Summations
-
Patients suffering from major affective disorders often require long term psychotropic drug maintenance.
-
Skillful and informed adjustment of maintenance medications is necessary in these patients.
-
Shifting between depression, mania, or a mixed state may recur in the course of affective disorders and various hypotheses have been advanced to explain the mechanism behind such mood shifting.
-
This review summarizes and examines the pharmacological properties of psychotropics in view of their reported treatment induced mood changes.
Considerations
-
Shifting in mood states or unstable mood may occur spontaneously in the course of some patients suffering from major affective disorders independent of drug treatment.
-
The relationship between the specific pharmacological properties of psychotropics and their induced mood shifting will be required to be tested in well designed studies.
-
The pharmacological properties and mechanism of actions of existing antidepressant drugs may not differ enough to show a quantifiable difference in treatment induced mood shifting.
Introduction
Some patients suffering from mood disorders (AD) and in particular bipolar disorders (BD) experience unstable or shifting moods throughout their illness, with or without treatment. Mood shifting includes a shift from depression to hypomania or mania, or from hypomania and mania into depression, or into a mixed state consisting of both hypomanic, manic and depressive features. The cause of the shifting or switching between different mood states in these patients has been the subject of much discussion and various hypotheses on the mechanism have been advanced. The role of psychotropic drugs in inducing switching into mania and or a depressive state has also been the subject of much debate.
BD with unstable mood and energy states present a well-known therapeutic challenge for clinicians. In many patients, the treatment plan often has to be adjusted throughout the course of the illness. Clinicians often have to resort to polypharmacy, with their well-known metabolic and other side effect burdens. It is estimated that about one-fifth of patients with BD may receive four or more psychotropic medications. In actual practice, demand and pressure from patients and their families often resulted in the prescription and switching between various medication combinations, outpacing evidence in the literature (Fornaro et al., Reference Fornaro, De Berardis, Koshy, Perna, Valchera, Vancampfort and Stubbs2016a; Fung et al., Reference Fung, Overhage, Sylvia, Reilly-Harrington, Kamali, Gao, Shelton, Ketter, Bobo, Thase, Calabrese, Tohen, Deckersbach and Nierenberg2019; Goldberg, Reference Goldberg2019; Nestsiarovich et al., Reference Nestsiarovich, Kerner, Mazurie, Cannon, Hurwitz, Zhu, Nelson, Oprea, Unruh, Crisanti, Tohen, Perkins and Lambert2019).
While combinations of antidepressants, lithium and other mood stabilisers or anticonvulsants are often prescribed in practice, some treatment guidelines still caution that antidepressants may trigger mania or mood cycle acceleration. However, the risks of antidepressants have not been studied in well-designed and adequately powered trials (Grunze, Reference Grunze2005).
In many BD patients, depression occurs in the first episode of the illness. As a result, antidepressant drugs are usually administered in patients suffering from mood disorders. It has been estimated that at least 70% of BD patients fail to receive the correct diagnosis in the year following the first episode and (estimated at 35%) it may take up to 10 years for the correct diagnosis to be established (Lish et al., Reference Lish, Dime-Meenan, Whybrow, Price and Hirschfeld1994). In addition, a substantial majority of BD spectrum patients go unrecognised and undiagnosed and remain untreated or inadequately treated (Hirschfeld et al., Reference Hirschfeld, Lewis and Vornik2003; Fountoulakis et al., Reference Fountoulakis, Vieta, Young, Yatham, Grunze, Blier, Moeller and Kasper2017). It is therefore not unexpected to find that antidepressants are widely and may be inappropriately used for many patients. This may contribute to the complexity and confusion in understanding whether antidepressants induce the switch process, or the switching in mood states was just spontaneous and independent of the antidepressant drug treatment.
While the mood switching in an episode could be induced by drug treatment, the phenomenon of mood switching was historically described as ‘reactive hyperthymia’ over a century ago, long before the discovery of antidepressants (Angst & Sellaro, Reference Angst and Sellaro2000). The concept of periodic mania was also well known and probably referred to patients with BD (Mendel, Reference Mendel1881). Likewise, mania switching into depression was commonly reported as ‘reactive depression’. MacDonald (Reference MacDonald1918) noted that mild depression preceded or terminated manic attacks in most of the patients he had studied.
Another important clinical feature is the difference in the syndromal stability between male and female patients. Angst (Reference Angst1978) studied the first 20 episodes of BD in male and female patients and reported that female patients showed more depressive episodes and male patients more cyclic episodes. These syndromal proportions were remarkably stable over the 20 episodes. It was also suggested that ageing is not associated with an increase in the depressive component of the illness (Angst & Weiss, Reference Angst, Weiss, Brill, Cole and Deniker1967). This implies that the underlying pathophysiology of BD may be relatively stable once it is established and does not change over time, and unstable or shifting mood is the nature of this illness.
While this historical evidence does not exclude the possibility that mood switching could be treatment induced in some patients, it does emphasise the underlying natural history of the disorder as an important confounding factor to be considered in evaluating the biological basis of treatment-induced mood switching in AD, in particular BD.
Hypotheses on the mechanism of mood state switching in affective disorders
Various hypotheses have been proposed to explain the neurobiological mechanism of treatment-induced or spontaneous mood switching in AD.
Neurotransmitter and neuropathway imbalance hypotheses
These hypotheses can be traced back to about 6 decades ago when the important roles of several neurotransmitters serotonin (5HT), norepinephrine (NE), dopamine (DA) and acetylcholine (Ach) in mental disorders began to be discovered and reported widely in the literature. The symptoms of mania and depression appeared to be ‘directly opposite’ to each other, and it was therefore suspected that neuropathways/neurotransmitters with opposite or antagonistic functions dominated the depressed versus the manic states. A simple cholinergic−adrenergic hypothesis (Janowsky et al., Reference Janowsky, Davis, El-Yousef and Sekerke1972) was proposed to explain the contrasting states of mood disorders. This hypothesis depicted an imbalance between an adrenergic/noradrenergic for a high energy/mood state and a cholinergic (Ach) circuit for a calm or sedated state. With later research showing the importance of DA in the regulation of mood, as well as its important role in the mechanism of action of many psychotropic drugs, the early simple hypothesis was then replaced by the catecholaminergic-cholinergic hypothesis (Salvadore et al., Reference Salvadore, Quiroz, Machado-Vieira, Henter, Manji and Zarate2010; van Enkhuizen et al., Reference van Enkhuizen, Janowsky, Olivier, van Enkhuizen, Janowsky, Olivier, Minassian, Perry, Young and Geyer2015; Ashok et al., Reference Ashok, Marques, Jauhar, Nour, Goodwin, Young and Howes2017). This neurotransmitter out of balance hypothesis is comparable to the DA-Ach balance hypothesis in Parkinson’s disease (PD) (McKinley et al., Reference McKinley, Shi, Kawikova, Hur, Bamford, Sudarsana Devi, Vahedipour, Darvas and Bamford2019; Ztaou & Amalric, Reference Ztaou and Amalric2019; Myslivecek, Reference Myslivecek2021). In PD, the reciprocal control and balance between Ach and DA-containing neurons are lost with the death of DA-containing neurons. A simple introduction of anticholinergic drugs to restore the balance fell out of favour because of side effects and the introduction of novel treatment methodology (Ztaou & Amalric, Reference Ztaou and Amalric2019; Poppi et al., Reference Poppi, Ho-Nguyen, Shi, Daut and Tischfield2021).
The role of other neuropathways such as glutamine (Glx) has also been brought into this neurotransmitter imbalance concept in recent years. Patients with BD were shown to have significantly higher levels of Glx (Gigante et al., Reference Gigante, Bond, Lafer, Lam, Young and Yatham2012) in certain brain regions such as the anterior cingulate (Li et al., Reference Li, Xu, Zhang, Guan, Zhang, Xu, Shen, Xiao, Liang, Chen, Zhang and Wu2016). It was hypothesised that depressive and manic episodes may be characterised by modulation of the glutamine/glutamate ratio in opposite directions, possibly suggesting reduced versus elevated glutamate conversion to glutamine by glial cells, respectively (Yüksel & Öngür, Reference Yüksel and Öngür2010). Spectroscopy studies of the glutamate system and mitochondrial dysfunction in paediatric BD measurement of glutamate changes were proposed for the early detection of bipolar changes in paediatric patients (Kondo et al., Reference Kondo, Hellem, Shi, Sung, Prescot, Kim, Huber, Forrest and Renshaw2014). Similarly, higher GABA/creatinine levels were reported in euthymic BD outpatients compared to healthy controls (Brady et al., Reference Brady, McCarthy, Prescot, Jensen, Cooper, Cohen, Renshaw and Ongür2013), suggesting that a GABAergic dysfunction may also exist.
The interactions between neuropathways are dynamic, continuous and reciprocally adjusting. The sensitivity of neurotransmitter receptors, for example, may adjust or change over time. Chronic antidepressant drug treatment was shown decades ago to downregulate both 5HT and NE receptors (Tang et al., Reference Tang, Seeman and Kwan1981; Dumbrille-Ross & Tang, Reference Dumbrille-Ross and Tang1983; Helmeste & Tang, Reference Helmeste and Tang1983) and withdrawal may result in rebound hypersensitivity (Tang et al., Reference Tang, Helmeste and Stancer1979a, Reference Tang, Helmeste and Stancer1979b). Similarly, DA receptors may adjust with changes in neurotransmission. While the elevation of DA receptors may contribute to a hyperactive reward network in mania, secondary down-regulation of dopaminergic receptor sensitivity over time may shift the system into a depressive state. A repetition of the cycle may explain the cyclical nature of mood changes. A breakdown of DA receptor and transporter homeostasis might underlie the pathophysiology of unstable mood (Berk et al., Reference Berk, Dodd, Kauer-Sant’anna, Malhi, Bourin, Kapczinski and Norman2007; Ashok et al., Reference Ashok, Marques, Jauhar, Nour, Goodwin, Young and Howes2017). While the chronic antidepressant treatment-induced receptor subsensitivity and withdrawal rebound supersensitivity may explain the antidepressant-induced mood switching phenomenon, spontaneous mood switching in patients who are not on psychotropics may have a different mechanism.
The subsensitivity/rebound hypersensitivity switching may also be the mechanism behind the neuropathway oscillation model (Goldbeter, Reference Goldbeter2011, Reference Goldbeter2013) for explaining mood switching in BD. His hypothesis depicted the propensities to mania and depression as governed by the activities of two putative neural circuits that oscillate and self-inhibit each other. When mutual inhibition is sufficiently strong, the model predicts ‘bistability’, and lesser inhibition would result in a mixed bipolar state. About two-thirds of bipolar-depressed patients had concomitant manic symptoms (Henry et al., Reference Henry, M’Bailara, Lépine, Lajnef and Leboyer2010). While interesting, no experimental data is available to support the hypothesis and it may be difficult to use a variable strength reciprocal inhibiting dual neurocircuit model to explain the mixed symptoms in many bipolar states.
In summary, the translational aspect of the above hypothesis is that introduction of a psychotropic with potent action on one neurotransmitter pathway may upset a previously balanced mood state, or that the balance mood state may be lost over time with the development of neuropathway-receptor subsensitivity or supersensitivity, and drug−drug interactions through CYP enzyme inhibition or metabolic polymorphism. It will require a well-designed placebo-controlled on and off study of psychotropics with relatively targeted action on the specific neurotransmitter pathway in question to resolve this.
Circadian rhythm photoperiod length-induced neurocircuit changes
It is well known that changing daylight duration may induce a mood switch in some patients with affective disorders, exemplified in those with seasonal affective disorders (SAD). One of the proposals was the increased catecholaminergic expression during periods of high activity, changing over to increased somatostatin and corticotrophin-releasing factor during periods of low activity (Young & Dulcis, Reference Young and Dulcis2015). Perturbations of the circadian biological and social rhythms may also influence the expression of rapid cycling (Papadimitriou et al., Reference Papadimitriou, Calabrese, Dikeos and Christodoulou2005). Furthermore, associations of circadian gene polymorphisms, such as the CLOCK gene (OMIM 601851), with affective disorders have been reported. This model proposed a role of MAO-A inhibition with the decreasing sunlight effect, which leads to an increase in DA function leading to mania (Kripke et al., Reference Kripke, Nievergelt, Joo, Shekhtman and Kelsoe2009). While interesting, the efficacy of light therapy is only effective in a portion of patients with SAD (Pail et al., Reference Pail, Huf, Pjrek, Winkler, Willeit, Praschak-Rieder and Kasper2011) and thus can be considered as an adjunct treatment for selected patients.
For the selection of psychotropics for SAD, there have been few reports supporting differential efficacies of different types of antidepressant drugs on SAD. Bupropion is an example was once reported to be an effective preventive treatment (Westrin & Lam, Reference Westrin and Lam2007) but others did not find enough evidence to support the superior efficacy of any specific antidepressant agents for the treatment of SAD (Yildiz et al., Reference Yildiz, Batmaz, Songur and Oral2016; Pjrek et al., Reference Pjrek, Friedrich, Cambioli, Dold, Jäger, Komorowski, Lanzenberger, Kasper and Winkler2020).
Stress-induced neurobiological changes
Stressful life events are well known to trigger depression in previously well-maintained patients suffering from MD and BD. Negative life events do not seem to trigger mania, but life events involving goal attainment do appear to trigger manic symptoms (Johnson, Reference Johnson2005).
In animal models, experimental stress-induced both acute and long-term changes in the brain (McEwen & Gianaros, Reference McEwen and Gianaros2011). Changes in neurotransmitter functions and neurocircuits (Mahar et al., Reference Mahar, Bambico, Mechawar and Nobrega2014), neurogenesis (Lau et al., Reference Lau, Qiu, Helmeste, Lee, Tang, So and Tang2007; Qiu et al., Reference Qiu, Helmeste, Samaranayake, Lau, Lee, Tang and So2007), response to psychotropics (Hamidovic et al., Reference Hamidovic, Childs, Conrad, King and de Wit2010), receptor and gene expressions (Cattaneo & Riva, Reference Cattaneo and Riva2016) have all been reported in these models. Antidepressant drugs and physical exercises may protect the brain from the neurocircuit damaging effects of stress-induced cortisol elevation (Qiu et al., Reference Qiu, Helmeste, Samaranayake, Lau, Lee, Tang and So2007; Tang et al., Reference Tang, Chu, Hui, Helmeste and Law2008, Reference Tang, Helmeste and Leonard2012, Reference Tang, Tang and Leonard2017; Yau et al., Reference Yau, Lau, Tong, Wong, Ching, Qiu, Tang, Lee and So2011). Mood stabilisers, such as lithium and valproic acid (Wang et al., Reference Wang, Fessler and Chuang2011; Chiu et al., Reference Chiu, Wang, Hunsberger and Chuang2013) have been shown to protect the brain from stress-induced changes through glycogen synthase kinase-3 (GSK-3), a multifunctional protein kinase (Harwood & Agam, Reference Harwood and Agam2003).
It is important to caution that most of the data came from animal models, using various paradigms to model stress. In practice, antidepressant drugs and mood stabilisers are often adjusted in patients facing severe stressful life events. However, stressful life events as a trigger for depressive or manic symptoms still await further assessment within a longitudinal study (Johnson, Reference Johnson2005). When designing future studies, it would be important to define what constitutes ‘stress’ and how to quantify stress.
Episodic neuroinflammation
Inflammation has long been linked to affective disorders and suicide (Berk et al., Reference Berk, Williams, Jacka, O’Neil, Pasco, Moylan, Allen, Stuart, Hayley, Byrne and Maes2013; Franklin et al., Reference Franklin, Xu and Duman2018; Kageyama et al., Reference Kageyama, Kasahara, Kato, Sakai, Deguchi, Tani, Kuroda, Hattori, Yoshida, Goto, Kinoshita, Inoue and Kato2018; Leonard, Reference Leonard2018; Bergmans et al., Reference Bergmans, Kelly and Mezuk2019). There are several studies that have demonstrated an increase in proinflammatory cytokines in the blood of patients with BD (Bai et al., Reference Bai, Su, Tsai, Wen-Fei, Li, Pei-Chi and Mu-Hong2014). The proinflammatory cytokines are secreted from activated macrophages, which include microglia as well as T-lymphocytes and endothelial cells. This results in the activation of neutrophils, the proliferation of B cells, the synthesis of acute-phase proteins and vascular permeability is also increased. Other studies have concentrated on changes in the interleukins (IL’s), tumour necrosis factor, alpha (TNF) factor, the interferons and transforming growth factors. On accessing the CNS, the cytokines activate the microglia, astrocytes and oligodendroglia. In BD, the chronic activation of the glia changes the homeostatic balance of the glia to an inflammatory state, which contributes to neuronal damage (Watkins et al., Reference Watkins, Sawa and Pomper2014).
The most consistent change reported to occur in BD has been the elevation in the serum concentration of IL-6 (Remlinger-Molenda et al., Reference Remlinger-Molenda, Wójciak, Michalak and Rybakowski2012; Munkholm et al., Reference Munkholm, Weikop, Kessing and Vinberg2015). The concentration of IL-6 is reported to be higher during the manic phase than during the remission period while INF-gamma was higher during the acute depressive episode. The IL-6 concentration was correlated with the intensity of the manic state. By contrast, the concentration of the anti-inflammatory cytokine IL-10 was reported to be higher in the depressed phase during the remission period by Brietzke et al. (Reference Brietzke, Stertz, Fernandes, Kauer-Sant’anna, Mascarenhas, Escosteguy Vargas, Chies and Kapczinski2009), who also reported that the IL-6 levels were elevated in the depressive phase. The particular importance of IL-6 in BD relates to its pleiotropic properties, which result in the stimulation of T and B lymphocytes, and hepatocytes, which release acute-phase proteins such as C-reactive protein (CRP). Other investigators have confirmed an increase of IL-6, TNF-alpha (Kim et al., Reference Kim, Jung, Myint, Kim and Park2007) and IL-2 (Rapaport et al., Reference Rapaport, Guylai and Whybrow1999) in the manic phase and normalised after effective treatment. The anti-inflammatory cytokine, IL-4, was also found to be reduced during the manic phase and returned to normal on effective treatment (Kim et al., Reference Kim, Jung, Myint, Kim and Park2007). These studies confirm that there is an imbalance between pro- and anti-inflammatory cytokines in the two main phases of BPD.
The chronic elevation of the proinflammatory cytokines reduces the sensitivity of the glucocorticoid and insulin receptors thereby contributing to the increased incidence of metabolic syndrome (dyslipidaemia, diabetes, cardiovascular disease etc.) in BD patients (Goldstein et al., Reference Goldstein, Kemp, Soczynska and McIntyre2009).
In addition to the impact of the proinflammatory cytokines on the intermediary metabolism and ultimately on the integrity of the neuronal structure and function, they also disrupt monoamine neurotransmitter synthesis by reducing the availability of tetrahydrobiopterin, the key factor in monoamine synthesis (Felger & Miller, Reference Felger and Miller2012). Monoamine signalling is further disrupted by the increase in the expression of the serotonin and DA transporter proteins. Glutamate signalling is also affected as the proinflammatory cytokines increase the activity of indoleamine-2,3-dihydrogenase. This leads to the synthesis of the neurotoxic N-methyl-D-aspartate agonist quinolinic acid which potentiates excitotoxicity (Myint, Reference Myint2012). However, despite the evidence that the neuronal structure is affected by the inflammatory assault, there is evidence that BD is characterised by the glial pathology rather than neurodegenerative changes which characterise major depression (Rajkowska, Reference Rajkowska2002).
Recent meta-analyses implicate an increase in proinflammatory cytokines and a decrease in brain-derived neurotropic factor (BDNF) as crucial to the cellular pathology of BD, particularly during the manic phase of the episode. These markers appear to respond to the therapeutic effects of such mood stabilisers as lithium and valproate. However, despite the availability of data indicating changes in the manic and euthymic states, there appear to be no reports which would give an insight into specific changes associated with the switch process, or how this may induce a switch in mood states. Clearly, it is essential to undertake detailed studies, larger sample sizes, BD subtypes, family history and comorbidities to understand the immune complexity of BD (Muneer, Reference Muneer2016).
In summary, given the episodic nature of mood state switching, an ‘on and off’ neuro or systematic inflammatory process could possibly be one of the causes in some patients (Maletic & Raison, Reference Maletic and Raison2014; Fries et al., Reference Fries, Walss-Bass, Bauer and Teixeira2019). Genetic and systematic abnormalities in inflammatory factors have been reported to be associated with bipolar mood disorders (Sigitova et al., Reference Sigitova, Fišar, Hroudová, Cikánková and Raboch2017) and impulsivity (Kim et al., Reference Kim, Kang, Bahk, Jang, Hong and Baek2020). An association between low-grade inflammation and the clinical features of BD was also reported (Gan et al., Reference Gan, Wu, Liao, Wu, He, Yang and Zhang2019). Electroconvulsive treatment, an effective and safe treatment for all the states of severe and drug-resistant BD (Perugi et al., Reference Perugi, Medda, Toni, Mariani, Socci and Mauri2017), would cause an acute immuno-inflammatory response followed by a decrease in inflammation (Yrondi et al., Reference Yrondi, Sporer, Péran, Schmitt, Arbus and Sauvaget2018). However, neuroinflammation is non-specific to neuropathways (Goldstein et al., Reference Goldstein, Kemp, Soczynska and McIntyre2009; Tang et al., Reference Tang, Helmeste and Leonard2021) and has also been proposed to be associated with other brain disorders such as dementia. A hypothesis of episodic inflammation as the cause of mood state switching will require much more supporting evidence and a clear and unequivocal efficacy of anti-inflammatory agents with or without concomitant antidepressant drugs needs to be demonstrated. At this point, consideration of adjunctive anti-inflammatory agents may be useful in patients with unstable and hard-to-stabilise mood states who showed abnormal inflammatory markers.
Though anti-inflammatory agents have been and are being tested for their antidepressant effects, direct or indirect modulation of inflammatory response also has the potential to be tested as novel therapeutic approaches for patients with unstable moods or problematic mood switching (Pereira et al., Reference Pereira, Oliveira, Silva, Madeira, Pereira and Cruz2021).
Of the anti-inflammatory drugs investigated as adjunctive treatments for major depression and for BD, the cyclooxygenase-2 inhibitor, celecoxib, has received particular attention (Husain et al., Reference Husain, Strawbridge, Stokes and Young2017). Celecoxib is a non-steroidal anti-inflammatory which inhibits the formation of proinflammatory prostaglandins from arachidonic acid. As a consequence, arachidonic acid is shunted into a pathway leading to the synthesis of anti-inflammatory eicosanoids (Strauss, Reference Strauss2008) of which the omega-3 fatty acid, eicosapentaenoic acid has anti-inflammatory and possible antidepressant activity, as indicated by the behavioural, neurotransmitter and immune changes in the olfactory bulbectomised rat model of depression. The arachidonic cascade hypothesis has been advanced to explain the anti-manic actions of mood-stabilising drugs such as lithium, valproate and lamotrigine, all of which have been shown to downregulate the turnover of brain phospholipids. Clinical studies have demonstrated that the combination of antidepressants with celecoxib enhances the antidepressant action in the treatment of bipolar depression (Halaris et al., Reference Halaris, Cantos, Johnson, Hakimi and Sinacore2020) while Edberg et al. (Reference Edberg, Hoppensteadt, Walborn, Fareed, Sinacore and Halaris2018) demonstrated that the adjunctive celecoxib treatment also reduced the concentration of CRP concomitant with the antidepressant effect in BPD.
Clearly more extensive clinical studies need to be undertaken to validate the usefulness of the arachidonic acid cascade hypothesis in the development of potential mood-stabilising drugs. Presently there is no experimental or clinical evidence that the eicosanoids are specifically involved in the switch mechanism. However, an experimental study by Rapoport et al. (Reference Rapoport, Basselin, Kim and Rao2009) that antidepressants shown to switch patients into the manic phase also upregulate the arachidonic acid cascade. This further emphasises the need to clarify and extend these studies, both experimental and clinically, to target the metabolism of arachidonic acid in the two main phases of BD and in the switch process.
BDNF and thyroid hormone imbalance
A wealth of data supports a major role of BDNF in affective disorders. Antidepressant treatment and exercise (Tang et al., Reference Tang, Chu, Hui, Helmeste and Law2008) also elevate BDNF. Altered activity of BDNF has been proposed as a possible cause of mood instability (Tsai, Reference Tsai2004). A possible association between BDNF Val66Met polymorphism and BD has been reported (Neves-Pereira et al., Reference Neves-Pereira, Mundo, Muglia, King, Macciardi and Kennedy2002) but later found to be complex and inconsistent (Kanazawa et al., Reference Kanazawa, Glatt, Kia-Keating, Yoneda and Tsuang2007; Wang et al., Reference Wang, Li, Gao and Fang2014, González-Castro et al., Reference González-Castro, Nicolini, Lanzagorta, López-Narváez, Genis, Pool García and Tovilla-Zárate2015). Whether changes in brain BDNF is an important factor in mood shift or unstable mood in affective disorders still requires much further research.
Reports on thyroid dysfunction in bipolar patients date back to the 1990s, with Grade I (Bauer et al., Reference Bauer, Whybrow and Winokur1990), II, III (Kusalic, Reference Kusalic1992) hypothyroidism reported in rapid cyclers. However, the findings were not consistent, as no significant differences in thyroid function indices between rapid- and non-rapid-cycling cases were also reported (Valle et al., Reference Valle, Ayuso-Gutierrez, Abril and Ayuso-Mateos1999). As thyroxine is an important ‘activating’ hormone in energy metabolism, it is natural to question whether changes in thyroid function may trigger a switch in mood state. The literature reviewed argued that there were some clues that thyroxine (T3) could augment and accelerate treatment response with antidepressants and lithium and that it might protect against rapid-cycling BD, as well as against relapse (Parmentier & Sienaert, Reference Parmentier and Sienaert2018). Another double-blinded control study showed the benefit of adjunctive L-T4 in alleviating resistant depression, reducing time in mixed states and increasing time euthymic and adjunctive T3 did not show statistically significant evidence of benefit over placebo in reducing the time spent in disturbed mood states (Walshaw et al., Reference Walshaw, Gyulai, Bauer, Bauer, Calimlim, Sugar and Whybrow2018). Again, the thyroxine factor is likely to be only present in some patients with unstable mood and not a general phenomenon in all patients with mood switching.
Genetic susceptibility
There has been great progress in the application of pharmacogenomics in diagnosis and treatment (Fortinguerra et al., Reference Fortinguerra, Sorrenti, Giusti, Zusso and Buriani2019) and genetic susceptibility is an important factor. The genetic basis of BD appears to be complex. Earlier reports of simpler gene abnormalities are replacing by more complex ones. For example, not only coding but non-coding RNA been hypothesised to underlie the pathology of BD (Luykx et al., Reference Luykx, Giuliani, Giuliani and Veldink2019) more meta-analyses of both bipolar and unipolar mood patients suggested the risk of antidepressant-associated mood elevations in bipolar II disorder was intermediate between bipolar I and major depression (Bond et al., Reference Bond, Noronha, Kauer-Sant’Anna, Lam and Yatham2008). The risk of mania switch was more frequent with than without antidepressants, and in bipolar as compared to unipolar patients (Tondo et al., Reference Tondo, Vázquez and Baldessarini2010). The 5HT transporter gene promoter polymorphism has been suspected to underline the susceptibility to antidepressant-induced mania but more research is needed (Biernacka et al., Reference Biernacka, McElroy, Crow, Sharp, Benitez, Veldic, Kung, Cunningham, Post, Mrazek and Frye2012). A functional variant in the 5HT receptor 7 gene (HTR7) was shown to be associated with good response to SSRIs in bipolar and unipolar depression (Fortinguerra et al., Reference Fortinguerra, Sorrenti, Giusti, Zusso and Buriani2019; Wei et al., Reference Wei, McCarthy, Ren, Carrillo-Roa, Shekhtman, DeModena, Liu, Leckband, Mors, Rietschel, Henigsberg, Cattaneo, Binder, Aitchison and Kelsoe2020). The genetic factor, therefore, is unlikely to be a major factor behind mood state switching.
The mitochondria hypothesis of energy abnormality in mood disorders
It is well known that mitochondria are the powerhouse of the body and energy defects are a prominent symptom of depression while excessive energy is an important diagnostic symptom of mania. Reports supporting energy metabolism abnormalities in subjects with affective disorders dated back to the early 1990s and altered expressions of mitochondria-related genes were reported (Kasahara & Kato, Reference Kasahara and Kato2018). Additionally, environmental factors for these disorders, such as stresses, have been suggested to induce mitochondrial abnormalities. Moreover, animal studies have suggested that interactions of altered expression of mitochondria-related genes and environmental factors might be involved in mental disorders.
Mitochondrial dysfunction in affective disorders was organised into a hypothesis (Kato, Reference Kato2017; Allen et al., Reference Allen, Romay-Tallon, Brymer, Caruncho and Kalynchuk2018; Caruso et al., Reference Caruso, Benatti, Blom, Caraci and Tascedda2019). A 16–21% prevalence of BD was reported in mitochondrial diseases by several groups (Fattal et al., Reference Fattal, Budur, Vaughan and Franco2006; Mancuso et al., Reference Mancuso, Ricci, Choub, Filosto, DiMauro, Davidzon, Tessa, Santorelli, Murri and Siciliano2008; Inczedy-Farkas et al., Reference Inczedy-Farkas, Trampush, Perczel Forintos, Beech, Andrejkovics, Varga, Remenyi, Bereznai, Gal and Molnar2014), which is about 20 times higher than the general population. This suggests that having a mitochondrial disease is a strong risk factor for BD. Using an Induced pluripotent stem cells model for human BD, mitochondrial abnormalities were found in young neurons from patients with BD (Mertens et al., Reference Mertens, Wang, Kim, Yu, Pham, Yang, Zheng, Diffenderfer, Zhang, Soltani, Eames, Schafer, Boyer, Marchetto, Nurnberger, Calabrese, Ødegaard, McCarthy, Zandi, Alda, Nievergelt, Mi, Brennand, Kelsoe, Gage and Yao2015). All these findings regarding mitochondria defects raise the possibility of developing new bipolar drugs targeting mitochondria (Pereira et al., Reference Pereira, Chavarria, Vian, Ashton, Berk, Marx and Dean2018). The mitochondria hypothesis certainly offers an important conceptual framework to study the episodic or fluctuating energy phenomenon in mood disorders. How episodic mitochondrial changes can be used to explain the unstable and episodic changes in mood awaits further investigation. At this point, there is no pharmacotherapy targeting the mitochondria resulting in the stabilisation of mood.
Method
This review employed the traditional style of literature search. We searched the English language literature, including foreign-language publications with informative abstracts in English, up to August 31st, 2021, using PubMed (https://pubmed.ncbi.nlm.nih.gov), crossing the keywords ‘mood instability’, ‘antidepressant induced mania’, ‘treatment induced depression’, ‘mood switching’, ‘stress’, respectively and in turn with the following words: psychiatry, psychosis, psychiatric disorders, BD, anxiety disorders, brain circuits, neurotransmitters, psychotropics, brain areas, serotonin (5HT), DA, NE and mitochondria.
Various terminology has been used to describe the phenomenon of mood shifting in mood disorders. In this review, we used the term ‘mood switching’ to refer to both spontaneous (non-treatment-induced) and treatment-induced changes in the states of mood, including shifting and changes between depression, hypomania and mania.
As the available literature concerning this topic is substantial, manuscripts identified were included in this review only after evaluating the quality of the research and relevancy to the various sections of this review, namely the hypothesis of neurobiology and mechanism of mood shifting, and treatment-induced mood shifting.
Results
Animal models
Animal models have been developed for research into the mechanism of mood switching and the search for new therapeutic targets (Logan & McClung, Reference Logan and McClung2016). Though there are serious limitations, in that to what extent to which an animal model could recapitulate important features of AD and BD in the human, behavioural switching in animal models still may be useful to simulate switching of mood state into mania and vice versa. Animal models offer tremendous benefits for research into the biology of a drug-induced and spontaneous switching in mood states, as both in vivo and in vitro neuroanatomical, imaging, neurochemistry, genetic and pharmacological challenge approaches are all possible. Animal models likely would be more appropriate for testing energy changes induced by genetic, drugs and other environmental factors. Pharmacological challenges with antidepressant drugs of opposite or contrasting neurotransmitter profiles, for example, DA antidepressant drugs versus anticholinergic/5HT antidepressant drugs, may also help to unlock the mystery of antidepressant drug-induced switching in mood states.
However, developing an animal model of any psychiatric disorder is naturally difficult if not impossible. The symptoms of a disorder are usually broad, complex, mixed or variable and shifting in patients. Some crucial symptoms that are used to diagnose a disorder cannot be assessed in non-human models. How can feelings of guilt or worthlessness, expressions of sorrow be expressed in a rodent? New models should fulfil the three axes of validity: face validity, predictive validity and construct validity (Einat, Reference Einat2014). The ideal model should express all the main symptoms of BD but also be able to spontaneously switch between the manic and depressed state. We are nowhere near to producing such a model.
Some models of BD are listed here:
-
a. Changes in circadian rhythms and in the sleep-wake cyclic are diagnostic criteria for BD (Wirz-Justice, Reference Wirz-Justice2006). BD patients display rhythmical changes in general activity, sleep, body temperature, hormonal secretion and cellular regeneration all of which reflect fundamental alterations in the circadian rhythm (Bunney & Potkin, Reference Bunney and Potkin2008). These disruptions suggest that changes in clock genes are intimately involved (Wirz-Justice, Reference Wirz-Justice2006). Thus, several mouse models have been developed for BD based on the clock delta 19 mutant mouse. These mice carry a deletion of exon 19 of the clock gene, which results in a dominant protein which is unable to activate transcription (King et al., Reference King, Zhao, Sangoram, Wilsbacher, Tanaka, Antoch, Steeves, Vitaterna, Kornhauser, Lowrey, Turek and Takahashi1997) As a consequence, the mice exhibit manic-like behaviour, an altered sleep pattern, an increase in response to reward stimuli, reduced anxiety and depressive-like behaviour (McClung et al., Reference McClung, Sidiropoulou, Vitaterna, Takahashi, White, Cooper and Nestler2005; McClung, Reference McClung2007).
In addition to the behavioural changes that simulate important features of mania, mice with the defective Clock gene show an increase in DA release from neurons in the ventral tegmental region which reflects the dopaminergic cell firing rate (McClung et al., Reference McClung, Sidiropoulou, Vitaterna, Takahashi, White, Cooper and Nestler2005). This lends support to the view that hyperdopaminergic function is responsible for mania in BD (Berk et al., Reference Berk, Dodd, Kauer-Sant’anna, Malhi, Bourin, Kapczinski and Norman2007).
An important predictive feature of the Clock gene model is the response to lithium. The dysfunctional circadian rhythm is corrected by the administration of lithium and the change is correlated with the action of lithium on GSK-3, a key intra-cellular target for the drug (Coque et al., Reference Coque, Mukherjee, Cao, Spencer, Marvin, Falcon, Sidor, Birnbaum, Graham, Neve, Gordon, Ozburn, Goldberg, Han, Cooper and McClung2011).
Observations of depressive-like behaviour in the Clock gene mouse model are less frequent (Mukherjee et al., Reference Mukherjee, Coque, Cao, Kumar, Chakravarty, Asaithamby, Graham, Gordon, Enwright, DiLeone, Birnbaum, Cooper and McClung2010) but there is experimental evidence the Bcl-2 gene is disrupted. This gene is involved in neuronal development, plasticity and neurodegeneration. Indirectly, it also affects the circadian rhythm. Thus, both the manic and the depressive-like states may be affected in the clock delta 19 mouse model (Einat et al., Reference Einat, Yuan and Manji2005; Lien et al., Reference Lien, Flaisher-Grinberg, Cleary, Hejny and Einat2008).
-
b. For over a century, clinical observers have reported that the severity of the symptoms of BD increases with the frequency of the episode. For example, Kraepelin (Reference Kraepelin1909) observed that with increasing episodes of BD the course of the illness became worse and more frequent thereby suggesting that sensitisation of the condition occurred. It was later shown that behavioural sensitisation to psychostimulants also shortened the frequency between episodes in BD patients, cocaine and amphetamine being well established to produce these effects (Post, Reference Post1990). Manic-like behaviour that was initiated by amphetamine was attenuated by valproate, lithium and other mood stabilisers (Sharma et al., Reference Sharma, Fries, Galvez, Valvassori, Soares, Carvalho and Quevedo2016).
In experimental studies in rodents, the abrupt withdrawal of chronically administered psychostimulants results in a depressive-like state, accompanied by an increase in anxiety-like behaviour (Mutschler & Miczek, Reference Mutschler and Miczek1998; Barr & Phillips, Reference Barr and Phillips1999). These behavioural changes are associated with serotonergic super sensitivity (Baumann & Rothman, Reference Baumann and Rothman1998), a transient decrease in noradrenaline in the hypothalamus and a reduction in the responsiveness to the amphetamine stimulus (Paulson et al., Reference Paulson, Camp and Robinson1991). Schwartz et al. (Reference Schwartz, Ksir, Krab and Bloom1982) showed that a switch occurred in the beta-endorphin-induced locomotor activity in rodents from a hyper-to a hypo-responsiveness during the withdrawal of amphetamine. The prominent neurotransmitter changes associated with these states in rodents are predicted to be the catecholamines and Ach, respectively (Berk et al., Reference Berk, Dodd, Kauer-Sant’anna, Malhi, Bourin, Kapczinski and Norman2007; van Enkhuizen et al., Reference van Enkhuizen, Janowsky, Olivier, van Enkhuizen, Janowsky, Olivier, Minassian, Perry, Young and Geyer2015).
To date, the psychostimulant stimulation models in rodents are unique in developing both the manic-like and depressive-like phases of BD (Kato et al., Reference Kato, Kubota and Kasahara2007). However, it is already apparent that there are numerous different neurotransmitter pathways involved so that only limited information is currently available regarding any primary change(s) which are responsible for the key bipolar phenotype. This has helped to stimulate research into genetically based models involving ‘knock-down’ mice (Zhuang et al., Reference Zhuang, Oosting, Jones, Gainetdinov, Miller, Caron and Hen2001) in which the dopaminergic system is overexpressed by the use of the inducible lentivirus vector (Freund et al., Reference Freund, Thompson, Sonntag, Meda and Andersen2016).
-
c. Changes in central Dopaminergic function in rodent models of BD.
The DA system has received particular attention as both clinical and experimental evidence suggest that this system is vulnerable to change in BD (Berk et al., Reference Berk, Dodd, Kauer-Sant’anna, Malhi, Bourin, Kapczinski and Norman2007). Whereas the manic state appears to be associated with DA hyperactivity, the depressive state may result from a desensitisation of DA receptors in response to the excessive DA stimulation. In support of this observation, while clinical studies show that the manic state is usually responsive to neuroleptics which are DA receptor antagonists, the DA receptor agonist, bromocriptine, also improved the depressive state (Zarate et al., Reference Zarate, Payne, Singh, Quiroz, Luckenbaugh, Denicoff, Charney and Manji2004).
While rodent models of mania have mainly concentrated on the effects of stimulants, more recently inducible lentivirus vectors have been used to overexpress DA D1 receptors, for which there is evidence that their overactivity in the prefrontal cortex is associated with depressive-like behaviour (Freund et al., Reference Freund, Thompson, Sonntag, Meda and Andersen2016).
-
d. Rodent models based on changes in the activation of the maternal immune system following the administration of the human influenza virus, bacterial lipopolysaccharide or polyinosinic-polycytidine (poly-I C) to pregnant animals cause the disruption of latent inhibition, impaired working memory, stereotypic behaviour, increased anxiety-like behaviour and learned helplessness (Meyer et al., Reference Meyer, Feldon, Schedlowsky and Yee2005; Meyer, Reference Meyer2014; Ronovsky et al., Reference Ronovsky, Berger, Molz, Berger and Pollak2016; Rose et al., Reference Rose, Careaga, Van de Water, McAllister, Bauman and Ashwood2017). Changes in the striatal DA activity are correlated with these behavioural changes (Zuckerman et al., Reference Zuckerman, Rehavi, Nachman and Weiner2003). Thus, together with the immune changes widely reported to occur in the serum of patients with BD (Maes et al., Reference Maes, Minaylova, Kubera and Ringel2012), there is evidence that the immune system might also play an important part in the pathophysiology of BD.
However, despite the attraction of some of the rodent models in expressing manic and depressive-like states which simulate BD, so far no animal model has been developed to examine specifically the switch mechanism between these states.
Treatment-induced mood changes
As treatment-induced mood switching is an important clinical consideration, we reviewed the possible relationship between the pharmacological properties of common psychotropics used in the treatment of AD and treatment-induced mood switching, with reference to the various hypotheses proposed for the mood switching mechanism.
Clinical trials
Many randomised trials of combination pharmacotherapy for the management of unstable mood in BD focus on the utility of pairing a mood stabiliser with a new generation antipsychotic (Goldberg, Reference Goldberg2019). So far, there are no well-designed controlled trials with adequate numbers and power to demonstrate what constitutes an effective stable mood maintenance regimen in BD or patients with unstable mood.
Much hope was put in the new generation antipsychotic agents with dual 5HT and DA modulation properties and agents with partial DA agonist action such as aripiprazole and brexpiprazole. Lurasidone is currently the only treatment for bipolar depression approved in the United States as both a monotherapy and an adjunctive therapy with lithium or valproate. Lurasidone was studied both as a monotherapy and adjunctive treatment to lithium or valproate. It has also been studied in acute depression and prevention of recurrence of any mood episode in patients with BD, whether initially treated for bipolar depression or mania. However, data from trials of combining lurasidone to lithium or valproate for BD were inconsistent (Loebel et al., Reference Loebel, Xu, Hsu, Cucchiaro and Pikalov2015). Although some research findings indicated that it is effective for acute bipolar depression, long-term data is still needed (Pompili et al., Reference Pompili, Verzura, Trovini, Buscajoni, Falcone, Naim, Nardella, Sorice, Baldessarini and Girardi2018) and it has not demonstrated efficacy in relapse prevention when added to a mood stabiliser (Ali et al., Reference Ali, Tegin and El-Mallakh2020).
In patients with bipolarity and mixed features, a combination of antidepressant drugs and mood stabilisers or atypical antipsychotics is recommended, rather than antidepressant monotherapy. Regarding the selection of mood stabilisers, lamotrigine appeared to be the most reliable, lithium’s effect is modest, while clear evidence is lacking for valproate and carbamazepine (Shim et al., Reference Shim, Woo, Kim and Bahk2017). The most efficacious combination treatments in all phases of bipolar illness are also urgently needed (Zarate & Quiroz, Reference Zarate and Quiroz2003).
Non-conventional agents for maintenance of stable mood
The usefulness of some non-conventional agents, including tamoxifen, allopurinol, methoxyprogesterone, ketamine, modafinil, pramipexole, pregnenolone and armodafinil, celecoxib, lisdexamfetamine, memantine, N-acetylcysteine, as monotherapy and as combination therapy with lithium and other mood stabilisers, have been reported to be useful in some patients. While they may open ‘new horizons’ in the understanding of the mechanism of unstable mood, their efficacies still have to be tested in formal clinical trials (Fountoulakis et al., Reference Fountoulakis, Balaris, Nikolaou and Nimatoudis2016).
Drug-induced switching in mood states
The mean rates of antidepressant-associated mood elevations in studies comparing bipolar I disorder and bipolar II disorder were 14.2% and 7.1%, respectively, in acute trials (less than 16 weeks), and 23.4% and 13.9%, respectively, in maintenance studies.
Drug-induced changes in mood are a practical concern for most clinicians managing patients suffering from affective disorders as the use of antidepressant drugs to treat bipolar depression is often unavoidable. For most clinicians, an important question needed to be addressed is whether the neurotransmitter profile of an antidepressant drug is related to its tendency to induce a mood shift (Gijsman et al., Reference Gijsman, Geddes, Rendell, Nolen and Goodwin2004; Sidor & Macqueen, Reference Sidor and Macqueen2011; Zhang et al., Reference Zhang, Yang, Yang, Liang, Dai, Wang and Zhang2013; Baldessarini et al., Reference Baldessarini, Vázquez and Tondo2020). If this is true, then certain antidepressant drugs with or without certain neurotransmitter properties will be preferred over others.
Antidepressant-induced shift into mania in bipolar patients was claimed to be common and occurs early (Tondo et al., Reference Tondo, Vázquez and Baldessarini2010). As it has been suggested that there is DA and or NE hyperactivity in mania and that DA antagonists are anti-manic, one would expect antidepressant drugs that potentiate DA and or NE neurotransmission would possess a stronger tendency to induce a mania switch. However, this may not be the case (Carlson et al., Reference Carlson, Merlock and Suppes2004; Dell’Osso et al., Reference Dell’Osso, Ketter, Cremaschi, Spagnolin and Altamura2013; Corp et al., Reference Corp, Gitlin and Altshuler2014).
Reducing DA transporter functioning recreates many aspects of BD mania including hyper motivation in the animal model (van Enkhuizen et al., Reference van Enkhuizen, Henry, Minassian, Perry, Milienne-Petiot, Higa, Geyer and Young2014; Milienne-Petiot et al., Reference Milienne-Petiot, Kesby, Graves, van Enkhuizen, Semenova, Minassian, Markou, Geyer and Young2017). Kurita (Reference Kurita2016) suggested that NE plays a critical role in the manic switch, as well as in the reversal of depression in bipolar. Tricyclic antidepressant drugs usages were reported to be associated with a higher incidence of drug induce mania (10%) than other types (3.2%) of antidepressant drugs combined (Peet & Peters, Reference Peet and Peters1995; Gijsman et al., Reference Gijsman, Geddes, Rendell, Nolen and Goodwin2004; Mundo et al., Reference Mundo, Cattaneo, Russo and Altamura2006; Tondo et al., Reference Tondo, Vázquez and Baldessarini2010). Koszewska and Rybakowski (Reference Koszewska and Rybakowski2009) also reported that the risk of switching was higher during treatment with TCA than with non-TCA drugs (36% vs. 17%) and for individual TCAs, the highest with amitriptyline (42% of treated episodes), imipramine (40%) and clomipramine (35%). A retroactive electronic case register cohort study reported SSRIs and venlafaxine usage showed a significant association with an increased incidence of mania and BD (Patel et al., Reference Patel, Reiss, Shetty, Broadbent, Stewart, McGuire and Taylor2015). Some case reports (Freitas et al., Reference Freitas, Barranha, Abreu and Von Doellinger2019) also did not consistently support that the DA and NE type of antidepressant drugs being more inclined to induce a manic switch.
Duloxetine, a more balanced dual 5HT and NE uptake inhibitor (SNRI) than venlafaxine, was reported to show a lower incidence of drug-induced mania (Dunner et al., Reference Dunner, D’Souza, Kajdasz, Detke and Russell2005). Escitalopram, an SSRI with insignificant action on neurotransmitter pathways other than 5HT, was reported to induce mania/hypomania in a dose-related manner (Yamaguchi et al., Reference Yamaguchi, Kimoto, Nagahama and Kishimoto2018). Thus, there is no clear evidence to support that catecholamine (DA and NE) or 5HT activation would consistently induce mania, or which antidepressant drug is associated with the induction of mania. The number of reports and the N size of the reports were all too small to support the use or avoidance of any specific group of antidepressants in BD. A much larger subject size review of cases of antidepressant drug-induced mania and hypomania needs to be done to answer this clinically important question.
An explanation for the higher incidence for TCA-induced mania compared to other groups of antidepressant drugs may come from the observation of scopolamine’s (an anticholinergic drug) antidepressant effect (Furey & Drevets, Reference Furey and Drevets2006). This raises an interesting point in the development of antidepressant drug development. Modern antidepressant drugs had their origin from atropine/scopolamine which are anticholinergic/histaminergic molecules (Tang & Tang, Reference Tang and Tang2019). The second-generation antidepressant drugs such as SSRIs and SNRIs, derived from antihistamine backbones, were considered improvements over TCAs with the removal of the anticholinergic/antihistaminergic side effects. Some patients who did not respond to SSRI/SNRIs improved on TCAs. The effectiveness of oral scopolamine as an adjuvant to citalopram in alleviating the symptoms of major depression (Khajavi et al., Reference Khajavi, Farokhnia, Modabbernia, Ashrafi, Abbasi, Tabrizi and Akhondzadeh2012) may highlight the same essential anticholinergic components. Thus, the removal of anticholinergic property from the TCAs might have removed an important antidepressant pharmacological component. Most TCAs (except amitriptyline and amoxapine) (PDSP Ki Database, accessed September 1, 2020) do not possess significant 5HT7 antagonist properties, while both 5HT3 and 5HT7 antagonism have been found to be responsible for or to potentiate antidepressant action of antidepressant drugs (Perez-Palomar et al., Reference Perez-Palomar, Mollinedo-Gajate, Berrocoso, Meana and Ortega2018; Balcer et al., Reference Balcer, Seager, Gleason, Li, Rasmussen, Maxwell, Nomikos, Degroot and Witkin2019). It would be interesting to observe if 5HT3 and 5HT7 antagonism also tend to induce mania and this may shed light on the pathogenesis of mood state switching as well.
The emergence of loss of efficacy during antidepressant drug treatment is observed clinically (Fornaro et al., Reference Fornaro, Anastasia, Novello, Fusco, Pariano, De Berardis, Solmi, Veronese, Stubbs, Vieta, Berk, de Bartolomeis and Carvalho2019). Whether the cause is spontaneous mood switching or rapid cycling caused by other factors such as compliance or other external factors is unclear at present. In conjunction, it is important to mention that cases of mania appearance with various antidepressant cessation (Ali & Milev, Reference Ali and Milev2003; Andrade, Reference Andrade2004; Narayan & Haddad, Reference Narayan and Haddad2011; Verma & Mohapatra, Reference Verma and Mohapatra2015; Kwok & Lim, Reference Kwok and Lim2017) were also reported. While the withdrawal-induced mania seemed to be self-limiting and subsided with drug withdrawal, the presence of mood stabilisers may not protect against the induced mania and anti-manic treatments may be necessary (Goldstein et al., Reference Goldstein, Frye, Denicoff, Smith-Jackson, Leverich, Bryan, Ali and Post1999; Andrade, Reference Andrade2004). As chronic antidepressant administration was associated with receptor subsensitivity (Tang et al., Reference Tang, Seeman and Kwan1981), acute withdrawal may result in a rebound hypersensitivity state, manifested as hypomania or mania.
Stoll et al. (Reference Stoll, Mayer, Kolbrener, Goldstein, Suplit, Lucier, Cohen and Tohen1994) reported that MAOIs and bupropion may be associated with milder manic states than either tricyclic drugs or fluoxetine. They noted that antidepressant-associated mania appears to be a milder and more time-limited syndrome than spontaneous mania and argued that it may represent a distinct clinical entity.
Sporadic case reports of specific antidepressant-induced hypomania and mania, such as agomelatine (Thorpe et al., Reference Thorpe, Pannell and Nance2014; Tu & Lin, Reference Tu and Lin2014; Kennel et al., Reference Kennel, Baus, Dogui, Siebert and Riemenschneider2017), trazodone (Warren & Bick, Reference Warren and Bick1984; Arana & Kaplan, Reference Arana and Kaplan1985; Knobler et al., Reference Knobler, Itzchaky, Emanuel, Mester and Maizel1986; Ashford, Reference Ashford2019) sertraline (Kumar et al., Reference Kumar, Dubey and Sinha2000; Mendhekar et al., Reference Mendhekar, Gupta and Girotra2003) and bupropion (Goren & Levin, Reference Goren and Levin2000; Kahyacı Kılıç et al., Reference Kahyacı Kılıç, Görgülü, Köse Çınar and Sönmez2019) caused concerns of their usage in bipolar patients. Such case reports also continued to show with other individual SSRIs and new antidepressant drugs such as vortioxetine (Maud, Reference Maud2016; Sobreira et al., Reference Sobreira, Oliveira and Brissos2017). Others argued that they may not necessarily cause mood state switching with reintroduction or dose reduction (Jabeen & Fisher, Reference Jabeen and Fisher1991; Kahyacı Kılıç et al., Reference Kahyacı Kılıç, Görgülü, Köse Çınar and Sönmez2019) and their tendency to induce a switch into mania may also be mitigated in the presence of mood stabilisers (Wichniak et al., Reference Wichniak, Jarkiewicz, Okruszek, Wierzbicka, Holka-Pokorska and Rybakowski2015; Yatham et al., Reference Yatham, Vieta, Goodwin, Bourin, de Bodinat, Laredo and Calabrese2016).
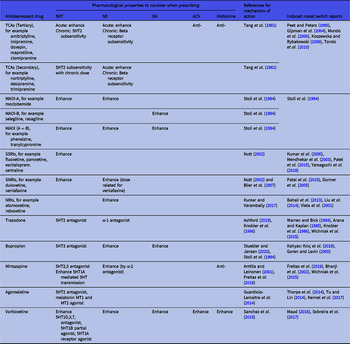
Irrespective of the inconsistent findings so far regarding antidepressant neurotransmitter profiles and their tendency to induce switching, it is still useful to use their profile as a guideline in switching to a drug with a different neurotransmitter profile when mood state switching occurs. We therefore summarised the neurotransmitter profile of antidepressant drugs in Table 1 for reference.
Table 1. Choosing antidepressant drugs in patients experiencing treatment-induced mania
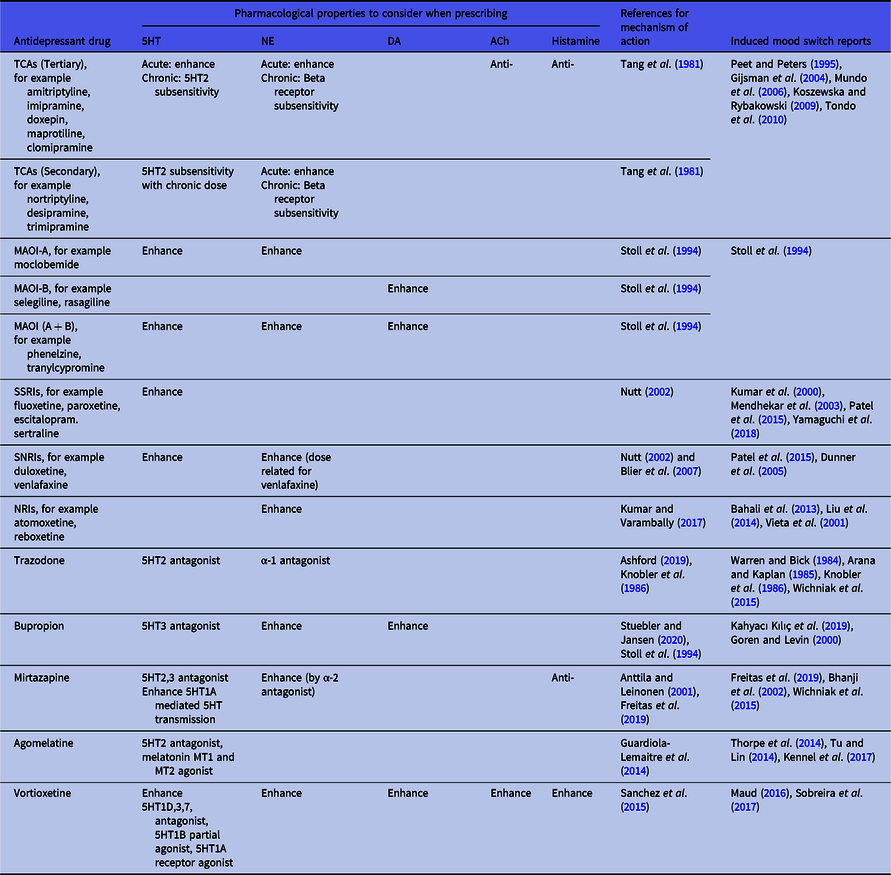
Pharmacokinetic, pharmacodynamic and drug−drug interaction factors
Changes in drug and drug target response due to pharmacokinetic and pharmacodynamic factors may be responsible for the switching of mood state in patients receiving treatment. Though the incidence of CYP enzyme-related extensive and slow metabolisers of psychotropics and G-glycoprotein drug transport abnormality of psychotropics may not be high, they are still important factors to exclude when mood switching began to occur in a patient previously well maintained. This is because patients with unstable mood, and in particular patients with BD, tend to be on polypharmacy and the introduction of additional medications is common over the course of treatment. CYP enzymes are also the major enzymes for the metabolism of psychotropics. Drug−drug interactions and metabolic pathway shifts (Albers et al., Reference Albers, Reist, Helmeste, Vu and Tang1996; Yoshioka et al., Reference Yoshioka, Ida, Yokota, Nishimoto, Shibata, Sugawara and Takiguchi2000; Tang & Helmeste, Reference Tang and Helmeste2008; Tang et al., Reference Tang, Helmeste and Leonard2017) definitely is an important aspect of therapeutics in polypharmacy situations.
Clinical profiles of patients with unstable mood
The clinical profiles of patients with unstable mood levels have been studied extensively. Niitsu et al. (Reference Niitsu, Fabbri and Serretti2015) summarised the risk factors as younger age, previous history of rapid cycling, severe manic symptoms, suicide attempts, amphetamine use and certain pharmacological and psychotherapeutic treatments. For the current depressive episode, the identified risk factors were mood elevation, multiple mania-associated symptoms with at least moderate severity and comorbid panic attacks.
With regard to diagnosis, the risk of antidepressant-induced mood elevations appeared to be greater in bipolar I disorder than bipolar II disorder and higher in bipolar II disorder than other mood disorders. Mood converted mostly to hypomania in bipolar II and mood disorders. Patients with bipolar I disorder would experience manias and hypomanias (Bond et al., Reference Bond, Noronha, Kauer-Sant’Anna, Lam and Yatham2008). It was suspected that between 1/3 to ¼ of bipolar patients may be ‘inherently susceptible’ to antidepressant-induced manias. Those with a strong genetic loading and whose illness began early are especially at risk. Identification of high-vulnerability subgroups (Visser & Van Der Mast, Reference Visser and Van Der Mast2005) and differentiate illness-specific from medication-specific factors in mood switching will be needed in future trials (Goldberg & Truman, Reference Goldberg and Truman2003). A similar mechanism may underly both the rapid mood switching in some forms of BD and the affective instability of borderline personality disorder and may even have the same genetic aetiology (MacKinnon & Pies, Reference Mackinnon and Pies2006).
Polypharmacy is defined here as the prescription of a combination of mood stabilisers (lithium, valproic acid), antipsychotics (DA antagonists or partial antagonists (aripiprazole), antidepressant drugs and benzodiazepines or the z drugs). The number of medications in polypharmacy has been studied (Visser & Van Der Mast, Reference Visser and Van Der Mast2005; Fornaro et al., Reference Fornaro, De Berardis, Koshy, Perna, Valchera, Vancampfort and Stubbs2016a, Reference Fornaro, Nardi, De Berardis and Carta2016b). The iterative addition of more and more drugs to the treatment regimen often is the pressure of suboptimal response in the earlier stage, but clearly without evidence from the literature. Interestingly, the personality of the patient (lower scores on openness, extraversion and lower conscientiousness) also appeared to invite polypharmacy (Sachs et al., Reference Sachs, Peters, Sylvia and Grunze2014).
Conclusion
We are still far from understanding the neurobiology underlying mood instability or mood switching in affective disorders. There is no single hypothesis that offers practical guidance for using psychotropics to manage patients with unstable mood so far. From the literature review, there is a poverty of double-blind placebo control studies with large patient numbers examining this issue. It is still inconclusive whether one antidepressant or groups of antidepressant drugs is safe from inducing mania or a mood state switching, with or without mood stabiliser coverage. Drug-induced mood level changes cannot yet be explained by simple activation of or antagonism between specific neuropathways. Case reports appeared to show that antidepressant drug-induced mania could be managed by dose reduction, withdrawal, or replacement of the antidepressant drug, or with anti-manic agents. However, the validity of observations from single case reports needs larger double-blind control studies to verify. It is likely that drug-induced or drug withdrawal-related mood changes, mania, hypomania or depression, have a different mechanism from the spontaneous mood state switching or switch caused by other factors such as seasonal changes. In this regard, no antidepressant or antipsychotic drug has been demonstrated to show an advantage over others. Much more research will be necessary to enable a clearer understanding of the nature of mood state switching in affective disorders and its treatment.