Summation
-
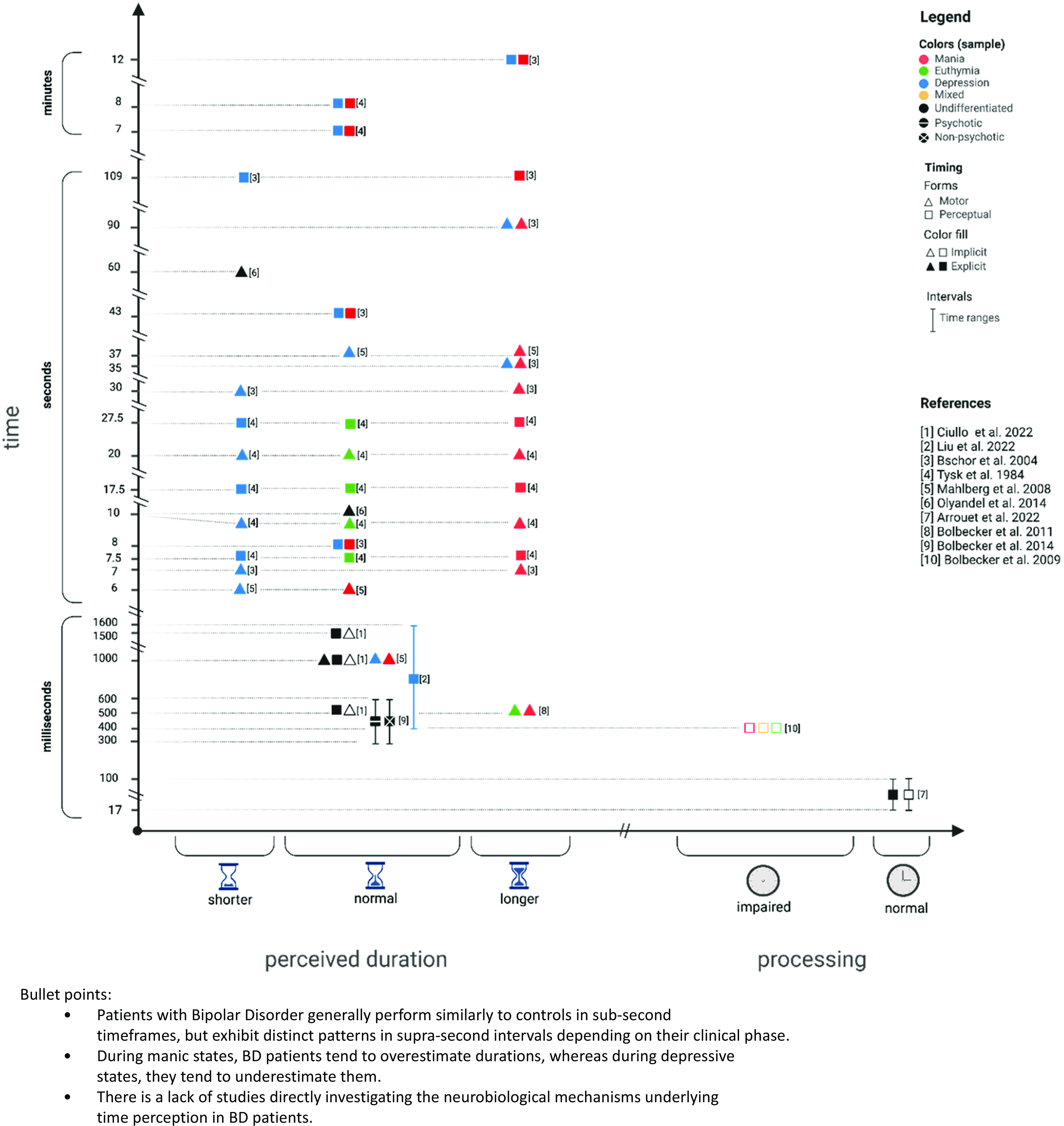
Patients with bipolar disorder generally perform similarly to controls in sub-second timeframes, but exhibit distinct patterns in supra-second intervals depending on their clinical phase.
-
During manic states, BD patients tend to overestimate durations, whereas during depressive states, they tend to underestimate them.
-
There is a lack of studies directly investigating the neurobiological mechanisms underlying time perception in BD patients.
Considerations
-
The review is limited by the small and heterogeneous sample size, potentially introducing confounds.
-
Many studies did not differentiate patients based on their clinical states and type of bipolar disorder, particularly in sub-second timeframes.
-
There is a significant lack of robust longitudinal research using experimental methodologies to assess temporal perception shifts across individual clinical phases.
Introduction
Bipolar disorder (BD) is a prevalent condition that affects 2–5% of the global population (Merikangas et al., Reference Merikangas, Akiskal, Angst, Greenberg, Hirschfeld, Petukhova and Kessler2007). It is a chronic and hereditary illness characterised by periodic episodes of mania or hypomania and depression, which may each present with or without mixed features (McIntyre et al., Reference Mcintyre, Berk, Brietzke, Goldstein, López-Jaramillo, Kessing, Malhi, Nierenberg, Rosenblat and Majeed2020a). The disorder manifests through dramatic shifts in mood, significant fluctuations in energy, disruptions in biological clocks, and irregularities in sleep and social rhythms. In addition, individuals with BD often demonstrate a reduced adaptability to stress and a heightened sensitivity to environmental triggers (Pagani et al., Reference Pagani, St Clair, Teshiba, Service, Fears, Araya, Araya, Bejarano, Ramirez, Castrillon, Gomez-Makhinson, Lopez, Montoya, Montoya, Aldana, Navarro, Freimer, Safaie, Keung, Greenspan, Chou, Escobar, Ospina-Duque, Kremeyer, Ruiz-Linares, Cantor, Lopez-Jaramillo, Macaya, Molina, Reus, Bearden, Takahashi J.S. and Freimer2016; McIntyre et al., Reference Mcintyre, Berk, Brietzke, Goldstein, Lopez-Jaramillo, Kessing, Malhi, Nierenberg, Rosenblat, Majeed, Vieta, Vinberg, Young and Mansur2020b). This disorder exhibits significant heterogeneity during clinical presentation, illness courses, treatment response, and functional outcome (McIntyre et al., Reference Mcintyre, Alda, Baldessarini, Bauer, Berk, Correll, Fagiolini, Fountoulakis, Frye, Grunze, Kessing, Miklowitz, Parker, Post, Swann, Suppes, Vieta, Young and Maj2022). The bipolar disorder spectrum is composed of BD type I, characterised by the occurrence of a syndromic manic episode, and BD type II, which is distinguished by the presence of a syndromic hypomanic episode and a major depressive episode. Although progress has been made to understand the pathophysiology of BD, the mechanisms underlying the disorder remain largely obscure (McIntyre et al., Reference Mcintyre, Berk, Brietzke, Goldstein, Lopez-Jaramillo, Kessing, Malhi, Nierenberg, Rosenblat, Majeed, Vieta, Vinberg, Young and Mansur2020b). Notably, emerging evidence suggests that disturbances in time perception may play a central role in the diverse symptomatology of BD (Northoff et al., Reference Northoff, Magioncalda, Martino, Lee, Tseng and Lane2018). To shed light on this topic, this work aims to conduct a systematic review of the experimental studies investigating time perception in patients with a diagnosis of BD. By synthesising the existing literature, this review provides a comprehensive understanding of this aspect and will potentially inspire further research in this promising direction.
Psychopathology of time
Because of the profound connection between the human sense of being, self-awareness, and the experience of time, the study of time has long been a central issue in both phenomenological psychopathology and philosophy. In recent decades, a growing body of evidence suggests that the clinical symptoms of psychiatric disorders can be understood as the manifestations of underlying disturbances within the fundamental elements of consciousness, including the perception of time (Fuchs, Reference Fuchs2013; Northoff, Reference Northoff2013; Arstila and Lloyd, Reference Arstila and Lloyd2014; Tewes and Stanghellini, Reference Tewes and Stanghellini2020). The initial investigations on time perception for people suffering from mental disorders focused on schizophrenia (De La Garza and Worchel, Reference De La Garza and Worchel1956, Goldstone and Lhamon, Reference Goldstone and Lhamon1956; Rabin, Reference Rabin1957, Ehrentheil and Jenney, Reference Ehrentheil and Jenney1960, Pearl and Berg, Reference Pearl and Berg1963; Simmel, Reference Simmel1963). They revealed a widespread alteration of time perception across short and long temporal intervals, with a dependence on arousal levels and rewards (for a review, see Ciullo et al., Reference Ciullo, Spalletta, Caltagirone, Jorge and Piras2016; Thoenes and Oberfeld, Reference Thoenes and Oberfeld2017; Amadeo et al., Reference Amadeo, Esposito, Escelsior, Campus, Inuggi, Pereira Da Silva, Serafini, Amore and Gori2022). Following these early studies on schizophrenia, a growing body of literature has explored the time domain in other neuropsychiatric disorders, including major depressive disorder (Thones and Oberfeld, Reference Thones and Oberfeld2015; Stanghellini et al., Reference Stanghellini, Ballerini, Presenza, Mancini, Northoff and Cutting2017), autism spectrum disorder (Vogel et al., Reference Vogel, Kramer, Schoofs, Kupke and Vogeley2018; Casassus et al., Reference Casassus, Poliakoff, Gowen, Poole and Jones2019), eating disorders (Stanghellini et al., Reference Stanghellini, Ballerini, Presenza, Mancini, Northoff and Cutting2017), and BD (see Results). Thus, numerous examples of abnormal time experiences are found in the classic phenomenological investigations of psychiatric disorders (Mezey and Cohen, Reference Mezey and Cohen1961; Lehmann, Reference Lehmann1967; Kitamura and Kumar, Reference Kitamura and Kumar1983; Tysk, Reference Tysk1984). This suggests that the disintegration of time has profound psychopathological effects on how one perceives oneself, the sensory world, and interactions with others (Kent and Wittmann, Reference Kent and Wittmann2021). About BD, literature highlights distorted subjective temporal experiences, such as perceiving the passage of time as faster during mania and slower during depression (Bschor et al., Reference Bschor, Ising, Bauer, Lewitzka, Skerstupeit, Muller-Oerlinghausen and Baethge2004; Moskalewicz and Schwartz, Reference Moskalewicz and Schwartz2020), and a past-focused time perspective during depression and a present/future-focused time perspective during mania (Ghaemi, Reference Ghaemi2007; Vogeley and Kupke, Reference Vogeley and Kupke2007; Gruber et al., Reference Gruber, Cunningham, Kirkland and Hay2012; Stanghellini et al., Reference Stanghellini, Ballerini, Presenza, Mancini, Raballo, Blasi and Cutting2016; Stanghellini et al., Reference Stanghellini, Ballerini, Presenza, Mancini, Northoff and Cutting2017).
Northoff’s Spatiotemporal Psychopathology model (STPP) proposed that the experience of individual time, as well as space, is directly related to the neuronal topography and dynamics of the brain’s spontaneous activity (Northoff et al., Reference Northoff, Magioncalda, Martino, Lee, Tseng and Lane2018; Northoff et al., Reference Northoff, Daub and Hirjak2023; Northoff and Hirjak, Reference Northoff and Hirjak2023). That is, the spatiotemporal configurations of the brain contribute to spatiotemporal changes on the psychological level. In turn, this leads to different kinds of psychopathological symptoms, including affective, perceptual, cognitive, and motor symptoms. Regarding BD, the model proposed that its typical oscillations in symptomatology are underlined by cyclic desynchronisation between self-time and world-time, which potentially corresponds to changes in neuronal variability within the somatomotor and sensory resting state networks (Martino et al., Reference Martino, Magioncalda, Huang, Conio, Piaggio, Duncan, Rocchi, Escelsior, Marozzi, Wolff, Inglese, Amore and Northoff2016; Northoff et al., Reference Northoff, Magioncalda, Martino, Lee, Tseng and Lane2018). By bridging the phenomenological level with neurobiology, this new perspective implies not only a meticulous analysis of the existing findings on time alterations in BD but also a shift from the phenomenological qualitative investigation of subjective time experience to quantifying its subjective features through experimental paradigms and objective measurements. Despite the existence of only limited research, this review will go beyond the phenomenological level and explore the results of quantitative studies investigating time perception in BD patients and consider psychopathological and cognitive correlates.
By examining relevant research about time perception (Grondin, Reference Grondin2008; Vatakis et al., Reference Vatakis, Balcı, Di Luca and Correa2018), findings will be discussed considering whether i) explicit or implicit time perception was investigated, ii ) the temporal intervals involved were sub-second or supra-second, and iii) a perceptual or motor timing paradigm was used. Indeed, there is a typical distinction in time literature between explicit and implicit time perception (Coull and Nobre, Reference Coull and Nobre2008; Piras and Coull, Reference Piras and Coull2011). Explicit time perception involves making explicit judgements about the temporal properties of external stimuli. For example, this includes comparing the duration of different stimuli, evaluating the temporal order of their appearance, or determining whether they are presented simultaneously or not. In contrast, implicit perception occurs automatically whenever we rely on intrinsic temporal information to solve other perceptual tasks. Indeed, some tasks involving perceptual judgements about other stimulus features implicitly require to use the temporal information intrinsic to stimulus presentation. An example is when individuals are asked to evaluate whether moving stimuli will collide: even though the task does not explicitly ask to evaluate time, temporal information, such as the speed of the sensory stimuli, is essential for predicting their eventual locations. Moreover, researchers have suggested that different mechanisms are involved depending on the temporal scale of external stimuli, specifically whether the intervals to be processed are above or below one second (e.g. evaluating the durations of stimuli lasting 10–200 ms or 10–200 s). Sub-second intervals reflect lower-level automatic timing processing, whereas supra-second intervals reflect higher-level cognitive timing processing (Mioni et al., Reference Mioni, Grondin, Bardi and Stablum2020; Li et al., Reference Li, Yu and Yuen2022). In addition, traditionally, the literature distinguishes between perceptual and motor timing (e.g. Keele et al., Reference Keele, Pokorny, Corcos and Ivry1985; Jones and Jahanshahi, Reference Jones and Jahanshahi2014). In perceptual timing, judgements of time are given through perceptual decisions. For instance, individuals typically state whether one temporal interval is shorter or longer than another, evaluate which stimulus appears first, or detect when the next stimulus will appear. In contrast, in motor timing, judgements of time are expressed through a motor response. Classic examples include production and reproduction tasks, where individuals are asked to produce or reproduce a duration using a sustained, delayed, or periodic motor act. For instance, participants may be asked to press a button for 1 s (production task), to press a button for the same duration as a sound presented through headphones, or reproduce a presented rhythm by tapping (i.e. reproduction tasks).
Literature search and study selection
Eligibility criteria
The current study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) recommendations (Page et al., Reference Page, Mckenzie, Bossuyt, Boutron, Hoffmann, Mulrow, Shamseer, Tetzlaff, Akl, Brennan, Chou, Glanville, Grimshaw, Hrobjartsson, Lalu, Li, Loder, Mayo-Wilson, Mcdonald, Mcguinness, Stewart, Thomas, Tricco, Welch, Whiting and Moher2021) to ensure a comprehensive inclusion of relevant articles on time perception in BD. We included studies published in English in peer-reviewed journals, without setting restrictions on the publication date. Since the goal of the systematic review is to illustrate quantitative results about time perception skills in BD, we have selected only studies encompassing an experimental paradigm to objectively measure the capacity of a subject to judge time. As such, the inclusion criteria were as follows: i) the presence of a bipolar and related disorders diagnosis based on the previous and latest ICD (World Health Organization, 2022) and DSM (Association, Reference Association2022) criteria; and ii) the use of experimental tasks to quantitatively investigate time perception as described in the manual “Timing and Time Perception: Procedures, Measures, and Applications” (Vatakis et al., Reference Vatakis, Balcı, Di Luca and Correa2018). The exclusion criteria were as follows: i) an absence of healthy controls; ii) a lack of a bipolar and related disorders diagnosis based on the previous and latest ICD (World Health Organization, 2022) and DSM (Association, Reference Association2022) criteria; iii) the lack of a timing assessment task as defined by “Timing and Time Perception: Procedures, Measures, and Applications” (Vatakis et al., Reference Vatakis, Balcı, Di Luca and Correa2018); iv) reviews, meta-analyses, case reports, conference posters, and other non-original studies; and v) studies in which the full text is not available. See Fig. 1 for the PRISMA flowchart and Supplementary Materials for the PRISMA check-list.

Figure 1. PRISMA flowchart of the literature search and study selection process.
Data sources
An extensive literature review was conducted by consulting PubMed, APA PsycNET, and Scopus. There were no limitations on the publication year, and the review covered the period up to July 2023. The search string used was (“bipolar disorder” OR “bipolar illness” OR “bipolar disease” OR “manic-depress*” OR “manic depress*” OR mania OR manic OR hypomanic* OR “mixed episode*” OR “mixed state*” OR “bipolar depress*” OR “bipolar spectrum” OR cyclothymic* OR “affective disorders” OR “mood disorders”) AND (“time perception” OR “temporal perception” OR “time estimation” OR “time judgment*” OR “subjective time” OR “subjective temporal” OR “temporal processing” OR “temporal binding” OR “timing” OR “tempo” OR “temporality” OR “duration judgment” OR “duration perception” OR “passage of time” OR “time perspective” OR “temporal perspective” OR “temporal order” OR “time sensitivity” OR “internal clock”).
Data extraction
The following variables were extracted: i) first author; ii) year of publication; iii) title; iv) PMID/doi; v) investigated time range; vi) diagnosis; vii) sample size; viii) type of controls; ix) number of controls; x) female percentage (sample/controls); xi) mean age (sample/controls); xii) sample characteristics (inpatients or outpatients); xiii) clinical characteristics (clinical phase, ongoing pharmacological treatment); xiv) implicit or explicit timing task; xv) perceptual or motor timing task; xvi) modality of time assessment; xvii) index used for time assessment; xviii) cognitive, psychopathological, and neurobiological assessments (if present) along with their correlation with timing task performance; and xix) main findings of the studies.
Study selection
The review process was facilitated by utilising Rayyan (Ouzzani et al., Reference Ouzzani, Hammady, Fedorowicz and Elmagarmid2016), an open-source software used to manage reviews. The authors who conducted the literature screening independently evaluated the titles and abstracts of all the search results (AE, YM, MG). Any discrepancies between the researchers were resolved through discussion (AE, MBA, and MG). Initially, the articles were screened based on their titles and abstracts. Subsequently, there was a full-text assessment. Duplicate articles, review articles, and those not meeting the search criteria were excluded from further analysis.
Assessment of methodological quality
The Appraisal Tool for Cross-Sectional Studies (AXIS) Scale (Downes et al., Reference Downes, Brennan, Williams and Dean2016) was used to assess the methodological quality of the studies included in the review. This choice was justified as the AXIS Scale is well-suited for evaluating the quality and risk of bias in cross-sectional research, ensuring an accurate interpretation of findings. It provides a structured approach to critically appraise studies based on several key criteria, including the clarity of aims, appropriateness of study design, justification of sample size, and the robustness of outcome measurement and statistical analysis.
Results
Literature search
The search yielded 1873 abstracts. After duplicates were removed, we screened 1291 records by title/abstract. Of these, 29 were relevant to our topic and included an experimental task to quantitatively assess time perception. Of these, 6 documents were excluded as they were not written in English. A full-text screening was performed on the remaining 23. Of these, 11 articles that were published between 1984 and 2022 were deemed eligible for inclusion. The process for selecting relevant articles is detailed in the Supplementary Materials (Table S1). The PRISMA flowchart, with details on screening and reasons for exclusion, is reported in Fig. 1. Results of the AXIS Scales for the assessment of methodological quality are reported in Supplementary Materials (Table S2). Studies show an overall high quality and low risk of bias, except for those by Tysk (Reference Tysk1984) and Ryu et al. (Reference Ryu, Kook, Lee, Ha and Cho2015) which have moderate quality and risk of bias.
Sample size and clinical characteristics
Table 1 reports details about sample size and clinical characteristics. The review comprised 11 case–control studies involving 455 individuals with bipolar disorder as well as 545 healthy controls. While three studies conducted cognitive assessments (Bschor et al., Reference Bschor, Ising, Bauer, Lewitzka, Skerstupeit, Muller-Oerlinghausen and Baethge2004; Bolbecker et al., Reference Bolbecker, Westfall, Howell, Lackner, Carroll, O.’donnell and Hetrick2014; Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022), the other nine focused on psychopathological assessments (Bschor et al., Reference Bschor, Ising, Bauer, Lewitzka, Skerstupeit, Muller-Oerlinghausen and Baethge2004; Mahlberg et al., Reference Mahlberg, Kienast, Bschor and Adli2008; Bolbecker et al., Reference Bolbecker, Mehta, Edwards, Steinmetz, O.’donnell and Hetrick2009; Bolbecker et al., Reference Bolbecker, Hong, Kent, Forsyth, Klaunig, Lazar, O.’donnell and Hetrick2011; Bolbecker et al., Reference Bolbecker, Westfall, Howell, Lackner, Carroll, O.’donnell and Hetrick2014; Ryu et al., Reference Ryu, Kook, Lee, Ha and Cho2015; Arrouet et al., Reference Arrouet, Polgári, Giersch and Joos2022; Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022; Liu et al., Reference Liu, Guo, Ma, Liu, Wang, Zhao, Tan, Tan, Yang and Wang2022); no study investigated neurobiological aspects. Regarding the BD patients’ clinical phases, the studies included 105 individuals during euthymia, 121 subjects in manic or hypomanic states, 108 individuals in depressed states, and 8 individuals in mixed states. Notably, two studies did not specify the clinical phase of the BD patients (Bolbecker et al., Reference Bolbecker, Westfall, Howell, Lackner, Carroll, O.’donnell and Hetrick2014; Oyanadel and Buela-Casal, Reference Oyanadel and Buela-Casal2014). Most studies did not mention comorbidities in their BD samples, with the exception of Ciullo et al. (Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022), where three subjects had comorbid borderline personality disorder, and Liu et al. (Reference Liu, Guo, Ma, Liu, Wang, Zhao, Tan, Tan, Yang and Wang2022); Arrouet et al. (Reference Arrouet, Polgári, Giersch and Joos2022), and Ryu et al. (Reference Ryu, Kook, Lee, Ha and Cho2015) who explicitly reported no comorbid conditions. The other studies, such as Bolbecker et al. (Reference Bolbecker, Hong, Kent, Forsyth, Klaunig, Lazar, O.’donnell and Hetrick2011, Reference Bolbecker, Westfall, Howell, Lackner, Carroll, O.’donnell and Hetrick2014, Reference Bolbecker, Mehta, Edwards, Steinmetz, O.’donnell and Hetrick2009), Mahlberg et al. (Reference Mahlberg, Kienast, Bschor and Adli2008), Bschor et al. (Reference Bschor, Ising, Bauer, Lewitzka, Skerstupeit, Muller-Oerlinghausen and Baethge2004), and Tysk (Reference Tysk1984), either did not assess or did not provide information about comorbidities. In terms of the BD samples, one study included 50% of patients with psychotic features (Bolbecker et al., Reference Bolbecker, Westfall, Howell, Lackner, Carroll, O.’donnell and Hetrick2014). Three studies included a sample of SZ subjects (Bolbecker et al., Reference Bolbecker, Westfall, Howell, Lackner, Carroll, O.’donnell and Hetrick2014; Oyanadel and Buela-Casal, Reference Oyanadel and Buela-Casal2014; Arrouet et al., Reference Arrouet, Polgári, Giersch and Joos2022). Moreover, three others included a sample of subjects with depressive symptoms (Tysk, Reference Tysk1984; Oyanadel and Buela-Casal, Reference Oyanadel and Buela-Casal2014; Liu et al., Reference Liu, Guo, Ma, Liu, Wang, Zhao, Tan, Tan, Yang and Wang2022). Further information is reported in Table 1.
Table 1. Sample characteristics of selected studies

Features of time perception
Based on the literature review, we found that 10 studies involved explicit time perception (Tysk, Reference Tysk1984; Bschor et al., Reference Bschor, Ising, Bauer, Lewitzka, Skerstupeit, Muller-Oerlinghausen and Baethge2004; Mahlberg et al., Reference Mahlberg, Kienast, Bschor and Adli2008; Bolbecker et al., Reference Bolbecker, Hong, Kent, Forsyth, Klaunig, Lazar, O.’donnell and Hetrick2011; Bolbecker et al., Reference Bolbecker, Westfall, Howell, Lackner, Carroll, O.’donnell and Hetrick2014; Oyanadel and Buela-Casal, Reference Oyanadel and Buela-Casal2014; Ryu et al., Reference Ryu, Kook, Lee, Ha and Cho2015; Arrouet et al., Reference Arrouet, Polgári, Giersch and Joos2022; Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022; Liu et al., Reference Liu, Guo, Ma, Liu, Wang, Zhao, Tan, Tan, Yang and Wang2022), three studies involved implicit time perception (Bolbecker et al., Reference Bolbecker, Mehta, Edwards, Steinmetz, O.’donnell and Hetrick2009; Arrouet et al., Reference Arrouet, Polgári, Giersch and Joos2022; Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022), six studies investigated sub-second temporal intervals (Bolbecker et al., Reference Bolbecker, Mehta, Edwards, Steinmetz, O.’donnell and Hetrick2009; Bolbecker et al., Reference Bolbecker, Hong, Kent, Forsyth, Klaunig, Lazar, O.’donnell and Hetrick2011; Bolbecker et al., Reference Bolbecker, Westfall, Howell, Lackner, Carroll, O.’donnell and Hetrick2014; Arrouet et al., Reference Arrouet, Polgári, Giersch and Joos2022; Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022; Liu et al., Reference Liu, Guo, Ma, Liu, Wang, Zhao, Tan, Tan, Yang and Wang2022), seven studies investigated supra-second temporal intervals (Tysk, Reference Tysk1984; Bschor et al., Reference Bschor, Ising, Bauer, Lewitzka, Skerstupeit, Muller-Oerlinghausen and Baethge2004; Mahlberg et al., Reference Mahlberg, Kienast, Bschor and Adli2008; Oyanadel and Buela-Casal, Reference Oyanadel and Buela-Casal2014; Ryu et al., Reference Ryu, Kook, Lee, Ha and Cho2015; Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022; Liu et al., Reference Liu, Guo, Ma, Liu, Wang, Zhao, Tan, Tan, Yang and Wang2022), eight studies involved perceptual timing (Tysk, Reference Tysk1984; Bschor et al., Reference Bschor, Ising, Bauer, Lewitzka, Skerstupeit, Muller-Oerlinghausen and Baethge2004; Bolbecker et al., Reference Bolbecker, Mehta, Edwards, Steinmetz, O.’donnell and Hetrick2009; Bolbecker et al., Reference Bolbecker, Westfall, Howell, Lackner, Carroll, O.’donnell and Hetrick2014; Ryu et al., Reference Ryu, Kook, Lee, Ha and Cho2015; Arrouet et al., Reference Arrouet, Polgári, Giersch and Joos2022; Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022; Liu et al., Reference Liu, Guo, Ma, Liu, Wang, Zhao, Tan, Tan, Yang and Wang2022), and seven studies focused on motor timing (Tysk, Reference Tysk1984; Bschor et al., Reference Bschor, Ising, Bauer, Lewitzka, Skerstupeit, Muller-Oerlinghausen and Baethge2004; Mahlberg et al., Reference Mahlberg, Kienast, Bschor and Adli2008; Bolbecker et al., Reference Bolbecker, Hong, Kent, Forsyth, Klaunig, Lazar, O.’donnell and Hetrick2011; Oyanadel and Buela-Casal, Reference Oyanadel and Buela-Casal2014; Ryu et al., Reference Ryu, Kook, Lee, Ha and Cho2015; Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022). Table 2 summarizes the studies based on time perception features.
Table 2. Comparative studies on explicit and implicit timing tasks by temporal interval and response type

Main findings
The main findings for each study are reported in Table 3. Table 4 divides the studies by experimental paradigm and interval range investigated, and Fig. 2 represents a graphical summary of their results.

Figure 2. Results of each study are represented based on BD performance at specific temporal intervals. Left: perceived duration, right: time processing performance. X-axes: shorter = underestimation compared to controls, normal = estimation similar to controls, longer = overestimation compared to controls; impaired = worst performance compared to controls, normal = similar performance compared to controls. Y-axes: timeframes investigated. Colours: red = BD during manic state, green = BD during euthymic state, blue = BD during depressed state, black = BD with no other specifications, black with white line = BD with psychotic features, black with white cross = BD without psychotic features. Forms: triangle = motor timing, square = perceptual timing. Fill: filled = explicit time perception, empty = implicit time perception.
Table 3. Assessment details and main findings of selected studies

BD, bipolar disorder; YMRS, Young Mania Rating Scale (Young, et al., Reference Young, Biggs, Ziegler and Meyer1978); HAM-D, Hamilton Depression Rating Scale (Hamilton, Reference Hamilton1960); CPZE, chlorpromazine equivalents; PANSS, Positive and Negative Syndrome Scale (Kay, et al., Reference Kay, Fiszbein and Opler1987); BPRS, the Brief Psychiatric Rating Scale (Pull & Overall, Reference Pull and Overall1977); MADRS, the Montgomery–Åsberg Depression Scale (Montgomery & Åsberg, Reference Montgomery and Åsberg1979); CGI, Clinical Global Impression; BRMS, Bech–Rafaelsen Mania Scale (Bech, et al., Reference Bech, Rafaelsen, Kramp and Bolwig1978).
Table 4. List of studies by experimental paradigm and temporal interval range

Explicit timing
Brief overview of each study.
- Ciullo et al. (Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022) investigated temporal perception by administering various explicit motor and perceptual paradigms to BD patients (BD type I, n = 20; BD type II, n = 10) involving both sub-second and supra-second stimuli. Specifically, they conducted the following tasks: i) duration discrimination task: participants were tasked with judging the relative duration (shorter, equal to, or longer) of two consecutive visual dots that lasted either 540 ms, 1080 ms, or 1620 ms; ii) temporal bisection task: participants determined whether the perceived duration of an empty interval was longer or shorter than their internal representation of 1s; and iii) temporal reproduction task: participants were required to reproduce, using a motor response (finger tapping), the perceived duration of an empty 1s interval. Compared to control participants (n = 30), individuals with BD showed no significant differences in measures of duration discrimination and temporal bisection. However, while their accuracy (i.e. mean reaction times (RT)) was comparable, BD participants exhibited reduced precision (i.e. greater standard deviation of RTs) in reproducing a one-second duration relative to the control group.
- Liu et al. (Reference Liu, Guo, Ma, Liu, Wang, Zhao, Tan, Tan, Yang and Wang2022) performed an explicit task of perception of time using both sub-second and supra-second stimuli in BD individuals with a current major depressive episode (n = 30), healthy controls (n = 30), and individuals with a diagnosis of major depressive disorder (n = 30). The task consisted of a temporal bisection task divided into two phases. During the training phase, participants were required to memorise two temporal intervals showed through visual stimuli (short: 400 ms, long: 1600 ms). During the testing phase, participants were instructed to rate as “Short” or “Long” the length of a given temporal interval (ranging from 400 to 1600 ms). While the BD group showed a tendency to overestimate sub-second intervals and underestimate supra-second intervals, there were no significant differences in precision (i.e. difference limen and Weber ratio) and accuracy (i.e. point of subject equality (PSE)) compared to the control group.
- Arrouet et al. (Reference Arrouet, Polgári, Giersch and Joos2022) tested sub-second temporal skills of BD patients (n = 20), individuals with schizophrenia (n = 24), and healthy controls (n = 31) using a temporal order judgement task. The latter consisted of asking participants to determinate the order of presentation of two visual stimuli (stimulus onset asynchrony could be 17 ms or 100 ms). Differently from most other paradigms mentioned in this review, this kind of task examines a different domain of time, that is the processing of temporal structure of events. Interestingly, while patients with schizophrenia performed worse as compared to controls, patients with BD showed a performance in-between that of controls and patients with schizophrenia, with no differences compared to either groups.
- Ryu et al. (Reference Ryu, Kook, Lee, Ha and Cho2015) examined temporal estimation and reproduction skills in the supra-second domain in BD patients, splitting the sample based on the clinical episode; a manic state (n = 22) and euthymic state (n = 24). Since authors were interested in the relationship between emotions and temporal perception, they choose as stimuli standardised affective pictures with different valence and level of arousal. In the temporal estimation task, participants identify the duration of presentation of each picture (2s, 4s, or 6s) on a range from 1 to 10 s in a visual analogue scale. In the temporal reproduction task, they were asked to reproduce the duration by pushing a button. While for both tasks the overall performance of euthymic subjects and healthy individuals similarly varied based on valence and level of arousal, this was not the case for the manic group. Indeed, both euthymic patients and healthy controls consistently overestimated positive low-arousal and negative high-arousal pictures and consistently underestimated positive high-arousal and negative low-arousal pictures. Instead, for manic patients there was no effect of positive low-arousal pictures during the estimation task, and there was a reversed effect of all emotional stimuli during the reproduction task. Since supra-second durations require more attentional resources than sub-seconds ones (Droit-Volet et al., Reference Droit-Volet, Fayolle, Lamotte and Gil2013), and based on previous literature which relates attentional components to emotional processing (e.g. Angrilli et al., Reference Angrilli, Cherubini, Pavese and Mantredini1997; Strakowski et al., Reference Strakowski, Eliassen, Lamy, Cerullo, Allendorfer, Madore, Lee, Welge, Delbello, Fleck and Adler2011), the authors suggested that impaired attentional processes may affect temporal judgements of emotional stimuli during manic phase.
- Bolbecker et al. (Reference Bolbecker, Westfall, Howell, Lackner, Carroll, O.’donnell and Hetrick2014) investigated explicit duration for sub-second intervals in subjects with non-psychotic BD (n = 37), BD with psychotic features (n = 34), schizophrenia (n = 66), and schizoaffective disorder (n = 31), as well as in a healthy control group (n = 73). The task was a temporal bisection that required participants to rate as “Short” of “Long” auditory durations of tones ranging from 300 to 600 ms. The testing phase was preceded by a training and a practice session where participants learned the anchor durations (i.e. 300 ms and 600 ms). While accuracy (i.e. bisection point) was consistent across all groups, all BD patients and individuals with schizophrenia exhibited significantly increased variability (i.e. worst precision as revealed by higher difference limen and Weber ratio).
- Oyanadel and Buela-Casal (Reference Oyanadel and Buela-Casal2014) investigated explicit supra-second perception of time with a time estimation task (time interval and analyses not specified) and a time production task (10s, and 60s) in BD subjects (n = 42), patients with major depressive disorder (n = 70), schizophrenia (n = 30), cluster B personality disorder (n = 25), and healthy participants (n = 167). While groups did not significantly differ from each other in the estimation task (details of results not provided) and production of 10s interval, patients with BD and major depressive disorder underestimated time when producing intervals of 60s.
- Bolbecker et al. (Reference Bolbecker, Hong, Kent, Forsyth, Klaunig, Lazar, O.’donnell and Hetrick2011) evaluated BD patients (n = 42) in a manic episode (n = 17) or euthymia (n = 25), and healthy controls (n = 42), using a production timing task involving sub-second stimuli. This involved a finger-tapping task where participants were directed to tap their index finger or alternate their thumbs in sync with a paced auditory stimulus (synchronization phase with a 500 ms inter-tap interval). Subsequently, they were instructed to continue tapping at the same rhythm even after the auditory stimulus ceased (continuation phase).The BD group tapped at a faster rate than controls, specifically when using alternating thumbs, and showed an overall greater timing variability (i.e. higher standard deviation of inter-tap interval). When analysing mood states within the BD group separately, no significant differences between manic and euthymic patients emerged.
- Mahlberg et al. (Reference Mahlberg, Kienast, Bschor and Adli2008) conducted a temporal reproduction task involving supra-second stimuli with BD patients, either in acute mania (n = 28) or acute depression (n = 28), and with a control group of healthy individuals (n = 30). Participants were asked to press a key to reproduce stimuli of 1s, 6s or 37s. Although no differences emerged for the very short temporal span (i.e. 1s), manic BD patients generally perceived durations as shorter when compared to their depressed counterparts. Yet, the pattern of response diverged between short and long temporal spans. Manic patients accurately reproduced the short interval (6s), but under-reproduced the long interval (37s). Conversely, depressed patients reproduced the long interval accurately, but over-reproduced the short interval. Although it can sound counterintuitive, it is important to keep in mind that, in motor timing task, to produce/reproduce a shorter time span than demanded is considered overestimation, and to produce/reproduce a longer time span than demanded is defined as underestimation (Bindra and Waksberg, Reference Bindra and Waksberg1956).
- Bschor et al. (Reference Bschor, Ising, Bauer, Lewitzka, Skerstupeit, Muller-Oerlinghausen and Baethge2004) investigated the temporal estimation and temporal production of supra-second stimuli in BD patients with a manic episode (n = 30), in BD patients with a depressive episode (n = 32), and controls (n = 31). After a training session, they were asked to evaluate the duration of visual stimuli lasting 8 s, 43 s, and 109 s, and to produce by pressing a key durations as long as 7s, 35s, and 90s. They were also asked to verbally report the duration of a short movie (12 min and 40s).
The results indicated that all participants performed similarly in the estimation task for the shorter temporal intervals (i.e. 8 s and 43 s). However, for the longer interval (i.e. 109 s), there were differences among BD groups: those in a manic episode reported longer durations, while those in a depressive episode reported shorter durations. When judging the duration of the short movie, depressed BD patients, and even more strongly manic BD patients, reported a significantly longer duration (i.e. 28 min±9 min) compared to controls.
In the production task, all BD patients produced shorter temporal intervals when evaluating longer temporal spans (i.e. 35 s and 90 s, but not 7 s). This effect was significantly more pronounced in BD patients with a manic episode compared to those with a depressive episode. Conversely, for the shorter intervals (i.e. 7 s), those with a manic episode produced significantly shorter durations, while those in a depressive episode longer durations. While not classified as an experimental paradigm according to the review criteria, it is worth mentioning that Bschor et al., explored also subjective time experience by asking participants to evaluate how slow or fast she or he experienced the flow of time on the day of the investigation. The control group conveyed a relatively balanced perception of time. In contrast, the depressed group reported a significant perception of time slowing down, while the manic group felt a marked acceleration.
- Tysk (Reference Tysk1984) recruited healthy individuals (n = 60) alongside a substantial cohort of patient with affective disorders. This included BD subjects in (hypo)manic (n = 11 ), depressed (n = 8), or euthymic (n = 9) states; major depressive patients with (n = 9) and without (n = 9) melancholic features; and individuals with dysthymic disorder (n = 10). Different tasks were used to explicitly evaluate supra-second perception of time: metronome adjustment (tempo range 40 to 208 beats/minute and subjects had to set it to one beat per second), verbal estimation (7.5 s, 17.5 s, 27.5 s and 7/8 min), and motor reproduction (10 s, 20 s, and 30 s). In all tasks, patients with BD during a depressive episode and melancholic subjects tended to under-estimate the short time intervals, while (hypo)manic individuals tended to count seconds too fast and to overestimate time. For the longer interval verbal estimation (i.e. 7/8 min), both BD patients during manic and depressive states were comparable to the control group. Moreover, all other patient groups in the study, including BD patients in remission, generally showed results similar to those of the healthy participants.
Summary of results for sub-second intervals. In the domain of sub-second explicit timing, individuals with BD generally do not display significant alterations in perceptual timing compared to HC (Arrouet et al., Reference Arrouet, Polgári, Giersch and Joos2022; Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022; Liu et al., Reference Liu, Guo, Ma, Liu, Wang, Zhao, Tan, Tan, Yang and Wang2022). In turn, similar results are observed in temporal discrimination (Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022), temporal bisection (Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022; Liu et al., Reference Liu, Guo, Ma, Liu, Wang, Zhao, Tan, Tan, Yang and Wang2022), and temporal order judgement (Arrouet et al., Reference Arrouet, Polgári, Giersch and Joos2022). However, one study (Bolbecker et al., Reference Bolbecker, Westfall, Howell, Lackner, Carroll, O.’donnell and Hetrick2014) documented increased timing variability in a temporal bisection task in BD compared to HC; the researchers interpreted it as reduced precision in sub-second time estimation. Only one study investigated sub-second explicit motor timing; it determined that greater timing variability (higher standard deviation) and faster tapping rate characterised both euthymic and manic BD patients compared to HC (Bolbecker et al., Reference Bolbecker, Hong, Kent, Forsyth, Klaunig, Lazar, O.’donnell and Hetrick2011).
Summary of results for supra-second intervals. With regard to intervals from 1 to 2 s, no differences emerged in perceptual timing (Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022; Liu et al., Reference Liu, Guo, Ma, Liu, Wang, Zhao, Tan, Tan, Yang and Wang2022). However, the opposite results were documented for motor timing, with either similar performance between BD patients and controls (Mahlberg et al., Reference Mahlberg, Kienast, Bschor and Adli2008) or reduced precision in BD patients partially explained by the patients’ number of previous depressive episodes (Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022).
Supra-second timing for temporal intervals between 2 s and 5 min did instead reveal more consistent differences between groups. Within this temporal span, although some authors reported some similar results between individuals with BD and healthy controls in perceptual (for 8 s and 43 s: (Bschor et al., Reference Bschor, Ising, Bauer, Lewitzka, Skerstupeit, Muller-Oerlinghausen and Baethge2004)) and motor (for 10 s: (Oyanadel and Buela-Casal, Reference Oyanadel and Buela-Casal2014)) explicit timing, most studies noted a general divergence between mania and depression. Manic BD patients tend to overestimate temporal spans while depressed BD patients tend to underestimate them. Specifically, Tysk (Reference Tysk1984) reported an overestimation during manic episodes and an underestimation in melancholic states in both the verbal estimation and motor production tasks encompassing temporal intervals between 7 and 30 s. According to Mahlberg et al. (Reference Mahlberg, Kienast, Bschor and Adli2008), depressed subjects showed a tendency towards over-reproduction (i.e. underestimating) for 6 s temporal intervals, while manic patients showed a tendency towards under-reproduction (i.e. overestimating) for 37 s temporal intervals. Similarly, although all patients were found to overestimate 35 s and 90s intervals (production task), manic patients tended to overestimate while depressed patients tended to underestimate at durations of 7 s (production task) and 109 s (estimation task), according to the results by Bschor et al. (Reference Bschor, Ising, Bauer, Lewitzka, Skerstupeit, Muller-Oerlinghausen and Baethge2004). In this case, the overestimation of time in the production task observed in manic BD patients correlated with impaired performance on the Trail Making Test part-A; in turn, the underestimation of depressed patients in both estimation and production tasks was predicted by the number of previous depressive episodes they had experienced. Oyanadel and Buela-Casal (Reference Oyanadel and Buela-Casal2014) determined that BD patients underestimate 60 s during a production task, but they did not take into account illness phases. Within this temporal span, the findings by Ryu et al. (Reference Ryu, Kook, Lee, Ha and Cho2015) demonstrated that emotional stimuli affected time perception differently in manic BD patients compared to euthymic patients and control individuals. However, these results are only partially related to the context of this review as they involved affective stimuli and investigated emotional effects on time perception rather than time perception. Only two studies considered longer time intervals (longer than 5 min). Tysk (Reference Tysk1984) found no difference between manic and depressed BD patients and healthy controls in temporal estimation. Subsequently, Bschor et al. (Reference Bschor, Ising, Bauer, Lewitzka, Skerstupeit, Muller-Oerlinghausen and Baethge2004) found that depressed and even more strongly manic individuals exhibited temporal overestimation.
Implicit timing
Brief overview of each study. Ciullo et al. (Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022), in addition to the explicit paradigms, tested BD patients (BD type I, n =20; BD type II, n =10) with a predictive timing task involving both sub-second and supra-second stimuli. Participants were instructed to detect as quickly as possible visual targets, which appeared after one of three intervals (540 ms, 1080ms, or 1620ms). Temporal expectations were modulated using symbolic cues with an 80% validity rate. Patients did not differ from controls.
- As described in the Section 3.4.1.1, Arrouet et al. (Reference Arrouet, Polgári, Giersch and Joos2022) administered an explicit temporal order judgement task to BD patients (n = 20), individuals with schizophrenia (n = 24), and healthy controls (n = 31). However, authors conducted specific analyses based on trial n-1 which allowed to draw conclusions about the implicit mechanisms that optimise the processing of successive stimuli based on previous ones. Interestingly, the implicit order effects, which lead to improved performance, were preserved and similar across groups.
- Bolbecker et al. (Reference Bolbecker, Mehta, Edwards, Steinmetz, O.’donnell and Hetrick2009) administered a delay eyeblink conditioning task using sub-second auditory stimuli to a group of BD patients (n = 28) during manic (n = 9), mixed (n = 8), and euthymic (n = 11) states, and a group of healthy participants (n = 28). The procedure involved assessing the acquisition and latency of conditioned eyeblink responses to an air puff (i.e. an unconditioned stimulus lasting 50 ms) that was paired with a tone (i.e. a conditioned stimulus lasting 400 ms). This kind of paradigm, similarly to Arrouet et al. (Reference Arrouet, Polgári, Giersch and Joos2022), investigates the temporal structure of how participants process external events. While all participants responded to the conditioning, the BD group exhibited a lower percentage of conditioned responses compared to controls. Upon grouping the BD group based on episodes, this trend was particularly pronounced for BD individuals in a mixed episode. More relevant to the topic of the review, BD patients differed from healthy participants regarding the latency of the conditioned response; specifically, they demonstrated faster latencies. This meant less adaptively timed responses for patients, as the timing of the conditioned response should gradually align more precisely with the onset of the unconditioned stimulus during the conditioning phase. Consequently, faster conditioned responses are less adaptive compared to those that occur later and closer to the onset of the unconditioned stimulus. Even in this case, BD patients during mixed episode performed worse than all other groups, failing to improve as the experiment progressed too. As for the peak latency of the unconditioned response, it decreased with experience similarly for all groups. Authors discussed these findings as evidence of poor temporal coordination of information processing, specifically in patients with mixed episode.
Summary of results for sub-second and supra-second intervals. Regarding implicit timing, one study reported a similar sensitivity between BD patients and controls to temporal expectations during a predictive timing task within the sub-second and supra-second range (Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022) and no alterations in sub-second implicit mechanisms underlying temporal processing were observed in another study using a temporal order judgement task (Arrouet et al., Reference Arrouet, Polgári, Giersch and Joos2022). However, one study reported lower conditioned responses and faster latency in eye-blink reflex conditioned responses in the sub-second domain, especially for BD during mixed episodes (Bolbecker et al., Reference Bolbecker, Mehta, Edwards, Steinmetz, O.’donnell and Hetrick2009).
Psychopathological correlates of time perception
The psychopathological assessment section mentioned only measures for which the association between the psychopathological indices and temporal performance had been tested.
Only one study compared BD type I and BD type II; it found similar results between the two groups (Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022). Although only a few studies analysed the performance of patients during different clinical states separately, those that did all highlight some significant differences between patients with depressive and manic episodes during supra-second tasks. Overall, manic patients tended to overestimate time, depressed patients tended to underestimate it, and patients in remission behaved similarly to the controls (Mahlberg et al., Reference Mahlberg, Kienast, Bschor and Adli2008; Tysk, Reference Tysk1984; Bschor et al., Reference Bschor, Ising, Bauer, Lewitzka, Skerstupeit, Muller-Oerlinghausen and Baethge2004). In terms of differences between euthymic and manic patients, Bolbecker et al. (Reference Bolbecker, Hong, Kent, Forsyth, Klaunig, Lazar, O.’donnell and Hetrick2011) found no significant differences in sub-second finger-tapping performance, as well as Bolbecker et al. ( Reference Bolbecker, Mehta, Edwards, Steinmetz, O.’donnell and Hetrick2009), when investigating the presence and temporal latency of eyeblink conditioning responses to auditory stimuli during an implicit sub-second timing task (here, the timing deficit was predominantly observed in patients with mixed episodes).
When considering the number and/or duration of previous clinical episodes, these variables seem to be crucial only for depressive states. Indeed, Ciullo et al. (Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022) found that the number of previous depressive episodes was a predictor of BD patients’ precision in the temporal reproduction of 1 s and Bschor et al. (Reference Bschor, Ising, Bauer, Lewitzka, Skerstupeit, Muller-Oerlinghausen and Baethge2004) found that poorer performance at supra-second estimation and production tasks was linked with the number of previous episodes depressed participants had experienced.
Only one study (Bolbecker et al., Reference Bolbecker, Westfall, Howell, Lackner, Carroll, O.’donnell and Hetrick2014) directly addressed the impact of the presence of psychotic symptoms, but they found no differences between patients with and without psychotic symptoms during the sub-second bisection task. One other study indirectly investigated the role of psychotic features by testing patients with the PANSS; however, the scores at its sub-scales did not predict temporal performance (Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022).
A few studies evaluated the impact of medications and considered, for instance, whether patients were on antipsychotic medications. Interestingly, they all revealed no significant results (Bolbecker et al., Reference Bolbecker, Mehta, Edwards, Steinmetz, O.’donnell and Hetrick2009; Bolbecker et al., Reference Bolbecker, Hong, Kent, Forsyth, Klaunig, Lazar, O.’donnell and Hetrick2011; Ryu et al., Reference Ryu, Kook, Lee, Ha and Cho2015; Arrouet et al., Reference Arrouet, Polgári, Giersch and Joos2022; Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022; Liu et al., Reference Liu, Guo, Ma, Liu, Wang, Zhao, Tan, Tan, Yang and Wang2022).
Some studies considered the association between temporal performance and psychopathological scales (i.e. the Young Mania Rating Scale (YMRS) (Young et al., Reference Young, Biggs, Ziegler and Meyer1978), the Hamilton Depression Rating Scale (HAM-D) (Hamilton, Reference Hamilton1960), the Positive and Negative Syndrome Scale (PANSS) (Kay et al., Reference Kay, Fiszbein and Opler1987), the Brief Psychiatric Rating Scale (BPRS) (Pull and Overall, Reference Pull and Overall1977), the Montgomery–Åsberg Depression Scale (MADRS) (Montgomery and Åsberg, Reference Montgomery and Åsberg1979), the Clinical Global Impression (CGI), and the Bech–Rafaelsen Mania Scale (BRMS) (Bech et al., Reference Bech, Rafaelsen, Kramp and Bolwig1978)). Most of them failed to find a significant association between patients’ scores on the scales and their temporal skills (Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022) (Bolbecker et al., Reference Bolbecker, Mehta, Edwards, Steinmetz, O.’donnell and Hetrick2009; Bolbecker et al., Reference Bolbecker, Westfall, Howell, Lackner, Carroll, O.’donnell and Hetrick2014; Liu et al., Reference Liu, Guo, Ma, Liu, Wang, Zhao, Tan, Tan, Yang and Wang2022) (Mahlberg et al., Reference Mahlberg, Kienast, Bschor and Adli2008) (Bschor et al., Reference Bschor, Ising, Bauer, Lewitzka, Skerstupeit, Muller-Oerlinghausen and Baethge2004). Only one study found that the performance of manic patients at supra-second estimation task correlated with manic and illness symptom severity as measured by the YMRS and the CGI scales (Ryu et al., Reference Ryu, Kook, Lee, Ha and Cho2015). For details about each study, see Supplementary Materials.
Cognitive correlates of time perception
Three of the selected studies investigated the cognitive correlates of time perception in BD. Two of them found significant results concerning the relationships between cognitive skills and temporal performance (Bschor et al., Reference Bschor, Ising, Bauer, Lewitzka, Skerstupeit, Muller-Oerlinghausen and Baethge2004; Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022) while the third one did not (Bolbecker et al., Reference Bolbecker, Westfall, Howell, Lackner, Carroll, O.’donnell and Hetrick2014). Specifically, the precision at temporal reproduction of BD patients was found to be influenced by working memory as measured by the Delayed Item Recognition task (DIR) (Ciullo et al., Reference Ciullo, Vecchio, Gili, Spalletta and Piras2018b; Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022). In addition, executive and motor speed assessed with the Trail Making Test part-A (TMT-A) (Reitan, Reference Reitan1992) correlated with the overestimation of time in manic patients during an estimation task (Bschor et al., Reference Bschor, Ising, Bauer, Lewitzka, Skerstupeit, Muller-Oerlinghausen and Baethge2004). However, IQ information derived from the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, Reference Wechsler1999) was not related to precision at a temporal bisection task (Bolbecker et al., Reference Bolbecker, Westfall, Howell, Lackner, Carroll, O.’donnell and Hetrick2014). For details about each study, see Supplementary Materials.
Discussion
Time perception alterations are characteristic of severe mental disorders that exhibit diverse clinical and neurobiological manifestations (Giersch et al., Reference Giersch, Lalanne and Isope2016; Stanghellini et al., Reference Stanghellini, Ballerini, Presenza, Mancini, Raballo, Blasi and Cutting2016; Tschacher et al., Reference Tschacher, Giersch and Friston2017). We aim to provide a thorough analysis of the literature concerning time perception in patients with BD. We included only studies that employed an experimental paradigm to objectively evaluate temporal performance. This allowed us to systematically categorise explicit/implicit and perceptual/motor timing alterations across sub-second/supra-second time intervals. To the best of our knowledge, this represents the first systematic review on the topic.
When addressing BD, the literature often refers to a subjective acceleration in manic individuals and a deceleration in patients with depression in the minute-to-day range. This conception is grounded in extensive psychopathological literature (Ghaemi, Reference Ghaemi2007; Vogeley and Kupke, Reference Vogeley and Kupke2007; Gruber et al., Reference Gruber, Cunningham, Kirkland and Hay2012; Stanghellini et al., Reference Stanghellini, Ballerini, Presenza, Mancini, Raballo, Blasi and Cutting2016; Stanghellini et al., Reference Stanghellini, Ballerini, Presenza, Mancini, Northoff and Cutting2017). Although it seems to be a hypothesis with significant theoretical bases, and even if it sounds this way to clinicians in their daily interactions with patients, this review reveals that the experimental literature is still quite limited. Moreover, so far, it has yielded conflicting results.
Within sub-second temporal ranges, the subjects’ performance in explicit and implicit timing mostly aligns with controls. Notably, these findings appeared coherent. However, they are very few and almost none of them focused on patients with BD during manic states. When considering manic BD patients, their sub-second explicit motor timing is altered (i.e. increased variability and a faster tapping rate in euthymic and manic BD patients) (Bolbecker et al., Reference Bolbecker, Hong, Kent, Forsyth, Klaunig, Lazar, O.’donnell and Hetrick2011) and so is their implicit perceptual timing (i.e. they have less adaptive eyeblink time-conditioned responses) (Bolbecker et al., Reference Bolbecker, Mehta, Edwards, Steinmetz, O.’donnell and Hetrick2009). As for supra-second temporal spans, the most consistent findings involve BD patients’ tendency towards overestimation of duration during manic states and towards underestimation during depressive states (see Fig. 3). Overall, this tendency characterises both perceptual and motor explicit timing. To this date, supra-second implicit time perception has not been investigated. Moreover, only two studies focused on the minute temporal range but they did not come to unanimous conclusions.

Figure 3. Graphical representation of main results. During depressive states, patients tend to estimate supra-second durations (i.e. objective time) as shorter (i.e. perceived duration) due to slowed-down subjective time flow. During manic states, patients tend to estimate supra-second durations as longer due to accelerated subjective time flow. During euthymic states, perceived durations and subjective time flow of patients tend to be more closely related to objective durations.
Studies show notable variations in both gender and age distribution of participants. For instance, the percentage of female participants ranges from 43.3% (Liu et al., Reference Liu, Guo, Ma, Liu, Wang, Zhao, Tan, Tan, Yang and Wang2022) to as high as 81% (Oyanadel and Buela-Casal, Reference Oyanadel and Buela-Casal2014). In terms of age, the sample in Liu et al. ( Reference Liu, Guo, Ma, Liu, Wang, Zhao, Tan, Tan, Yang and Wang2022) includes younger participants with a mean age of around 26 years, while studies such as Mahlberg et al. (Reference Mahlberg, Kienast, Bschor and Adli2008) and Bschor et al. (Reference Bschor, Ising, Bauer, Lewitzka, Skerstupeit, Muller-Oerlinghausen and Baethge2004) focus on older populations, with mean ages exceeding 45 years. These differences in gender and age distributions could potentially affect the generalizability of findings. Indeed, gender may influence clinical presentations, and age may reflect different stages of illness and treatment histories, which may impact the interpretation of perceptual outcomes across studies.
While the characteristics of clinical episodes affect the time perception skills of BD patients overall, only two studies controlled for the presence of psychotic features and found no significant associations (Bolbecker et al., Reference Bolbecker, Mehta, Edwards, Steinmetz, O.’donnell and Hetrick2009; Bolbecker et al., Reference Bolbecker, Hong, Kent, Forsyth, Klaunig, Lazar, O.’donnell and Hetrick2011; Ryu et al., Reference Ryu, Kook, Lee, Ha and Cho2015; Arrouet et al., Reference Arrouet, Polgári, Giersch and Joos2022; Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022). Moreover, while patients with schizophrenia were impaired in a temporal order judgement task, this was not the case with BD patients, who performed in the range between controls and schizophrenic patients (Arrouet et al., Reference Arrouet, Polgári, Giersch and Joos2022). Similarly, implicit predictive timing was found to be impaired in schizophrenia (Ciullo et al., Reference Ciullo, Piras, Vecchio, Banaj, Coull and Spalletta2018a), but it was not altered in BD patients (Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022). These results suggest that the alteration of time may be linked with the expression of psychotic symptoms. In line with this hypothesis, a recent meta-analysis of six studies suggested a potential relationship between positive symptoms and altered time perception; this also suggests that psychosis might be associated with the overestimation of timing intervals (Ueda et al., Reference Ueda, Maruo and Sumiyoshi2018). Given these premises, it is possible that time perception varies depending on the severity of bipolar symptoms, with more pronounced alterations potentially being linked to the expression of psychotic features or fluctuations in mood states. However, future research must explore the role of psychotic features in the time perception of BD patients, accounting for cognitive impairments as they play a significant role in both psychosis and timing alterations.
Most studies mentioned in this review did not explore the relationship between timing and cognitive functions. However, the few existing results suggest that time perception alterations may be linked with broader cognitive deficits. This underscores the importance of assessing cognitive functions when conducting studies on time perception in psychiatric disorders. The perception of time is not an isolated function; rather, it is intricately connected with multiple other processes in our brain; for example, supra-second timing is connected with attention and working memory skills (Grondin, Reference Grondin2010). Interestingly, in individuals with schizophrenia, the disruption of time perception occurs independently of broader cognitive impairments (Ciullo et al., Reference Ciullo, Spalletta, Caltagirone, Jorge and Piras2016).
As regards the impact of medications, while the effects of pharmacological agents on time perception are often discussed, no significant results emerged from studies included in the review (Bolbecker et al., Reference Bolbecker, Mehta, Edwards, Steinmetz, O.’donnell and Hetrick2009; Bolbecker et al., Reference Bolbecker, Hong, Kent, Forsyth, Klaunig, Lazar, O.’donnell and Hetrick2011; Ryu et al., Reference Ryu, Kook, Lee, Ha and Cho2015; Arrouet et al., Reference Arrouet, Polgári, Giersch and Joos2022; Ciullo et al., Reference Ciullo, Piras, Banaj, Vecchio, Piras, Sani, Ducci and Spalletta2022; Liu et al., Reference Liu, Guo, Ma, Liu, Wang, Zhao, Tan, Tan, Yang and Wang2022). Several other studies suggest that certain drugs, especially those acting on the dopaminergic system, can influence temporal perception. For instance, dopamine antagonists, such as antipsychotics, have been shown to slow the internal clock (Escelsior et al., Reference Escelsior, Trabucco, Radicati, Murri, Da Silva, Serafini and Amore2023). Preclinical evidence also points to valproic acid as a cause of deficits in seconds-to-minutes temporal processing, as demonstrated in animal models (Acosta et al., Reference Acosta, Campolongo, Höcht, Depino, Golombek and Agostino2018). Lithium has been found to have no significant effects on time perception within short intervals (Kolk et al., Reference Kolk, Kathmann and Greil1993), while other substances like imipramine impair the estimation of longer intervals (Wittenborn et al., Reference Wittenborn, Flaherty, Mcgough, Bossange and Nash1976), and diazepam disrupts the estimation of intervals around 10 s (Unrug-Neervoort et al., Reference Unrug-Neervoort, Kaiser and Coenen1992). Additionally, bromazepam has been shown to increase error in time judgements within the 4–9 s range (Silva et al., Reference Silva, Marinho, Magalhaes, Farias, Gupta, Barbosa, Velasques, Ribeiro, Cagy and Bastos2022). Despite these findings, the available literature remains sparse and fragmented, especially when considering shorter temporal intervals.
Several limitations should be acknowledged when interpreting the results of this review. First, given the limited number of studies included, the sample analysed was both small and heterogeneous. This restriction could introduce potential confounds, such as small effect sizes in results and a lack of significant differences due to merging for clinical phases or types of BD (type I/type II). Studies exploring shorter temporal spans have suggested no differences between BD patients and controls; however, these studies did not differentiate patients based on their clinical states. Another significant limitation identified in the reviewed studies is the lack of robust longitudinal research employing experimental methodologies to assess the temporality shifts in BD patients based on specific individual clinical phases. Indeed, we found that only three studies (Mezey and Knight, Reference Mezey and Knight1965; Elsass et al., Reference Elsass, Mellerup, Rafaelsen and Theilgaard1979; Tysk, Reference Tysk1985) conducted longitudinal assessments, but none of these met the criteria for this systematic review (see the Supplementary Materials). However, these studies are either anecdotal, characterised by a very small sample size, or have unspecified follow-up periods. Collectively, they yield inconclusive results, not allowing to formulate a hypothesis supported by experimental evidence regarding the state or trait nature of potential alterations in temporal perception. At the same time, the main significant shortcoming is that the available results do not delve into the neurobiological correlates of timing performances and, to our knowledge, there are no available articles that directly investigate the neurobiological mechanisms associated with time perception. In their study on implicit timing, Bolbecker et al. (Reference Bolbecker, Mehta, Edwards, Steinmetz, O.’donnell and Hetrick2009) posited a link between impaired sub-second time processing and alterations in cerebellar circuitry in BD patients. Future research should focus on the multimodal neuroimaging of the cerebellum to explore its association with timing alterations and its potential therapeutic target, as has been suggested for schizophrenia (Escelsior and Belvederi Murri, Reference Escelsior and Belvederi Murri2019; Escelsior et al., Reference Escelsior, Belvederi Murri, Calcagno, Cervetti, Caruso, Croce, Grassi and Amore2019). The study of the neurobiological correlates of temporality in BD patients must also consider the investigation of the neurotransmitter systems involved in time perception. Indeed, several neurotransmitter systems contribute to this function, most notably the dopaminergic (DA) system (Escelsior et al., Reference Escelsior, Trabucco, Radicati, Murri, Da Silva, Serafini and Amore2023), and it is known that BD is widely characterised by abnormalities in DA neurotransmission (Grande et al., Reference Grande, Berk, Birmaher and Vieta2016).
Conclusions
The existing literature suggests that BD patients are mostly similar to controls in time perception within sub-second temporal intervals. In turn, manic BD patients tend to overestimate time and depressed BD patients tend to underestimate it when dealing with longer temporal spans. However, due to the multifaceted nature of time perception, not enough studies on the topic exist to draw specific conclusions. Moreover, the underlying neurobiological mechanisms of time perception in BD patients remain obscure. Based on this literature review, future studies on the topic should carefully consider the clinical episodes of patients with BD along with their cognitive skills. Given the current lack of reliable biomarkers in psychiatry that bridge neurobiological mechanisms and clinical symptoms, time perception should be further investigated as it could serve as a valuable intermediate phenotype. This has the potential to enhance research, diagnosis, and therapeutic interventions in BD, ultimately contributing to more effective public health strategies.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/neu.2024.57.
Acknowledgements
This work was supported by #NEXTGENERATIONEU (NGEU) and funded by the Italian Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRPP), project MNESYS (PE0000006) - A Multiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022).
This work was developed within the framework of the DINOGMI Department of Excellence of MIUR 2018–2022 (law 232; 2016).
Author contribution
AE: conceptualisation, methodology, data curation, visualisation, writing – original draft. MBA: methodology, writing – original draft. AI: methodology, writing – review and editing. MaG and GM: investigation. YM and AT: investigation, writing – original draft. BP: resources. GN: conceptualisation, validation, writing – review and editing. MoG, MA, and GS: validation, supervision, project administration.
Funding statement
No funding source contributed directly to this work.
Competing interests
The authors declare no conflict of interest.