1 million and takes about 1 year. This is highly onerous given the number of active chemicals used as legal highs. One approach to dealing with this problem is to require manufacturers and distributors to prove that their products pose a low risk of harm before they receive approval (**Reference Winstock and RamseyWinstock 2010). Unfortunately, this is unlikely to be effective when products are ordered internationally on the internet and when they are being imported supposedly as plant food, solvents or industrial chemicals.
Treatment services for legal highs are being developed, although this is a very recent problem. Abstinence-based options are usually offered as for cannabis or stimulants.
GHB
GHB (gamma-hydroxybutyric acid) is a commonly misused, short-acting sedative drug that produces disinhibition and euphoria similar to the effects of alcohol. It is a typical example of a ‘legal high’ and is relatively simple to synthesise at home. It is often used in ‘raves’ and has been implicated in date rapes.
A withdrawal state with acute delirium has been reported and treatment with benzodiazepines or baclofen has been used (**Reference Zvosec, Smith and PorrataZvosec 2011). GHB intoxication is a problem for accident and emergency departments, although long-term treatment strategies are being developed in a similar fashion to other legal highs.
Benzodiazepine dependence
By the mid-1970s, benzodiazepines had become the most widely prescribed psychotropic drug. Since the discovery in the 1970s of the dependence liability of benzodiazepines such as diazepam, most responsible expert guidelines have advised against the long-term prescribing of these agents. Similar advice is also given in relation to other sedative-hypnotics, such as zopiclone and zolpidem. Nevertheless, there remain a large number of people who are dependent on prescribed benzodiazepines and misuse of these drugs is widespread. For example, the British National Formulary (**August 2014) states that benzodiazepines should only be used short term (2–4 weeks) in severe and disabling anxiety (not mild anxiety or insomnia) (Box 2). Despite repetition of this advice by virtually all responsible professional organisations, dependence on prescribed and illicit benzodiazepines is common. This may partly be due to determined pressure from individual patients to continue or restart prescriptions, especially patients with other substance use disorders.
BOX 2 Indications for benzodiazepine prescription
-
• Benzodiazepines are indicated for the short-term relief (2–4 weeks only) of anxiety that is severe, disabling or causing the patient unacceptable distress, occurring alone or in association with insomnia or short-term psychosomatic, organic or psychotic illness
-
• The use of benzodiazepines to treat short-term ‘mild’ anxiety is inappropriate
-
• Benzodiazepines should be used to treat insomnia only when it is severe, disabling or causing the patient extreme distress
Benzodiazepines are one of the largest classes of misused drugs. Estimates suggest that 2% of the adult populations of the USA and UK have used benzodiazepines regularly for more than 12 months – around half of these for 5–10 years. In 2010, the UK National Institute for Health and Care Excellence (NICE) estimated that 10–30% of chronic benzodiazepines users were physically dependent on them (NICE 2010).
The idea that ‘pharmacological’ dependence on prescribed benzodiazepines is somehow different from other forms of ‘addiction’ is spurious. Benzodiazepine withdrawal usually produces uncomfortable but not life-threatening symptoms, with insomnia, anxiety, tremor, perspiration and tinnitus. This may persist for months and craving can continue for years. Consequently, many patients find that the benefits of cessation are not justified by the effort required to abstain, including chronic withdrawal symptoms.
Are benzodiazepine detoxification or maintenance justified?
It has yet to be demonstrated that either short-term (detoxification) or long-term (maintenance) prescribing of benzodiazepines is justified following dependence on either illicit or prescribed benzodiazepines. Unlike heroin, alcohol or cocaine dependence, benzodiazepine misuse is not associated with high levels of acquisitive crime, regular injecting or physical complications such as cirrhosis, blood-borne virus infection or cardiovascular disease. Consequently, it is difficult to demonstrate significant benefits of treatment and it is hard to justify its cost (which is comparable to that of opioid substitution therapy in terms of staff time and dispensing arrangements). Many specialist addiction services therefore advise that detoxification should take place gradually in primary care or will provide a time-limited (3-month) community detoxification regime on a once-only basis.
The UK clinical guidelines on the management of substance misuse state:
‘There is little evidence to suggest that long-term substitute prescribing of benzodiazepines reduces the harm associated with benzodiazepine misuse and there is increasing evidence that long-term prescribing (especially of more than 30 mg diazepam equivalent per day) may cause harm’ (Department of Health (England) 2007: p. 60).
Managing benzodiazepine detoxification involves transferring onto a long-term agent, typically diazepam, and reducing the dose by one-quarter to one-eighth every 2 weeks, with frequent dispensing. In practice, this is almost always a protracted and uncomfortable process and may take years. Furthermore, there are high rates of relapse among patients who previously used illicit benzodiazepines, because of their low cost and relatively high availability (e.g. via the internet).
The evidence base
A meta-analysis of discontinuation from prescribed benzodiazepines reported that gradual dose reduction and brief interventions were superior to ‘routine care’ at achieving cessation (**Reference Parr, Kavanagh and CahillParr 2008). The odds ratios were ~6 for dose tapering (although this was based primarily on one study by **Reference Oude Voshaar, Gorgels and MolOude Voshaar et al (2003)) and ~4 for brief interventions. Brief interventions might include sending letters to patients suggesting that they reduce their benzodiazepine prescriptions. The duration of withdrawal had a mean of 49 days (maximum 70 days), with variable periods of post-treatment follow-up, from 3 to 12 months. Typical abstinence rates at the end of treatment were 10% in the routine care group (maximum 15%), 18% in the brief intervention group (maximum 40%) and 37% in the gradual reduction group (maximum 80%). Reference Oude Voshaar, Gorgels and MolOude Voshaar et al (2003) compared gradual (3-month) dose reduction with treatment as usual for 180 patients and reported cessation rates of 45% v. 29% at 3 months. Additional cognitive–behavioural therapy (CBT) did not improve outcomes. Unfortunately, ‘routine care’ in these trials is ill-defined. Furthermore, many of the patients were in receipt of prescribed benzodiazepines rather than obtaining these drugs from illicit suppliers (which is more typical of patients attending drug and alcohol services).
A meta-analysis (**Reference Parr, Kavanagh and CahillParr 2008) found that three studies have shown brief interventions in primary care to be effective in achieving benzodiazepine abstinence among people on prescribed agents. However, the overall effectiveness remained modest, with 5% successful detoxification in the control group v. 22% in the treatment group. It also found that additional psychological therapy (based on CBT) significantly increased rates of abstinence: to 85% compared with routine care (48%) or gradual dose reductions (54%). However, the high rates of cessation in the enhanced treatment group fell to 50–60% at follow-up. These studies also involved primary care patients dependent on prescribed benzodiazepines. A further 14 studies showed little additional benefit of other adjuvant medication (11 different additional medications were tried, including melatonin, valproate, trazodone and paroxetine).
High-dose benzodiazepine users, who often also use other illicit drugs and/or alcohol, present a difficult problem because gradual benzodiazepine detoxification can often become long-term maintenance. There was little benefit of additional CBT over simple dose tapering (27% v. 13% discontinuation). However, patients who continued to use reported a halving in benzodiazepine consumption (**Reference Vorma, Naukarinen and SarnaVorma 2003). Contingency management has also been used to address benzodiazepine misuse in patients on opioid substitution therapy, although the benefits are lost when the reinforcement is removed (**Reference Stitzer, Bigelow and LiebsonStitzer 1982).
Long-term benzodiazepine prescription in anxiety disorders and alcohol dependence
There have been intermittent suggestions that long-term benzodiazepine prescriptions are justified in generalised anxiety disorder, panic disorder and chronic insomnia – usually as a last resort (Reference StarcevicStarcevic 2012). Evidence to support their long-term use is controversial, especially as it is almost impossible to distinguish benzodiazepine withdrawal from relapse (rebound anxiety symptoms). The discovery that benzodiazepines cause dependence and pronounced withdrawal and that they are frequently misused means that there are few studies of long-term benzodiazepine use in anxiety disorders. Consequently, non-addictive antidepressants, especially selective serotonin reuptake inhibitors, are usually recommended in preference to benzodiazepines in anxiety disorders. Using antidepressants and benzodiazepines at the start of treatment for anxiety disorders will often relieve symptoms quickly, although the subsequent plan to discontinue the benzodiazepine after 6–10 weeks ‘is not always easy. (Reference StarcevicStarcevic 2012). Hence, NICE guidelines state that ‘benzodiazepines are associated with a less good outcome in the long term and should not be prescribed for the treatment of individuals with panic disorder’ and also that ‘benzodiazepines should not usually be used for more than 2 to 4 weeks for the treatment of generalised anxiety disorder’ (NICE 2014).
A working group of the Royal College of Psychiatrists and the British Association for Psycho pharmacology offers less Spartan advice regarding patients with anxiety symptoms:
‘If there is no history of drug dependence, and positive indicative “lifestyle” factors are present, a conscious decision to continue benzodiazepine treatment may be more reasonable than the alternatives, provided the patient periodically attempts to slowly reduce the dosage at regular intervals and tries to stop altogether when or if possible’ (**Reference Baldwin, Aitchison and BatesonBaldwin 2013).
However, the group explicitly states that longer-term prescribing is appropriate only for those with no history of drug dependence. In relation to anxiety disorders it states:
‘There are clinical circumstances in which longer-term prescription of benzodiazepines might be considered desirable because the alternatives are probably worse than the continued use […] In rare instances longer-term prescriptions […] may be seen as a form of harm reduction in patients who would otherwise consume illicit benzodiazepines’ (**Reference Baldwin, Aitchison and BatesonBaldwin 2013).
There is little evidence that long-term benzodiazepines assist abstinence in alcohol dependence.
Summary
Overall, treatment for benzodiazepine dependence remains unsatisfactory. This is partly due to the iatrogenic nature of the dependence, the investment required to detoxify patients, patients’ ambivalence about stopping, poor outcome of treatment and also the relatively low harm associated with benzodiazepine dependence compared with alcohol or intravenous drug use.
Gabapentin and pregabalin
These anticonvulsant drugs are now being widely prescribed for pain, especially for more intractable neuropathic pain, to avoid the risk of dependence associated with opioids. Gabapentin and pregabalin enhance the action of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) in a similar manner to benzodiazepines. There are now many reports of misuse of pregabalin and gabapentin, which produce euphoria and intoxication similar to the effects of alcohol (**Reference Schifano, D'Offzi and PiccioneSchifano 2011). This problem is only just emerging and there is no consensus on how it should be managed. However, restrictions are now being placed on the prescribing of pregabalin in the USA (Drug Enforcement Administration 2011).
Conclusions
Political issues have major bearing on treatment in substance misuse. This ranges from the stated preference for abstinence (‘recovery’) rather than methadone maintenance and funding for expensive and controversial treatments such as injectable opioids to political efforts to restrict sales of legal highs. In general, it would appear that obstacles preventing diversion of prescribed drugs of misuse and proliferation of legal highs are insurmountable – it is extremely difficult to legislate against a multitude of synthetic drugs of misuse, and placing restrictive regulations on the use of opioid pain killers and benzodiazepines seems unlikely. Methadone has come under scrutiny again because of ECG abnormalities and high death rates from diverted prescriptions. However, the principal theme emerging over the past decade is the gradual failure of prohibition, especially with regard to diversion of prescribed medication and legalisation of cannabis.
MCQs
Select the single best option for each question stem
-
1 Of the following list, the most common cause of avoidable death in the world is:
-
a tobacco
-
b alcohol
-
c heroin
-
d cocaine
-
e cannabis.
-
-
2 The second most common class of illicit drugs of misuse in the USA in 2011 was:
-
a cannabis
-
b cocaine
-
c prescription opioids
-
d heroin
-
e ketamine.
-
-
3 Over the past decade, supervised consumption has reduced deaths from methadone overdose by about:
-
a fourfold
-
b tenfold
-
c half
-
d 10%
-
e twentyfold.
-
-
4 The proportion of the adult populations of the USA and UK estimated to have used benzodiazepines regularly for 12 months or over is:
-
a 20%
-
b 0.1%
-
c 2%
-
d 40%
-
e 50%.
-
-
5 Regarding ‘legal highs’, testing a single compound to determine whether it is likely to cause harm:
-
a typically costs £10 000 and takes 1 month
-
b typically costs £10 00 and takes 6 months
-
c typically costs £100 000 and takes 6 months
-
d typically costs £100 000 and takes 1 year
-
e typically costs £1 000 000 and takes 1 year.
-
MCQ answers
| 1 | a | 2 | c | 3 | a | 4 | c | 5 | e |





eLetters
No eLetters have been published for this article.