Significant outcomes
-
– Fibromyalgia women showed a correlation between sleep hours and melatonin levels.
-
– Fibromyalgia and healthy control groups did not show differences in pineal gland volumes.
-
– Fibromyalgia and healthy control groups show differences in cyst prevalence.
Limitations
-
– Only the correlation study between melatonin and pineal volume in the fibromyalgia population could be performed.
-
– The study sample consisted only of women and was relatively small.
-
– During manual segmentation, adjacent structures can be included, which may affect pineal volume.
-
– Some drugs used and comorbidities associated with FM can alter the production and secretion of melatonin.
-
– This study did not consider the influence of the menstrual cycle on melatonin variation.
Introduction
Fibromyalgia (FM) is a chronic disease, which affects between 2.9% and 4.7% of the European population (Branco et al., Reference Branco, Bannwarth, Failde, Carbonell, Blotman, Spaeth, Saraiva, Nacci, Thomas and Caubère2010), most of them being women (Walitt et al., Reference Walitt, Nahin, Katz, Bergman and Wolfe2015). FM is characterised by widespread musculoskeletal pain associated with different symptoms, such as sleeping problems, fatigue, depression, anxiety, stiffness and poor physical fitness (Wolfe et al., Reference Wolfe, Clauw, Fitzcharles, Goldenberg, Katz, Mease, Russell, Russell, Winfield and Yunus2010). All these symptoms have a significant impact on both the ability to perform activities of daily living (Huijnen et al., Reference Huijnen, Verbunt, Meeus and Smeets2015) and the quality of life of women with FM (Mas et al., Reference Mas, Carmona, Valverde and Ribas2008). Sleeping problems have become one of the most important associated symptoms. In this regard, FM patients experience sleep disorder (Wolfe et al., Reference Wolfe, Smythe, Yunus, Bennett, Bombardier, Goldenberg, Tugwell, Campbell, Abeles and Clark1990) and sleep disturbances (Wu et al., Reference Wu, Chang, Lee, Fang and Tsai2017), and almost 80% of FM population have reported poor sleep (Bennett et al., Reference Bennett, Jones, Turk, Russell and Matallana2007; Jacobson et al., Reference Jacobson, Simpson, Lubahn, Hu, Belden, Davis, Nicholson, Long, Osredkar and Lorton2015). This is relevant since sleep-related problems are strongly associated with the severity of the symptoms (Choy, Reference Choy2015).
The pineal gland is a key neuroendocrine organ which mainly synthesises and secretes melatonin (N-acetyl-5-methoxytryptamine; Macchi & Bruce, Reference Macchi and Bruce2004). This relevant hormone is involved in several processes, such as the circadian rhythm (Korf, Reference Korf1994; Acer et al., Reference Acer, Turgut, Yalçın and Duvernoy2011), the sleep–wake rhythm (Arendt, Reference Arendt2005; Acer et al., Reference Acer, Turgut, Yalçın and Duvernoy2011), mood regulation, anxiety, appetite, immune responses, cardiac functions (Comai & Gobbi, Reference Comai and Gobbi2014) and pain (Danilov & Kurganova, Reference Danilov and Kurganova2016). It acts as a neuroprotector or antioxidant (Alghamdi, Reference Alghamdi2018). In this regard, reliable non-invasive methods, such as saliva sampling, and urinary metabolite of melatonin sampling have been developed in both research and clinical fields (Benloucif et al., Reference Benloucif, Burgess, Klerman, Lewy, Middleton, Murphy, Parry and Revell2008). These methods allow us to evaluate the levels of melatonin production. In this regard, salivary melatonin can be considered as a biomarker of circadian rhythm, thus improving the diagnosis and treatment of circadian rhythm sleep–wake disorders (Keijzer et al., Reference Keijzer, Smits, Duffy and Curfs2014). Regarding melatonin levels of FM patients, controversial results have been found when compared with healthy controls (HCs), thus showing normal (Press et al., Reference Press, Phillip, Neumann, Barak, Segev, Abu-Shakra and Buskila1998; Senel et al., Reference Senel, Baygutalp, Baykal, Erdal and Ugur2013), increased (Korszun et al., Reference Korszun, Sackett-Lundeen, Papadopoulos, Brucksch, Masterson, Engelberg, Haus, Demitrack and Crofford1999) and decreased (Wikner et al., Reference Wikner, Hirsch, Wetterberg and Röjdmark1998) melatonin levels.
Regarding pineal gland morphology, alterations have been reported in physiological and pathological conditions, including a decrease of the pineal gland in obese people compared to lean people (Grosshans et al., Reference Grosshans, Vollmert, Vollstaedt-Klein, Nolte, Schwarz, Wagner, Leweke, Mutschler, Kiefer and Malte Bumb2016) and a reduced pineal volume in people with primary insomnia (Bumb et al., Reference Bumb, Schilling, Enning, Haddad, Paul, Lederbogen, Deuschle, Schredl and Nolte2014), Alzheimer’s disease (Matsuoka et al., Reference Matsuoka, Imai, Fujimoto, Kato, Shibata, Nakamura, Yokota, Yamada and Narumoto2017), schizophrenia (Fındıklı et al., Reference Fındıklı, Fatih Inci, Gökçe, Avni Fındıklı, Altun and Fatih Karaaslan2015) and major depressive disorder (Zhao et al., Reference Zhao, Zhu, Zhang, Zhang, Wang, Yang, Bai, Zhu and Yu2019). Moreover, in healthy individuals, sleep rhythm disturbance correlated with smaller pineal volume (Liebrich et al., Reference Liebrich, Schredl, Findeisen, Groden, Bumb and Nölte2014).
As previously mentioned, the main function of the pineal gland is the production and secretion of melatonin (Macchi & Bruce, Reference Macchi and Bruce2004). However, this function is altered by the calcification, a frequent clinical finding that occurs in this small structure and which has been associated with pathological conditions and ageing (Tan et al., Reference Tan, Xu, Zhou and Reiter2018). The pineal gland has one of the highest calcification rates among organs and tissues (Yalcin et al., Reference Yalcin, Ceylan, Bayraktutan, Sonkaya and Yuce2016; Tan et al., Reference Tan, Xu, Zhou and Reiter2018). Nevertheless, the mechanism that produces calcification is not known with certainty, although several theories have been proposed (Tan et al., Reference Tan, Xu, Zhou and Reiter2018).
In the case of Alzheimer’s disease, it has also been observed that reduced pineal volume and pineal gland calcifications may contribute to reduce melatonin secretion, sleep problems (Song, Reference Song2019) and, therefore, cognitive impairment (Krause et al., Reference Krause, Simon, Mander, Greer, Saletin, Goldstein-Piekarski and Walker2017).
Another frequent magnetic resonance imaging (MRI) finding is pineal cysts that usually occur between the ages of 21 and 30 years, decreasing, its prevalence, with age (Nolte et al., Reference Nolte, Brockmann, Gerigk, Groden and Scharf2010). It appears that cysts may also have a negative effect on melatonin secretion by compressing the pineal parenchyma (Nölte et al., Reference Nölte, Lütkhoff, Stuck, Lemmer, Schredl, Findeisen and Groden2009). In this regard, both calcifications and cysts are considered hormonally inactive tissues and its volume is usually excluded from the total pineal volume. This is based on the results of previous studies (Nölte et al., Reference Nölte, Lütkhoff, Stuck, Lemmer, Schredl, Findeisen and Groden2009; Liebrich et al., Reference Liebrich, Schredl, Findeisen, Groden, Bumb and Nölte2014; Sigurdardottir et al., Reference Sigurdardottir, Markt, Sigurdsson, Aspelund, Fall, Schernhammer, Rider, Launer, Harris and Stampfer2016) which showed that volumetric alterations may affect melatonin activity, mainly related to the pineal parenchymal volume (PPV) which is referred to hormonal active pineal tissue.
Although sleep disorders in FM have been widely studied there is, to the best of our knowledge, no study that evaluates the volume of the pineal gland and its relation to sleep hours and melatonin levels in women with FM. Therefore, this study was aimed to assess the volume of the pineal gland as well as the relation between this gland and levels of melatonin and sleep quality in women with FM. We hypothesised that reduced pineal volumes would be correlated with reduced levels of melatonin and sleep hours.
Subjects and methods
Participants
In this study, a total of 50 participants participated in this study. A total of 30 women with FM were recruited by the Extremadura Association of Fibromyalgia (AFIBROEX) in Cáceres by telephone calls. All participants met the following inclusion criteria: (a) being a 30- to 75 years old woman; (b) have been diagnosed with FM by a rheumatologist according to American College of Rheumatology 2010 criteria (Wolfe et al., Reference Wolfe, Clauw, Fitzcharles, Goldenberg, Katz, Mease, Russell, Russell, Winfield and Yunus2010) and (c) being able to communicate with the study staff effectively; and (d) have understood and signed an informed consent conform to the updated Declaration of Helsinki. Moreover, participants were excluded if: (a) they were pregnant; (b) they had any cerebral injury and (c) illegible MRI sequences were obtained. In this sense, three participants were excluded due to poor visibility of the pineal gland. Therefore, a total of 27 participants of the FM were included in the present study.
A total of 20 HC participants were selected by the OASIS-3 data set (LaMontagne et al., Reference Lamontagne, Keefe, Lauren, Xiong, Grant, Moulder, Morris, Benzinger and Marcus2018). The participants met the following inclusion criteria: (a) female and ages between 42 and 60 years, (b) height between 152 and 178 cm, (c) weight between 40 and 120 kg, (d) being cognitively normal and (e) have T1w MRI scan with 3 tesla scanner.
According to the power calculation estimates using the PASS-11 software (version 11; NCSS, LLC, Kaysville, UT, USA), a group sample of 27 and 20 achieve a 100% power to detect a difference of −7.7 at the 0.05 significance level (alpha) using a two-sided Mann−Whitney test after 2000 Monte Carlo simulations.
All participants were verbally informed about the details of the study and gave written informed consent to participate. Participants underwent MRI in January 2018. All procedures were approved by the Research Ethics Committee of the University of Extremadura (approval reference: 62/2017) and were conducted in accordance with the tenets of the updated Declaration of Helsinki.
Image acquisition
The FM MRI scans were performed with a 3.0 tesla (T) system (Achieva 3.0T TX, Philips Medical Systems, Best, Netherlands) with eight channel receiver head coil. MRI data were obtained using 3D T1-weighted Turbo Field Echo (T1-w TFE) sequence (time repetition/time to echo = 11.51/2.8 ms; matrix size= 288 × 288; flip angle= 10°; slice thickness= 0.9 mm; number of averages = 1). For the HC group, a system equipped with a 16-channel head coil (Siemens TIM Trio or BioGraph mMR PET-MR, Erlangen, Germany) was used. The MP-RAGE protocol of TIM Trio scanner used the following parameters: TR/TE = 2400/3.16 ms, ± 176 axial slices without slice gap and 1.0 mm nominal isotropic resolution (Field-of-view (FOV) = 256 × 256 mm). The MP-RAGE sequence of BioGraph mMR PET-MR scanner used the following parameters: TR/TE = 2300/2.95 ms, ± 176 axial slices without slice gap and 1.2-mm nominal isotropic resolution (FOV = 256 × 256 mm).
Image processing
The 3D Slicer version 4.11.0 Nightly Build free software, to see at https://www.slicer.org/, was used for processing the imaging data. The 3D mask of each pineal gland was manually drawn by one researcher (J.L.L.-L.), who was blind to the diagnosis and supervised by an experienced neuroradiologist, 10 years in neuroradiology. Anatomical structures, such as the quadrigeminal cisterna posteriorly, the posterior part of the third ventricle anteriorly, the corpus callosum above, the superior colliculus below and pulvinars laterally (Moeller & Reif, Reference Moeller and Reif2007), were used as limits to draw the pineal gland. The pineal glands were also classified according to the size of the cyst, following the recommendations by Pu et al. (Reference Pu, Mahankali, Hou, Li, Lancaster, Gao, Appelbaum and Fox2007): type 1 (pineal gland with no visible cyst), type 2 (pineal gland with a visible cyst < 2 mm) and type 3 (pineal gland with a visible cyst > 2 mm).
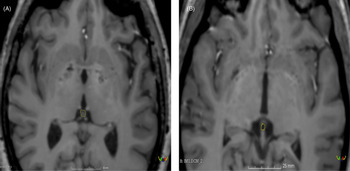
Volumetric measurements of each pineal gland were manually drawn via slice-by-slice segmentation using the ‘Draw Effect’ tool in the axial plane, with simultaneous side-by-side view of the sagittal and coronal plane. The sum of these areas was added up to determine the volumes of the pineal gland. The total pineal gland area was obtained using ’Segment Statistics’ module to determine the real volume (RV) of the pineal gland in cubic millimetres (see Fig. 1). The cyst pineal volume (CPV) was also measured following the same procedure used to measure the RV (see Fig. 1). Additionally, the reference value of the pineal gland volume (TPV) was estimated using the Hasegawa method by the formula 0.5 × H × L × W (where W is the maximum width, L is the maximum length and H is the maximum height of the gland; Hasegawa et al., Reference Hasegawa, Ohtsubo and Mori1987).
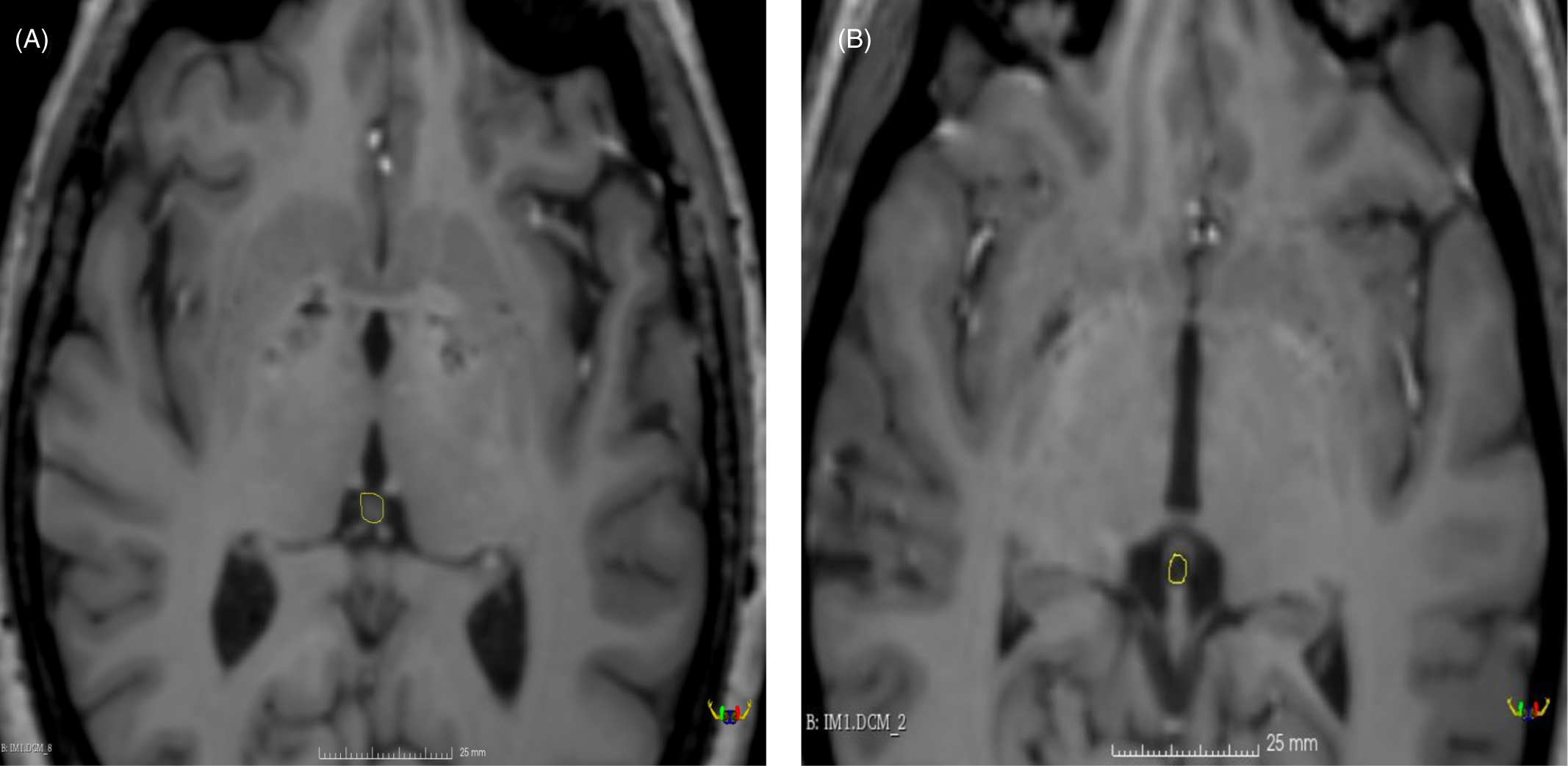
Fig. 1. T1-weighted Turbo Field Echo (T1-w TFE) imaging of pineal region illustrating: (A) solid pineal gland measured manually (hiperintense) and (B) cystic pineal gland measured manually (hipointense).
PPV was calculated as the subtraction of CPV-RV in the cases where cysts were found. Moreover, when no cyst was observed the PPV was calculated as follows: PPV = RV. Pineal calcifications were not estimated when using T1 sequences, since it does not allow a reliable identification (Adams et al., Reference Adams, Böker, Bender, Diederichs, Fallenberg, Wagner, Hamm and Makowski2017).
A total of 20 participants, from the whole sample, were randomly selected to determine the reliability of the pineal gland measurements. These participants were measured twice with a time gap of one month.
All T1-weighted images were analysed on a HP 840 eliteBook (Version Windows 10, 8 GB, 2.60 GHz, Intel Core i7).
Fibromyalgia Impact Questionnaire
The revised Spanish version of the Fibromyalgia Impact Questionnaire was used (Salgueiro et al., Reference Salgueiro, García-Leiva, Ballesteros, Hidalgo, Molina and Calandre2013) to evaluate the impact of FM-related symptoms on daily living skills and general health status. The questionnaire has 21-item, and it is self-administered. The total score was measured from 0 to 100, thus indicating the impact of the disease, 100 being the highest score and meaning the worst state.
Pittsburgh sleep quality index
The Pittsburgh sleep quality index (PSQI) enabled us to assess the sleep quality and disturbances of the participants over 1 month. Seven components, with subscales ranging from 0 to 3, make up the global score, which ranges from 0 to 21: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication and daytime dysfunction (Buysse et al., Reference Buysse, Reynolds, Monk, Berman and Kupfer1989). Higher scores represent poorer subjective sleep quality. We used the Spanish version of the PSQI, which has been validated for its use in patients with FM. (Hita-Contreras et al., Reference Hita-Contreras, Martínez-López, Latorre-Román, Garrido, Santos and Martínez-Amat2014).
Melatonin measurement
Participants’ salivary samples were collected in sterile biological liquid collection tubes (Deltalab, Barcelona, Spain) on two consecutive days at home (24 p.m.) in order to measure melatonin levels. All collected samples were directly stored in a refrigerator at −80°C. In order to avoid variability, all samples were analysed in the same batch. Direct Saliva Melatonin ELISA (Bühlmann, Schönenbuch, Switzerland) was used to measure melatonin concentrations.
Participants were requested to follow their usual sleep habits and to collect the salivary sample with dim lighting (<30 lux). Moreover, they were instructed not to smoke, eat or drink during the 60 min before the collection and to rinse their mouths with water before collecting saliva.
No data were obtained on melatonin and sleep hours in HC since this information was not available in the OASIS-3 data set. In the same way, no data were available on the impact of FM, since it did not apply to this population.
Statistical analysis
The SPSS statistical package (version 24.0 SPSS, Inc., Chicago, IL, USA) was used to analyse the data. The data were analysed with non-parametric methods for the results obtained from the Shapiro−Wilk test. Intraclass correlation coefficient (ICC) two-way random effects, consistency, single measurement was used to explore the reliability of the measures (Weir, Reference Weir2005).
The Mann−Whitney U-test was conducted to examine the differences between groups (FM vs. HCs and FM with and without pineal cyst) for each variable. Furthermore, chi-square test was used to evaluate the prevalence of the existence of cysts between groups as well as to evaluate the impact of the type of cyst on PPV, years with FM, PSQI and sleep hours in people with FM.
Finally, Spearman’s correlation coefficient was used for correlation analysis of PPV with sleep hours, melatonin level at night and sleep quality index in FM. Statistical significance was set at p = 0.05.
Results
Reliability or the pineal gland measurements
The reliability of the PGV and the RV was high. For the RV, the ICC was 0.97 (95% CI 0.92–0.98), showing ‘excellent’ reliability. Moreover, the ICC for the PGV was 0.90 (95% CI 0.76–0.96), showing ‘moderate to excellent’ reliability. The classification by Koo and Li (Reference Koo and Li2016) was used for reliability estimation.
Demographic characteristics of the participants and differences between groups, fibromyalgia impact and sleep quality
Table 1 shows the main characteristics of the participants of both groups. Differences between FM and HC group were only observed in height (p-value < 0.004; see Table 1). FM participants had a mean of 10.46 (6.10) years from FM diagnosis. The fibromyalgia impact questionnaire revised (FIQ-R) indicated that participants had a value of 56.01 (17.94), which corresponds to mild severity symptoms. The mean hours of sleep were 5.54 (0.97) (see Table 1).
Table 1. Descriptive characteristics of the participants
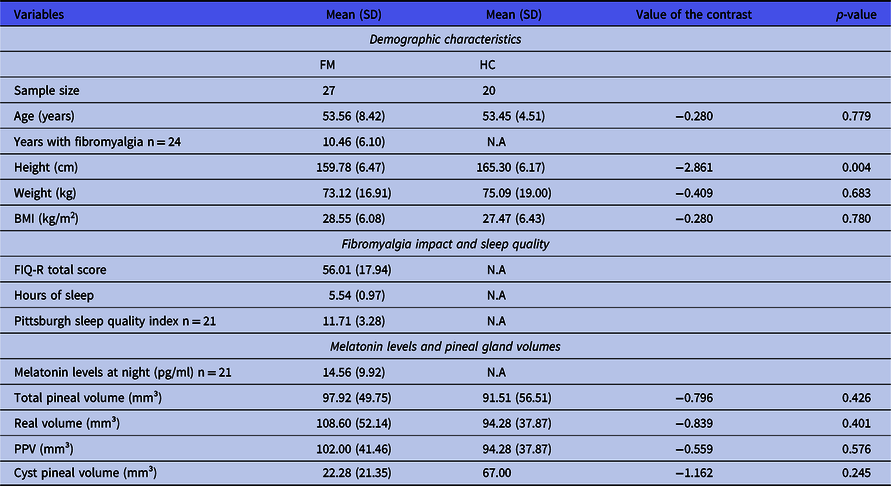
BMI, body mass index; FIQ-R, fibromyalgia impact questionnaire revised; FM, fibromyalgia; HC, healthy control; PPV, pineal parenchymal volume.
Pineal gland volumes and melatonin levels
Table 1 shows the different pineal volumes measured by Hasegawa method (TPV) and manually (RV and PPV). Cyst volume is also reported (see Table 1).
The mean levels of melatonin at night were 14.56 (9.92) pg/ml (see Table 1).
Table 2 shows the differences, within the FM group, between those people in whom a cyst was detected or not. In this regard, people with FM with a pineal cyst showed statistically significant more sleep hours than people with FM without pineal cyst (p-value < 0.028).
Table 2. Differences between people with fibromyalgia in whom a cyst was detected or not in the pineal parenchymal volume, years with fibromyalgia, Pittsburgh sleep quality index and sleep hours in people with fibromyalgia
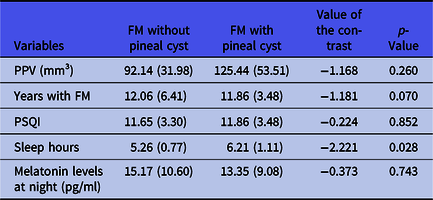
FM, fibromyalgia; PPV, pineal parenchymal volume; PSQI: Pittsburgh sleep quality index.
Prevalence and types of cyst
In the visualisation MRI sequences, 19 types I cyst (no visible cyst), 2 types II (< 2 mm) cyst and 6 types III (>2 mm) cyst were found in the FM. Therefore, a 29.60% of cyst prevalence was established in the FM group sample evaluated. On the other hand, 19 types I cyst and 1 type III cyst were found in the HC group, showing a 5.00% of cyst prevalence (see Table 3). Differences between FM and HC group were only observed in cyst prevalence (p-value = 0.034).
Table 3. Prevalence and types of cysts found among different groups
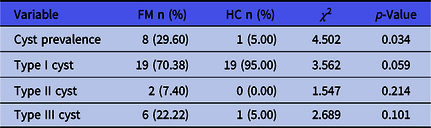
FM, fibromyalgia; HC, healthy control; n, number of participants.
Table 4 shows the impact, within the FM group, of the type of pineal cyst on PPV years with FM, PSQI and sleep hours in people with FM. Significant differences were not found in any of the studied variables (p-value > 0.05).
Table 4. Impact of type of cyst on pineal parenchymal volume, years with fibromyalgia, Pittsburgh sleep quality index and sleep hours in people with fibromyalgia

FM, fibromyalgia group; PPV, pineal parenchymal volume; PSQI, Pittsburgh sleep quality index.
Correlation analyses
Table 5 shows the correlation analyses between pineal gland volumes, sleep hours and melatonin levels in the FM group. A significant correlation was found between PPV and sleep hours (p-value = 0.041). Furthermore, a significant correlation was also found between melatonin levels and the PPV (p-value = 0.027).
Table 5. Relationship between pineal parenchyma volume (PPV) with sleep hours, nocturnal average melatonin level and total score in the PSQI in the FMG

FM, fibromyalgia; PPV, pineal parenchymal volume; PSQI: Pittsburgh sleep quality index.
* p-value lower than 0.05. For correlation analyses, Spearman’s correlation coefficient was used.
Discussion
The present study was aimed to investigate the pineal volume of women with FM as well as to establish relationships between sleep hours and nocturnal melatonin levels in this population.
To date, no volume reference data have been found in women with this age range analysed by MRI. As a result, the mean PPV was 102.00 with a prevalence of 29.60% cyst between women with FM. Moreover, significant correlations were found between the PPV and both sleep hours and melatonin level at night. In addition, a non-significant tendency between PPV and the sleep quality index obtained by PSQI was also noticed. Finally, no significant differences were observed in PPV, TPV and RV between the FM and the HC group. Only differences were observed in the prevalence of cysts between the groups. Results from the present study are relevant, since this is the first study that has reported on correlations between variables, such as pineal gland volumes, cyst prevalence and relationships between PPV and sleep hours and melatonin levels in women with FM. In addition, no difference in pineal volume was observed in comparison with healthy women.
Our study showed a significant correlation between the active pineal gland tissue (PPV) and both hours of sleep and melatonin levels. Regarding pineal gland volume and sleep hours, previous studies showed a significant correlation between sleep quality and pineal gland volume in primary insomnia (Bumb et al., Reference Bumb, Schilling, Enning, Haddad, Paul, Lederbogen, Deuschle, Schredl and Nolte2014), major depressive disorder and HCs (Zhao et al., Reference Zhao, Zhu, Zhang, Zhang, Wang, Yang, Bai, Zhu and Yu2019). Moreover, our results are also in line with previous studies focused on melatonin levels. In particular, these previous studies found associations between the production of melatonin and the PPV in healthy people (Liebrich et al., Reference Liebrich, Schredl, Findeisen, Groden, Bumb and Nölte2014; Nölte et al., Reference Nölte, Lütkhoff, Stuck, Lemmer, Schredl, Findeisen and Groden2009). In addition, Liebrich et al. (Reference Liebrich, Schredl, Findeisen, Groden, Bumb and Nölte2014) found an inverse correlation between active pineal tissue and sleep rhythm disorder. In the same way, the pineal volume was correlated with the morning 6-sulfatoxymelatonin levels (Sigurdardottir et al., Reference Sigurdardottir, Markt, Sigurdsson, Aspelund, Fall, Schernhammer, Rider, Launer, Harris and Stampfer2016) in older adults. Moreover, Riemann et al., (Reference Riemann, Klein, Rodenbeck, Feige, Horny, Hummel, Weske, Al-Shajlawi and Voderholzer2002) found reduced nocturnal plasma melatonin levels comparing primary insomnia and healthy individuals and poor quality of sleep in primary insomnia patients. In a whole, considering our study and previous studies, the pineal gland volume of women with FM could be related to reduced sleep hours. Moreover, it might be associated with reduced nocturnal melatonin releases, which may lead to a reduced quality of sleep. However, more studies are needed to confirm this hypothesis.
FM patients usually experience sleep disorders (Bennett et al., Reference Bennett, Jones, Turk, Russell and Matallana2007; Jacobson et al., Reference Jacobson, Simpson, Lubahn, Hu, Belden, Davis, Nicholson, Long, Osredkar and Lorton2015), and this is associated with the severity of the symptoms (Wolfe et al., Reference Wolfe, Smythe, Yunus, Bennett, Bombardier, Goldenberg, Tugwell, Campbell, Abeles and Clark1990; C-oté & Moldofsky, Reference C-Oté and Moldofsky1997; Mease et al., Reference Mease, Arnold, Choy, Clauw, Crofford, Glass, Martin, Morea, Simon and Strand2009; Mork & Nilsen, Reference Mork and Nilsen2012; Choy, Reference Choy2015). The pineal gland is a key neuroendocrine organ that is located in the centre of the brain and synthesises and secretes melatonin (N-acetyl-5-methoxytryptamine; Macchi & Bruce, Reference Macchi and Bruce2004) in its active tissue (PPV). The melatonin is involved in circadian rhythm processes (Korf, Reference Korf1994; Acer et al., Reference Acer, Turgut, Yalçın and Duvernoy2011), and therefore it is in relation with sleep quality (Ferracioli-Oda et al., Reference Ferracioli-Oda, Qawasmi and Bloch2013). Thus, the reduction of pineal volume is usually associated with a decrease in melatonin levels, which can affect the lack of sleep (Song, Reference Song2019) and pain (Danilov & Kurganova, Reference Danilov and Kurganova2016). Therefore, we hypothesised that women with FM would show a reduced pineal gland volume. Previous studies using MRI in healthy populations reported that women have slight smaller pineal gland volumes than men (Sun et al., Reference Sun, Wang, Tang, Fan, Lin, Yu, Qi, Li and Liu2009) and old males have bigger volumes than young (Sigurdardottir et al., Reference Sigurdardottir, Markt, Sigurdsson, Aspelund, Fall, Schernhammer, Rider, Launer, Harris and Stampfer2016). Results from our study show that the average PPV, 102.00 (41.46), is consistent with previous literature and with the values obtained in the HC group. Comparing this result with previous studies, we found that participants’ pineal gland volumes were slightly lower than the pineal gland volumes reported by Liebrich et al. (Reference Liebrich, Schredl, Findeisen, Groden, Bumb and Nölte2014) 113 mm³ with 3.0 T MR and Nölte et al. (Reference Nölte, Lütkhoff, Stuck, Lemmer, Schredl, Findeisen and Groden2009) 125 mm³ by 1.5 T MR in healthy young male population, but slightly high compared to the HC values 94.28 (37.87). Bumb et al. (Reference Bumb, Schilling, Enning, Haddad, Paul, Lederbogen, Deuschle, Schredl and Nolte2014) reported a pineal gland volumes much smaller than us in primary insomnia, and Matsuoka et al. (Reference Matsuoka, Imai, Fujimoto, Kato, Shibata, Nakamura, Yokota, Yamada and Narumoto2017) controlling cyst volumes found 70, 90 and 111 mm³ in adults (mixed men and women) older than 70 years old with Alzheimer’s disease, mild cognitive impairment and healthy, respectively. Due to the high variability of methods (e.g. MRI with different brands and analysis) and populations in previous studies regarding pineal gland volumes, the comparisons between studies have been cautious. Therefore, the current study could not conclude that women with FM have smaller pineal gland than healthy population, thought women with FM reported sleep disorders and pain. Further studies are warranted to fully determine the mechanisms of the impact of FM in the size of this relevant neuroendocrine gland.
Due to the shape variability of the pineal gland through the volume data obtained in this study using the Hasegawa method, it seems that it is not the most appropriate method to establish the volume since an underestimation is performed, finding similarities with previous studies (Sun et al., Reference Sun, Wang, Tang, Fan, Lin, Yu, Qi, Li and Liu2009). In the present study, 3D T1-w TFE image sequences were used to assess pineal gland volumes, excluding cystic tissue, since it can be considered as hormonally inactive (Nölte et al., Reference Nölte, Lütkhoff, Stuck, Lemmer, Schredl, Findeisen and Groden2009). A pineal gland cyst was observed in the 29.60% of women with FM. These results are similar to the findings reported by Pu et al. (Reference Pu, Mahankali, Hou, Li, Lancaster, Gao, Appelbaum and Fox2007) 23% and Sun et al. (Reference Sun, Wang, Tang, Fan, Lin, Yu, Qi, Li and Liu2009) 25% in healthy adults. However, only 5% of the sample of healthy women in this study showed a cyst. Pineal cyst is common and frequently detected as an incidental finding (Petitcolin et al., Reference Petitcolin, Garcier, Mohammedi, Ravel, Mofid, Viallet, Vanneuville and Boyer2002; Nolte et al., Reference Nolte, Brockmann, Gerigk, Groden and Scharf2010). Our results showed that people with FM whom a cyst was detected reported more sleep hours than those with FM in whom a pineal cyst was detected. However, no significant differences in PPV and melatonin levels at night were observed. Furthermore, when the FM sample was divided between those who did not have a cyst, who had a cyst of less than 2 mm and those who had a cyst of more than 2 mm, no significant differences were observed in any of the variables studied (PPV, years with FM, PSQI and sleep hours in people with FM). In this regard, although pineal cysts are considered a hormonally inactive tissue, further research on the effects of pineal cyst on pineal function is needed to elucidate this question (Nölte et al., Reference Nölte, Lütkhoff, Stuck, Lemmer, Schredl, Findeisen and Groden2009). Moreover, pineal calcifications were not estimated when using T1 sequences, since it does not allow a reliable identification (Adams et al., Reference Adams, Böker, Bender, Diederichs, Fallenberg, Wagner, Hamm and Makowski2017). Therefore, future studies should evaluate the impact of pineal cysts in the melatonin releases as well as the prevalence of pineal calcifications increasing the sample size of women with FM. For that it would be interesting to use computed tomography and/or susceptibility-weighted MRI, which allow detection of pineal gland calcifications (Adams et al., Reference Adams, Böker, Bender, Diederichs, Fallenberg, Wagner, Hamm and Makowski2017).
The present study has some limitations, which should be considered. First, no analysis could be performed in the HC group on sleep hours and melatonin levels since the OASIS-3 data set does not have this information. Second, we included only women, so our results cannot be generalised to male patients with FM. Third, the relatively small sample size likely reduced the statistical significance of some of our results; it is possible that only the largest differences had enough statistical power to reach significance. Fourth, when performing manual segmentation, it is possible to include adjacent structures to the pineal gland, and this affects the final volume value. Therefore, we recommend to increase sample size and to include male patients with FM. Fifth, there are numerous factors that can influence the production and secretion of melatonin, such as age, pathologies, pharmacotherapy, exposure to light and consumption of substances (alcohol or caffeine; Aulinas, Reference Aulinas2019). In people with FM, one of the most important factors might be pharmacotherapy, since some medications are used to reduce some symptoms of FM and have been shown to cause an alteration in the secretion and production of melatonin. For example, some drugs used for the treatment of anxiety and depression show increases in melatonin production. In the same line, other drugs such as benzodiazepines, also used for the treatment of anxiety, have shown a decrease in melatonin levels. On the other hand, some non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen or aspirin, often used to reduce pain, have been shown to decrease melatonin production and secretion (Aulinas, Reference Aulinas2019). Nevertheless, both serotonin noradrenaline reuptake inhibitors and NSAIDs are commonly used in the treatment of FM (Derry et al., Reference Derry, Wiffen, Haeuser, Mücke, Tölle, Bell and Moore2017; Welsch et al., Reference Welsch, Üçeyler, Klose, Walitt and Haeuser2018) and it is rare to find patients who do not use these drugs. Sixth, there are possibly a number of comorbidities, which could be manifested along lifetime, associated with insomnia that could impact FM symptoms such as depression, anxiety and chronic pain. In this regard, it is important that future studies take into account medications and comorbidities to clarify how they can alter melatonin production and secretion. Finally, another aspect that has not been controlled in this study has been the menstrual period of the participants; however, to the best of our knowledge, it appears that the current data to date on melatonin and its variation during the menstrual cycle are inconsistent (Aulinas, Reference Aulinas2019).
Our present study found significant correlations between the PPV and both sleep hours and melatonin levels at night in women with FM. Moreover, a mean PPV of 102.00 (41.46) mm³ was observed, with a prevalence of 29.62% cyst in this population. Results from the present study are relevant, since this is the first study that has reported pineal gland volumes, cyst prevalence and correlative relationships between PPV and sleep hours and melatonin levels in women with FM.
Acknowledgements
The support and help of the Servicio de Técnicas Aplicadas a la Biociencia of the University of Extremadura is greatly acknowledged. We thank Dr. Laura Martín for her help and supervision with pineal gland measurements. We are also grateful to the Extremadura Association of Fibromyalgia (AFIBROEX) in Cáceres for helping recruit the participants for this study. The figure was made by the author JLLL using 3D Slicer software version 4.11.0 Nightly Build available in https://www.slicer.org/. HCG data used in this article were obtained from the OASIS-3 database of which the principal investigators are T. Benzinger, D. Marcus, J. Morris and have the following grant numbers: P50 AG05681, P01 AG03991, P01 AG026276, R01 AG021910, P20 MH071616 and U24 RR021382. The OASIS investigators did not participate in analysis or writing this work. The data of the HC group presented in this study are openly available in https://www.oasis-brains.org/.
Author contributions
JLLL: design, drafting the article, analysis and interpretation of data. NG: concept, design, and drafting the article. SV: acquisition of data, analysis and interpretation of data. PRD: review of literature. AMG: design, analysis and interpretation of data. NG, SV, AMG and PRD: reviewing and revising the manuscript. SV: consultation and reviewing statistical analysis and interpretation of data. All authors contributed to and approved the final version.
Financial support
The author AMG was supported by a grant from the Spanish Ministry of Education, Culture and Sport (FPU17/03130). The author JLLL was supported by a grant from the Spanish Ministry of Education, Culture and Sport (FPU18/05655). The funders played no role in the study design, the data collection and analysis, the decision to publish or the preparation of the manuscript. In the framework of the Spanish National R + D + i Plan, the current study was co-funded by the Spanish Ministry of Sciences and Innovation (reference PID2019-107191RB-I00/AEI/10.13039/501100011033). This study was also funded by the Research Grant for Groups (GR18155) funded by the Junta de Extremadura (Regional Government of Extremadura) and the European Regional Development Fund (ERDF/FEDER) ‘a way of doing Europe’. This study was supported by the Biomedical Research Networking Center on Frailty and Healthy Aging (CIBERFES) and FEDER funds from the European Union (CB16/10/00477). This work was also supported by 4IE+ project (0499_4IE_PLUS_4_E) funded by the Interreg V-A España-Portugal (POCTEP) 2014–2020 programme ‘a way of doing Europe’.
Competing interests
The authors declare no competing interests.