Significant outcomes
-
The left inferior frontal gyrus pars opercularis of tic disorder patients shows increased global efficiency compared to controls.
-
The left inferior frontal gyrus pars opercularis shows enhanced resting-state functional connectivity with its right counterpart in patients compared to controls.
Limitations
-
The small number of enrolled patients is a limitation of this study.
-
The cross-sectional nature of our study precluded the verification of our results over a prospective period.
Introduction
Tic disorders typically manifest at an early age and involve repetitive, involuntary movements or vocal sounds. They are notable prevalent with an incidence rate as high as 5.6% (Knight et al., Reference Knight, Steeves, Day, Lowerison, Jette and Pringsheim2012). The most severe manifestation of tic disorders is called Tourette syndrome, characterised by the concurrence of motor and vocal tics for over one year. While not life-threatening, tic disorders are associated with impaired self-esteem, social life, and academic performance. These issues may persist into adulthood, adversely influencing career choices and interpersonal relationships. Tic disorders are also linked to a higher prevalence of attention-deficit hyperactivity disorder (ADHD), which can independently lead to various psychiatric and behavioural problems. These challenges significantly impact not only the patients but often their entire families.
Our current understanding of tic disorders, derived from various imaging, genetic, and postmortem studies, suggests that tic disorders are associated with abnormalities in the corticospinal tract, particularly within the striatum. Imaging studies documented decreases in volume in the striatum and the globus pallidus in patients with tic disorders (Makki et al., Reference Makki, Govindan, Wilson, Behen and Chugani2009; Y. Worbe et al., Reference Worbe, Marrakchi-Kacem, Lecomte, Valabregue, Poupon, Guevara, Tucholka, Mangin, Vidailhet, Lehericy, Hartmann and Poupon2015). Intriguingly, an ongoing study on tic disorder patients reported that the severity of the condition was predicted not by striatal volumes but by hippocampal volumes (Black et al., Reference Black, Kim, Schlaggar and Greene2020). A relatively recent study proposed a model dividing the underlying mechanism into two discrete networks: the expression network and the control network (Yael et al., Reference Yael, Vinner and Bar-Gad2015). The expression network, associated with behavioural symptoms, includes motor-related areas such as the pre- and primary motor cortices, putamen, and limbic areas, as highlighted by functional magnetic resonance imaging (fMRI) studies (Neuner et al., Reference Neuner, Werner, Arrubla, Stöcker, Ehlen, Wegener, Schneider and Shah2014). Conversely, the control network, which modulates the expression of these behavioural symptoms, is thought to encompass the frontal cortical areas. This hypothesis is supported by a study showing correlations between tic suppression and increased regional homogeneity of the left inferior frontal gyrus (Ganos et al., Reference Ganos, Kahl, Brandt, Schunke, Bäumer, Thomalla, Roessner, Haggard, Münchau and Kühn2014). Premonitory urges, on the other hand, are believed to span both networks, mediated by sensory and limbic brain regions (Neuner et al., Reference Neuner, Werner, Arrubla, Stöcker, Ehlen, Wegener, Schneider and Shah2014; Z. Wang et al., Reference Wang, Maia, Marsh, Colibazzi, Gerber and Peterson2011). Church et al. also investigated the resting-state functional connections in 33 adolescents with tic disorder and 210 healthy individuals and found that patients showed many less mature functional connections in the fronto-parietal network and the cingulo-operculo network (Church et al., Reference Church, Fair, Dosenbach, Cohen, Miezin, Petersen and Schlaggar2009). The authors defined ‘maturity’ as how developed each functional brain connection is in the patient group compared to typical development in people without the disease. Such defects in brain maturation was further supported by a study on the functional connectivity of 59 adult Tourette syndrome patients and 27 controls, revealing stronger functional integration and global disorganisation in sensory motor, associative, and limbic cortico-basal ganglia networks among patients (Yulia Worbe et al., Reference Worbe, Malherbe, Hartmann, Pélégrini-Issac, Messé, Vidailhet, Lehéricy and Benali2012).
Our research endeavoured to extend these earlier studies by employing graph theory to conceptualise the brain as a network of interconnected nodes. Investigations on animal brains deducted that nervous systems possess properties of small-world networks, making them suitable for analysis using graph theory (Watts and Strogatz, Reference Watts and Strogatz1998; Hilgetag et al., Reference Hilgetag, Burns, O’Neill, Scannell and Young2000). The advent of fMRI facilitated practical applications of graph theoretical analysis on the human brain. Early studies already reported small-world structures within cortical and subcortical regions of the brain network (Salvador et al., Reference Salvador, Suckling, Coleman, Pickard, Menon and Bullmore2005). This methodology has been adopted in various clinical studies, exploring a range of psychiatric disorders, such as depression and schizophrenia, in greater depth (Su et al., Reference Su, Hsu, Lin and Lin2015; Ye et al., Reference Ye, Yang, Qing, Lei, Qiu and Liu2015). The application of network analysis offers a comprehensive perspective on the brain’s connectivity, capturing both anatomical pathways and correlations in activity, providing a more nuanced understanding of whole-brain effective connectivity and the inherent dynamics of brain activity (Gilson et al., Reference Gilson, Kouvaris, Deco, Mangin, Poupon, Lefranc, Rivière and Zamora-López2019; J. Wang et al., Reference Wang, Zuo and He2010). This was applied in tic disorder research by Wen et al., who constructed whole-brain, region of interest (ROI)-level functional connectivity networks for 29 drug-naive tic disorder children and 37 healthy controls, and used graph theory to investigate topological disruptions (Wen et al., Reference Wen, Liu, Rekik, Wang, Chen, Zhang, Zhang, Peng and He2018). The identified regions involved the sensorimotor, visual, language, and default-mode areas. They also analysed white matter networks in 44 tic disorder children and 41 healthy controls, finding that patients exhibited decreased global and local efficiency, increased path length, and disrupted network balance (Wen et al., Reference Wen, Liu, Rekik, Wang, Zhang, Zhang, Peng and He2017).
Despite this extensive amount of existing research, a comprehensive understanding of this complex disorder remains elusive. This study aimed to investigate the resting-state functional neuroimaging data of tic disorder patients through a data-driven network analysis followed by a hypothesis-driven functional connectivity analysis of the prior results.
Material and methods
Participants and clinical measures
A total of 39 patients with tic disorder and 37 healthy controls were initially enrolled in the study. All participants were between the ages of 6 and 18, psychotropic medication free for at least 3 weeks, and had no history of neurologic disorders including head trauma, tumours, or seizures. Patients were clinically diagnosed with tic disorders (Tourette’s disorder, persistent motor or vocal tic disorder, provisional tic disorder, other specified tic disorder, and unspecified tic disorder) based on the 5th edition of the Diagnostic and Statistical Manual of Mental Disorder (DSM-5) by child and adolescent psychiatrists. Intelligence quotients of all patients and controls were examined using the Korean version of the Wechsler Intelligence Scale for Children fourth edition (K-WISC-IV). Patients were recruited from the department of psychiatry of Korea University Guro Hospital. Healthy controls were recruited from local schools and kindergartens.
Patients were assessed using the Korean version of the Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (K-SADS-PL) for psychiatric comorbidities, and the Yale Global Tic Severity Scale (YGTSS) for tic disorder symptom severity.
The research processes were approved by the Institutional Review Board (IRB) of the Korea University Guro Hospital (2021GR0275). All research methods were performed in accordance with the relevant guidelines and regulations. Written consent was obtained from parents or legal guardians of each participant.
Functional MRI image acquisition
Participants were instructed to close their eyes but stay awake, relax, and remain motionless throughout the scan. The head was stabilised using sponges, and sedatives were administered in case of excessive movement. A 3.0-Tesla Siemens MR scanner (MAGNETOM Prisma; SIEMENS Healthineers, Erlangen, Germany) at Korea University Guro Hospital was used. Structural data were scanned using a T1-weighted magnetisation-prepared rapid gradient-echo sequence (repetition time: 2,300 ms; echo time: 2.32 ms; inversion time: 900 ms; flip angle: 8°; field of view: 230 mm; voxel size: 0.9 mm isotropic; 208 slices; generalised auto-calibrating partially parallel acquisition acceleration factor: factor of two along the phase-encoding direction; received bandwidth per pixel: 200 Hz/pixel; echo spacing: 7.1 ms). Resting-state fMRI scans were acquired through 200 contiguous echo-planar imaging volumes of the whole brain (repetition time: 2,000 ms; echo time: 30 ms; flip angle: 70°; field of view: 224 mm; voxel size: 2.0 mm isotropic; multi-slice mode; interleaved; simultaneous multi-slice factor: three; phase-encoding shift factor: two; received bandwidth per pixel: 2,480 Hz/pixel; echo spacing: 0.55 ms). Images were manually inspected for abnormalities and artefacts.
Brain image preprocessing and analysis
Data were preprocessed and analysed using the CONN-fMRI Functional Connectivity toolbox v20b (Whitfield-Gabrieli and Nieto-Castanon, Reference Nieto-Castanon2012) (http://www.nitrc.org/projects/conn), MATLAB R2019a (MathWorks, Natick, MA, USA), and SPM12 (The Wellcome Department of Cognitive Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm/software/spm12). All sequences were preprocessed using the default pipeline of the CONN toolbox to remove physiological confounds such as head movements: resampling to 2-mm voxels, realigning, unwarping, centring, slice time correction, normalisation using the Montreal Neurological Institute (MNI) echo-planar imaging template, smoothing using an 8-mm Gaussian kernel, and outlier detection. Data were denoised, and signal data from the cerebrospinal fluid and white matter were regressed out and processed with a band-pass filter of 0.008–0.09 Hz to reduce noise effects from physiological sources and scanner drift. Additional image scrubbing, using ART (artefact detection tools) in the CONN toolbox with a frame-wise displacement threshold of 0.5, outlier timepoints at 97th percentiles, global signal Z-scores of 5, and linear de-trending were performed.
Statistical analysis
ROI-to-ROI maps for the whole brain were constructed using the toolbox. All ROIs were defined using the toolbox, based on the Harvard-Oxford atlas. The mean time series of each seed region was used as a predictor in a multiple regression general linear model at each ROI. An analysis of covariance controlling for age, sex, and IQ was performed between the patients and controls. Graph theory parameters of the whole brain network were calculated using the toolbox with a false discovery rate (FDR) adjusted p < 0.05 as the threshold. The following parameters were analysed.
Degree
Degree refers to the number of edges from/to each node. It represents a measure of network centrality, showing the degree of local connectedness of the ROI (Nieto-Castanon, Reference Nieto-Castanon2020).
Betweenness centrality
Betweenness centrality also represents the centrality of a node. It is the proportion of times that a certain node is part of a shortest-path between any two nodes within the graph (Nieto-Castanon, Reference Nieto-Castanon2020).
Global efficiency
Global efficiency is the average of inverse-distances between a node and all other nodes in the network. It represents a measure of centrality within the network, showing the degree of global connectedness of the node (Nieto-Castanon, Reference Nieto-Castanon2020).
Local efficiency
Local efficiency refers to the global efficiency of a node within the neighbouring sub-graph of the node. It represents a measure of local coherence (Nieto-Castanon, Reference Nieto-Castanon2020).
ROIs that showed significant differences in the data-driven network analysis were considered to have high/low centrality within the network and were selected as seed areas for a hypothesis-driven ROI-to-ROI resting-state functional connectivity (rsFC) analysis. The threshold for ROI-to-ROI connections was set as uncorrected p < 0.05 for assessing statistical significance.
Statistical analyses of clinical and demographic data were performed using SPSS version 23 (IBM Corp., Armonk, NY, USA). The significance level was set at p < 0.05. Means and standard deviations for demographic and clinical data were calculated.
Results
Demographics and clinical characteristics
Several enrolled participants were excluded from analysis based on our exclusion criteria. Out of the 39 patients one patient was excluded due to an IQ under 70, one patient was excluded due to co-diagnosis of obsessive-compulsive disorder (OCD), two patients were excluded due to imaging artefacts, and 13 patients failed to undergo a full MRI scan due to motion artefacts. Two out of the 37 controls were reassigned to the patient group after clinical assessment, one participant was excluded due to poor image quality, and eight failed to undergo MRI scans. A total of 22 patients (14 males, 4 females) and 26 controls (13 males, 9 females) were included in the final analysis, and 6 patients were co-diagnosed with ADHD. Details are shown in table 1.
Table 1. Demographics and clinical measures

* p < 0.05.
Network analysis between patients and controls
All four graph theory measures were analysed for all ROIs. Degree, betweenness centrality, and local efficiency did not show any significant differences between patients and controls. The left inferior frontal gyrus pars opercularis showed significantly higher global efficiency in the patient group (β = 0.06, T = 3.96, p-FDR = 0.0428). All graph theory measures did not show significant associations with YGTSS scores or other clinical measures. Table 2 shows statistical details and Fig. 1 shows a graphical representation.

Figure 1. The inferior frontal gyrus pars opercularis showing significantly increased network hubness and resting state functional connectivity in tic disorder patients. Left: inferior frontal gyrus pars opercularis, left. Right: inferior frontal gyrus pars opercularis, both.
Table 2. Network analysis and ROI-to-ROI analysis between patients and controls
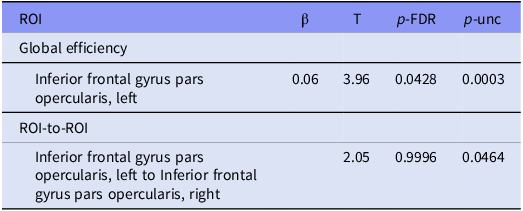
Abbreviations: ROI, region of interest; p-FDR, false discovery rate adjusted p-value; p-unc, uncorrected p-value.
ROI-to-ROI analysis between patients and controls
Tic disorder patients and healthy controls showed different functional connectivities between various brain areas. ROIs with significant centrality were used as seed ROIs for analysis. The rsFC between the left inferior frontal gyrus pars opercularis and the right inferior frontal gyrus pars opercularis (T = 2.05, p = 0.0464) was shown to be higher in the patient group. Table 2 includes detailed statistics on the ROI-to-ROI analysis and Fig. 1 shows a graphical representation. Fig. 2 shows connectome ring representations of the ROI of significant network centrality, as well as the connectome ring of all significant rsFC differences between patients and controls.

Figure 2. ROI-to-ROI connectome ring. Left: connectome ring of all significant ROI-to-ROI interactions; right: significant connection between left and right inferior frontal gyrus pars opercularis.
Discussion
One of the strengths of this study is in the participant selection. Although it is not a perfectly homogeneous group, efforts to maintain the highest possible level of homogeneity aimed to minimise the variability typically seen in more diverse groups. This approach ensures that the observed neuroimaging patterns are more confidently attributed to tic disorders rather than confounded by other co-existing psychiatric conditions. It is already widely known that a notable subset of tic disorder patients is co-diagnosed with OCD. Decades of research have been invested on this topic, and even though a clear explanation has yet to be provided, we are aware that tic disorder patients, OCD patients, and patients with both disorders show many significant differences (Coffey et al., Reference Coffey, Miguel, Biederman, Baer, Rauch, O’Sullivan, Savage, Phillips, Borgman, Green-Leibovitz, Moore, Park and Jenike1998; Lebowitz et al., Reference Lebowitz, Motlagh, Katsovich, King, Lombroso, Grantz, Lin, Bentley, Gilbert, Singer, Coffey, Kurlan and Leckman2012). We therefore decided to only include patients without OCD to maximise homogeneity.
Our analysis revealed heightened network centrality within the left inferior frontal gyrus pars opercularis. This elevated centrality indicates that this region exhibits increased hubness in patients with tic disorders, suggesting its enhanced significance within their neural network. The pars opercularis is the part of the inferior frontal gyrus that is posterior to the ascending ramus of the lateral sulcus, overlying the insular cortex. This region was known to associate with recognising spoken language as it overlaps with a segment of Broca’s area (Schremm et al., Reference Schremm, Novén, Horne, Söderström, van Westen and Roll2018) and was also believed to play a central role in executive control and cognitive inhibition (Aron et al., Reference Aron, Robbins and Poldrack2004). Recent advancements in neuroimaging have underscored the significance of this region in inhibitory controls (Swick et al., Reference Swick, Ashley and Turken2008). Notably, lesions in the right inferior frontal gyrus have been correlated with deficits in stop-signal inhibition (Aron et al., Reference Aron, Fletcher, Bullmore, Sahakian and Robbins2003). A non-invasive study employing theta burst transcranial magnetic stimulation on this region also revealed augmented inhibitory control in patients with nicotine addiction (Newman-Norlund et al., Reference Newman-Norlund, Gibson, McConnell and Froeliger2020). Based on these and other related studies, it has been concluded that this region is posited to have a unique association with motor inhibition. Interestingly, the inhibitory function of the right inferior frontal gyrus appears to manifest predominantly in later stages of life, while the left inferior frontal gyrus demonstrates heightened inhibitory activity in children (Bunge et al., Reference Bunge, Dudukovic, Thomason, Vaidya and Gabrieli2002). Similar to its right counterpart, lesions of the left inferior frontal gyrus were also linked to deficits in GoNoGo tasks (Swick et al., Reference Swick, Ashley and Turken2008). Altogether, these findings highlight the critical role of both right and left inferior frontal gyri in response inhibition, a hallmark characteristic that is affected in tic disorder. A recent study observing increased surface curvature of the pars opercularis and the pars triangularis in tic disorder patients compared to controls further substantiates this assertion (McCann et al., Reference McCann, Lam, Shiohama, Ijner, Takahashi and Levman2022). The authors also suggested that abnormalities of this region are possibly associated with vocal tics, since damage to this area may give rise to hesitant speech that is also evident in Broca’s aphasia, but it has to be noted that the brain regions responsible for language and inhibition do not completely overlap, and there is still a lack of research supporting the idea that regions associated with language also play a role in inhibition. A diffusion tensor imaging (DTI) study on adults with tic disorder showed decreased fractional anisotropy of several brain regions including the left pars opercularis, further suggesting that alterations of this area are related to the inhibitory impairments in tic disorder (Müller-Vahl et al., Reference Müller-Vahl, Grosskreutz, Prell, Kaufmann, Bodammer and Peschel2014). This was also demonstrated in a study that observed impaired ability to stop an action while the right pars opercularis was temporarily deactivated by repetitive transcranial magnetic stimulation (Chambers et al., Reference Chambers, Bellgrove, Stokes, Henderson, Garavan, Robertson, Morris and Mattingley2006). Collectively, these findings suggest the pars opercularis’s involvement in the inhibitory mechanism governing tics. Increased hubness of this area can be hypothesised to be an acquired change in response to frequent motor tics during the developmental ages. This ‘secondary neuroplastic compensatory mechanism’ in tic disorders was already proposed by many researchers including Muller-Vahl and others (Müller-Vahl et al., Reference Müller-Vahl, Grosskreutz, Prell, Kaufmann, Bodammer and Peschel2014). Such compensatory adaptations are thought to result from continuous tic suppression, also possibly linked to the known decline of tic symptoms with increasing age (Peterson et al., Reference Peterson, Staib, Scahill, Zhang, Anderson, Leckman, Cohen, Gore, Albert and Webster2001).
A study by Openneer et al., also investigated the brain network of tic disorder by comparing patients with tic disorder, ADHD, and healthy controls (Openneer et al., Reference Openneer, Marsman, van der Meer, Forde, Akkermans, Naaijen, Buitelaar, Dietrich and Hoekstra2020). Patients were also divided into groups with and without a co-diagnosis of ADHD. The authors reported that tic disorder patients with ADHD showed lower local efficiency and clustering coefficients in the default-mode network compared to controls, and in the fronto-parietal network when compared to ADHD patients. The pars opercularis, which was significant in our study, does not traditionally belong to one of those networks, so our results do not exactly match those of Openneer et al., Our results show increased network centrality in tic disorder patients whereas Openneer et al., reported decreased network centrality in the patient group. Of course, it must be noted that the two studies had different patient groups (all tic disorder patients vs. only tic disorder patients with ADHD), but the different results indicate that tic disorder is a complicated mixture of various brain activities.
Our study is not without limitations. First, the small number of enrolled patients is a major limitation of all tic disorder studies. The young age and frequent movement inherent to tic disorder patients make it difficult to obtain high quality MRI data. Nonetheless, it is crucial to highlight the high homogeneity and drug-naïve status of our patient group. Second, the cross-sectional design of the study made it impossible to verify our results through a prospective time period. Third, it can be observed that the YGTSS scores of the enrolled patients are not very high. In addition, to participate in the study, children and adolescents with motor tics had to be able to lie still and cooperate with the MRI for a certain amount of time. This was a limiting factor for participants with severe, uncontrolled tics. Therefore, some participants with severe tics were not able to participate in the study. This contributed to lower YGTSS scores in those who were able to participate. Fourth, there is a gender imbalance in the study population. While it is expected that more males are affected due to the nature of the disorder, it is undeniable that a more balanced sample would have been more appropriate for the study.
We did not separate patients with comorbid ADHD. Although some previous studies suggested that tic disorders with comorbid ADHD may have different pathophysiological mechanisms compared to those without ADHD, these patients were not excluded due to practical constraints in the study design. In future research, we aim to address this by using a larger sample size to enable such a distinction. Nevertheless, our study suggests that the brains of tic disorder patients may exhibit different developmental traces when compared to controls, as they are continuously inhibiting tics during their developmental ages. These findings will aid in unveiling the pathophysiology of tic disorders. Further research and follow-up studies are necessary to verify our results and to develop clinical applications.
Conclusion
This study revealed that tic disorder patients have increased network hubness in the left inferior frontal gyrus pars opercularis. This region also showed higher rsFC with its right counterpart in the patient group relative to controls. This area is considered to be linked with inhibitory mechanisms, and its increased hubness could be associated to the constant suppression of frequent tics during developmental years.
Data availability
Please contact the corresponding author for data and material.
Acknowledgements
None.
Author contributions
S.C contributed to drafting and data analysis.
Y.M., J.K., and J.G. contributed to data gathering and data analysis.
M.L. contributed to drafting and managed the whole project.
All authors reviewed and approved the final manuscript.
Financial support
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI21C0012).
Competing interests
The authors declare none.
Ethics standard
The research processes were approved by the Institutional Review Board (IRB) of the Korea University Guro Hospital (2021GR0275). All research methods were performed in accordance with the relevant guidelines and regulations.
Consent to participate
Written informed consent was obtained from parents or legal guardians (if parents not available) of all participants.
Consent for publication
Written informed consent for publishing was obtained from parents or legal guardians (if parents not available) of all participants.







