Significant outcomes
-
Cannabidiol does not bind orthosterically to A2AR.
-
Cannabidiol reduces the functionality of A2AR
Limitations
-
This is an in vitro study, and results cannot be directly extrapolated to in vivo conditions.
-
Putative allosteric binding of CBD to A2AR cannot be confirmed or ruled out with the luminescence-based techniques employed in this study.
Introduction
Cannabidiol (CBD) is a phytocannabinoid isolated from Cannabis sativa without psychoactive properties, but with potential benefits against multiple pathological conditions (ElSohly et al., Reference ElSohly, Radwan, Gul, Chandra, Galal, Kinghorn, Falk, Gibbons and Kobayashi)2017). Several preclinical reports demonstrated protective and anti-inflammatory effects of CBD in a wide spectrum of neurodegenerative diseases, neuroinflammatory processes, stroke, colitis, liver, kidney injury, cardiovascular disease, arthritis, sepsis, diabetes, cancer, and epilepsy models (Pacher et al., Reference Pacher, Kogan and Mechoulam2020). Furthermore, CBD exerted positive effects in experimental models of other neuropsychiatric disorders such as epilepsy, anxiety, schizophrenia, dementia, addiction, and neonatal hypoxic-ischemic encephalopathy (Devinsky et al., Reference Devinsky, Cilio, Cross, Fernández-Ruiz, French, Hill, Katz, Di Marzo, Jutras-Aswad, Notcutt, Martínez-Orgado, Robson, Rohrback, Thiele, Whalley and Friedman2014). Although its translation to clinical trials is somewhat limited to date, the successful case of Epidiolex®, an oral solution based on a botanical extract containing purified CBD, is notable. Epidiolex® was approved by the US Food and Drug Administration in 2018 for the treatment of Lennox-Gastaut and Dravet syndromes, two rare and debilitating genetic forms of epilepsy in children. Additionally, CBD is currently under clinical evaluation for other conditions, including different forms of pain, obsessive-compulsive disorders, and behavioural problems associated with intellectual disability or autism, among others (ClinicalTrials.gov database).
Despite growing interest in its potential clinical applications, the mechanism(s) of action of CBD require further exploration. CBD has a very low affinity for the orthosteric site of CB1 and CB2 receptors, the main G protein-coupled receptors (GPCRs) that belong to the endogenous cannabinoid system (McPartland et al., Reference McPartland, Duncan, Di Marzo and Pertwee2015). Alternatively, CBD can act on multiple targets, including TRPV1 channels and PPARγ, adenosine A2A, 5-HT1A, α3-glycine, α1-adrenal, dopamine D2, GABAA, μ- and δ-opioid receptors (McPartland et al., Reference McPartland, Duncan, Di Marzo and Pertwee2015). Additionally, CBD can inhibit the activity of GPR55 (Ryberg et al., Reference Ryberg, Larsson, Sjögren, Hjorth, Hermansson, Leonova, Elebring, Nilsson, Drmota and Greasley2007), an effect that has been associated with its antiepileptic activity (Sylantyev et al., Reference Sylantyev, Jensen, Ross and Rusakov2013). In the present study, we probed the putative direct effects of CBD on adenosine A2A receptors (A2ARs). The relevant role that A2ARs play in several of the neuropsychiatric disorders in which CBD could offer beneficial effects (i.e. dementia, schizophrenia, epilepsy, depression, anxiety) supports this interest (Domenici et al., Reference Domenici, Ferrante, Martire, Chiodi, Pepponi, Tebano and Popoli2019). Furthermore, previous preclinical evidence supports the participation of A2AR in CBD-mediated effects. Thus, A2AR antagonists blocked the anti-inflammatory effects of CBD (Liou et al., Reference Liou, Auchampach, Hillard, Zhu, Yousufzai, Mian, Khan and Khalifa2008; Ribeiro et al., Reference Ribeiro, Ferraz-de-Paula, Pinheiro, Vitoretti, Mariano-Souza, Quinteiro-Filho, Akamine, Almeida, Quevedo, Dal-Pizzol, Hallak, Zuardi, Crippa and Palermo-Neto2012; Mecha et al., Reference Mecha, Feliú, Iñigo, Mestre, Carrillo-Salinas and Guaza2013; Oláh et al., Reference Oláh, Tóth, Borbíró, Sugawara, Szöllõsi, Czifra, Pál, Ambrus, Kloepper, Camera, Ludovici, Picardo, Voets, Zouboulis, Paus and Bíró2014), or the ability of CBD to blunt Δ9-THC-induced cognitive impairment (Aso et al., Reference Aso, Fernández-Dueñas, López-Cano, Taura, Watanabe, Ferrer, Luján and Ciruela2019). Similarly, the genetic deletion of A2AR reduced the CBD-induced potentiation of the cataleptic and anxiolytic properties of Δ9-THC (Stollenwerk et al., Reference Stollenwerk, Pollock and Hillard2021). This A2AR-dependent activity of CBD was proposed to depend on the ability of CBD to bind to the equilibrative nucleoside transporter (ENT). Thus, inhibition of adenosine uptake would lead to indirect activation of A2AR (Pandolfo et al., Reference Pandolfo, Silveirinha, dos Santos-Rodrigues, Venance, Ledent, Takahashi, Cunha and Köfalvi2011). However, a direct effect of CBD on A2AR has not been further investigated. Here we aimed to evaluate the capacity of CBD to bind to the orthosteric site of A2AR and/or to modify its intrinsic activity by using state-of-the-art luminescence-based assays.
Materials and methods
Reagents
The ligands used were CGS21680, ZM241385, and CBD (Tocris Bioscience, Bristol, United Kingdom). MRS7396, a fluorescent selective A2AR orthosteric antagonist derived from SCH442416 and containing a BODIPY630/650 fluorophore, was previously described (Duroux et al., Reference Duroux, Ciancetta, Mannes, Yu, Boyapati, Gizewski, Yous, Ciruela, Auchampach, Gao and Jacobson2017). Other reagents used were Dulbecco’s modified Eagle’s medium (DMEM; Sigma Aldrich, St Louis, MO, USA), geneticin (Santa Cruz Biotechnology, Dallas, TX, USA), adenosine deaminase (ADA; Roche Diagnostics GmbH, Mannheim, Germany), zardaverine (Calbiochem, San Diego, CA, USA), and coelenterazine 400a (NanoLight Technologies, Pinetop, AZ, USA).
Plasmid constructs
To perform bioluminescence resonance energy transfer (BRET) experiments and cAMP accumulation assays, we used the A2AR NanoLuciferase (NanoLuc) sensor (A2ARNL), previously described (Lanznaster et al., Reference Lanznaster, Massari, Marková, Šimková, Duroux, Jacobson, Fernández-Dueñas, Tasca and Ciruela2019). To perform the NanoBiT™ assay, the cDNA encoding human A2AR was cloned at the BamHI/EcoRV restriction enzyme sites of pIREShyg3-SmBiT (Promega, Madison, WI, USA), as previously described (Sarasola et al., Reference Sarasola, Del Torrent, Pérez-Arévalo, Argerich, Casajuana-Martín, Chevigné, Fernández-Dueñas, Ferré, Pardo and Ciruela2022). The construct (A2ARSmBiT) was verified by DNA sequencing. The plasmid encoding the mini-Gαs (engineered GTPase domain of Gα subunit; LgBiTmini-Gαs) linked to LgBiT was previously described (Wan et al., Reference Wan, Okashah, Inoue, Nehmé, Carpenter, Tate and Lambert2018; Meyrath et al., Reference Meyrath, Palmer, Reynders, Vanderplasschen, Ollert, Bouvier, Szpakowska and Chevigné2021).
Cell culture and transfection
Human embryonic kidney (HEK)-293T cells were grown in DMEM supplemented with 1 mM sodium pyruvate (Biowest, Nuaillé, France), 2 mM L-glutamine (Biowest), 100 U/mL streptomycin (Biowest), 100 mg/mL penicillin (Biowest), and 5% (v/v) foetal bovine serum (Invitrogen Corporation, Camarillo, CA, USA) at 37°C and in an atmosphere of 5% CO2. Cells were transiently transfected with the indicated cDNA construct using polyethylenimine (PEI, 1 mg/mL, Sigma Aldrich), as previously described (Longo et al., Reference Longo, Kavran, Kim and Leahy2013). Finally, HEK-293T cells stably expressing A2ARNL were grown in the presence of geneticin (1 mg/mL).
NanoBRET experiments
The NanoBRET assay was performed as previously described (Lanznaster et al., Reference Lanznaster, Massari, Marková, Šimková, Duroux, Jacobson, Fernández-Dueñas, Tasca and Ciruela2019). Briefly, HEK-293T cells expressing the A2ARNL construct were resuspended in Hank’s balanced salt solution (HBSS; Thermo Fisher, Waltham, MA, USA) containing ADA (0.5 U/mL) and plated on white 96-well plates coated with poly-ornithine (Corning, Corning, NY, USA) at a density of 20,000 cells/well. After 24 h, cells were challenged with the fluorescent A2AR antagonist (MRS7396) in the absence/presence of ZM241385 or CBD and incubated for 1 h at 37°C. Subsequently, coelenterazine 400a was added at a final concentration of 1 μM, and the readings were performed after 15 min using a CLARIOStar microplate reader (BMG Labtech, Durham, NC, USA). Donor and acceptor emission were measured at 490 ± 10 nm and 650 ± 40 nm, respectively. The raw NanoBRET ratio was calculated by dividing the 650 nm emission by the 490 nm emission and the values fitted by nonlinear regression using GraphPad Prism 9 (GraphPad Software, La Jolla, CA, USA). The results were expressed as a percentage of the maximum signal obtained (mBU; miliBRET units).
NanoBiT assay
The NanoBiT™ assay (Promega) was performed as previously described (Sarasola et al., Reference Sarasola, Del Torrent, Pérez-Arévalo, Argerich, Casajuana-Martín, Chevigné, Fernández-Dueñas, Ferré, Pardo and Ciruela2022). Briefly, transient transfected HEK-293T cells with A2ARSmBiT and LgBiTmini-Gαs were resuspended in HBSS containing ADA (0.5 U/mL) and transferred (90 µl) into white 96-well plates (Corning). Subsequently, coelenterazine 400a was added (1 μM) to each well. After 15-minute incubation, basal luminescence was determined using a CLARIOstar plate reader (BMG Labtech). Immediately after the initial measurement (basal), the ligands were added, and the luminescent signal was measured every 5 min for 30 min. The luminescence signal (RLU) was normalised as follows: (RLUsample − RLUbasal) / RLUbasal.
cAMP assay
cAMP accumulation was measured using the LANCE® Ultra cAMP Kit (PerkinElmer, Waltham, MA, USA) as previously described (Lanznaster et al., Reference Lanznaster, Massari, Marková, Šimková, Duroux, Jacobson, Fernández-Dueñas, Tasca and Ciruela2019). Briefly, HEK-293T cells stably expressing the A2ARNL construct were first incubated for 1 h at 37°C with stimulation buffer (BSA 0.1%, ADA 0.5 units/mL, zardaverine 2 µM; in serum-free DMEM) and later with CGS21680 (100 nM) and increasing concentrations of ZM241385 or CBD for 30 min at 37°C. Subsequently, cells were transferred (1000 cells/well) into white 384-well plates (Corning), in which reagents were added following the manufacturer’s instructions. After 1 h at room temperature, time-resolved fluorescence resonance energy transfer (TR-FRET) was determined by measuring light emission at 620 nm and 665 nm using a CLARIOstar plate reader (BMG Labtech).
Statistics
Data are represented as mean ± standard error of mean (SEM) with statistical significance set at P < 0.05. The number of samples (n) in each experimental condition is indicated in the legend of the corresponding figure. Outliers were assessed using the ROUT method (Motulsky & Brown, Reference Motulsky and Brown2006); thus, no sample was excluded assuming a Q value of 1% in GraphPad Prism 9. Comparisons between experimental groups were performed using one-way factor analysis of variance (ANOVA) followed by Dunnett’s multiple comparisons post hoc test using GraphPad Prism 9 as indicated.
Results
To assess the impact of CBD on A2AR functionality, we initially evaluated whether CBD modified the binding of MRS7396, a fluorescent A2AR antagonist. To this end, we took advantage of a previously reported NanoBRET-based A2AR binding assay (Fig. 1a) (Lanznaster et al., Reference Lanznaster, Massari, Marková, Šimková, Duroux, Jacobson, Fernández-Dueñas, Tasca and Ciruela2019). HEK-293T cells permanently expressing the A2ARNL construct were challenged with increasing concentrations of MRS7396, which upon binding to the receptor can act as a compatible acceptor in a BRET process (Fig. 1a). As expected, a saturable hyperbolic curve was obtained for the total binding of MRS7396, which was blocked upon incubation with a saturating concentration (1 µM) of the unlabelled A2AR antagonist ZM241385 (nonspecific binding; Fig. 1b). The analysis of the specific binding of MRS7396 revealed a dissociation constant (K D) of 8.5 ± 3.2 nM and a maximum binding capacity (B max) of 98.9 ± 8.4 %. Next, upon the same experimental conditions, we examined the ability of CBD to attenuate MRS7396 binding. Differently from ZM241385, CBD (1 µM) was unable to modify the specific binding of MRS7396 to the A2AR (Fig. 1b). Accordingly, no significant differences in affinity (K D) and receptor capacity (B max) were found in the presence of CBD (KD = 8.2 ± 2.7 nM; Bmax = 96.9 ± 7.2 %; P = 0.992, F (2, 34) = 0.0079). In addition, we also assessed whether CBD could modulate orthosteric binding of A2AR by performing a competition binding assay with a fixed concentration of MRS7396 (30 nM). Again, while ZM241385 blocked A2AR binding, CBD did not significantly modify the NanoBRET signal (Fig. 1c; P = 0.269, F (4, 10) = 1.518). Collectively, these results indicate that CBD does not bind orthosterically to A2AR.
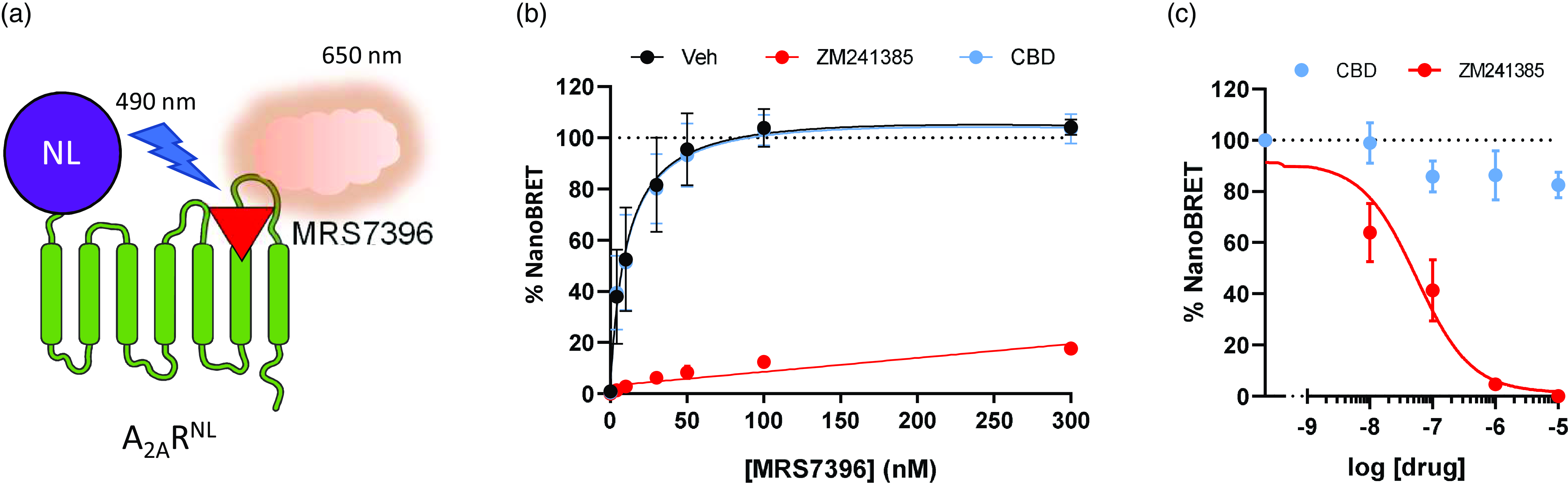
Figure 1. Determination of CBD effects on A2AR ligand binding affinity. (a) Schematic representation of the NanoBRET assay. A nanoluciferase is linked to the N-terminal part of the A2AR (A2ARNL). When the nanoluciferase substrate coelenterazine is added, A2ARNL (donor) emits light at 490–10 nm. Light excites the fluorescent selective A2AR ligand, MRS7396 (acceptor), which subsequently emits fluorescence at 650-80 nm. (b) NanoBRET saturation binding curves obtained by challenging A2ARNL expressing HEK-293T cells with increasing concentrations of MRS7396 in the absence/presence of CBD (1 µM) or ZM241385 (1 µM). (c) NanoBRET signals obtained by challenging A2ARNL expressing HEK-293T cells with a fixed concentration of MRS7396 (30 nM, normalised to 100%) in the presence of increasing concentrations of CBD or ZM241385. The represented data are mean ± SEM of three independent experiments each performed in triplicate.
Subsequently, we aimed to determine whether CBD affected A2AR signalling. To this end, we first evaluated the interaction of A2AR with mini-Gαs protein using the NanoBiT™ complementation assay (Sarasola et al., Reference Sarasola, Del Torrent, Pérez-Arévalo, Argerich, Casajuana-Martín, Chevigné, Fernández-Dueñas, Ferré, Pardo and Ciruela2022). Accordingly, cells were transiently transfected with the A2ARSmBiT and LgBiTmini-Gαs constructs, which once expressed allow the reconstitution of the split nanoluciferase and real-time recordings of receptor-effector coupling induced by agonists (Fig. 2a). Cells were challenged with the selective A2AR agonist CGS21680 (100 nM), which induced a rapid increase in the luminescent signal, reaching a peak at 15 min that remained stable for 30 min. This effect was absent in cells challenged with CBD alone (Fig. 2b). Notably, this agonist-dependent A2AR interaction with mini-Gαs protein was completely blocked when co-incubating cells with ZM241385 (50 nM, Fig. 2b). Next, we assessed CGS21680-induced A2AR coupling to Gαs protein in the presence of increasing concentrations of CBD. Interestingly, CBD dose-dependently blocked A2AR coupling to Gαs protein, both decreasing the maximum peak and the density of the effect (Fig. 2b). Of note, differently from ZM241385, CBD only led to a partial blockade of CGS21680-mediated effects.

Figure 2. Assessment of CBD effects on A2AR intrinsic activity. (a) Schematic representation of the NanoBIT™-based assay. The two fragments of nanoluciferase, small (SmBIT) and large (LgBIT), are fused to A2AR and mini-Gαs protein, respectively. Then, upon agonist binding, A2AR intrinsic activity is assessed by receptor recruitment of Gαs, which induces an increase on luminescence due to nanoluciferase reconstitution. (b) Representative time-course of A2AR agonist-mediated Gαs recruitment. The selective A2AR agonist CGS21680 was challenged to A2ARSmBiT and LgBiTmini-Gαs expressing HEK-293T cells in the absence/presence of increasing concentrations of CBD or ZM241385. The luminescent signal obtained after reconstitution of the nanoluciferase was assessed by calculating the area under the curve for each condition. Data are shown as mean ± SEM of three independent experiments with five replicates. *P < 0.05, ***P < 0.001, one-way ANOVA with Dunnett’s post hoc test. (c) cAMP accumulation was assessed on HEK-293T cells permanently expressing the A2ARNL. Cells were challenged with the selective A2AR agonist CGS21680 (100 nM, normalised to 100% of effect) in the absence/presence of increasing concentrations of CBD. Data are expressed as mean ± SEM of four independent experiments performed in triplicates. *P < 0.05, one-way ANOVA with Dunnett’s post hoc test.
Finally, to further characterise the effects of CBD on the intrinsic activity of A2AR, we evaluated agonist-induced cAMP accumulation in cells permanently expressing the A2ARNL construct and challenged with CGS21680 in the presence/absence of CBD. Interestingly, although CBD itself did not modify cAMP levels, it was able to dose-dependently block CGS21680-induced cAMP levels (Fig. 2c). Again, differently from ZM241385, a full-antagonist, CBD partially blocked A2AR agonist increase of cAMP levels. Overall, these results are compatible with the notion that CBD acts as a weak A2AR negative allosteric modulator.
Discussion
CBD is a promising drug for several pathologies (ElSohly et al., Reference ElSohly, Radwan, Gul, Chandra, Galal, Kinghorn, Falk, Gibbons and Kobayashi)2017). Accordingly, unravelling its precise mechanism of action is relevant in the progress towards its clinical development. Here, we reveal that CBD does not affect the binding of an A2AR orthosteric ligand, but it is capable of negatively modulating agonist-induced interaction with the Gαs protein at sub-micromolar concentrations (≥100 nM), thus reducing receptor signalling (i.e. cAMP generation). Therefore, we disclose a new non-competitive interaction of CBD with A2AR.
The effect of CBD on A2AR could operate through a new allosteric site at the receptor. However, further experiments (i.e. using labelled CBD) would be needed to confirm this hypothesis. On the other hand, we cannot rule out other mechanisms of action for CBD different from classical allosteric drugs. In this sense, previous evidence indicates that other lipids, including the endogenous cannabinoid anandamide at micromolar concentrations, might act as allosteric modulators of other GPCRs through a membrane-perturbing effect that is sensitive to receptor conformation (Lanzafame et al., Reference Lanzafame, Guida and Christopoulos2004; Van der Westhuizen et al., Reference Van der Westhuizen, Valant, Sexton and Christopoulos2015). Further studies are needed to assess this putative CBD-mediated membrane effect on A2AR-Gαs protein coupling. Similarly, CBD could indirectly modify A2AR functioning by interacting with equilibrative nucleoside transporter 1 (ENT1), as was previously demonstrated in striatal synaptosomes (Pandolfo et al., Reference Pandolfo, Silveirinha, dos Santos-Rodrigues, Venance, Ledent, Takahashi, Cunha and Köfalvi2011). However, this hypothetical CBD effect on ENT seems not to play a relevant role in vivo, since a recent study demonstrated that CBD lacks the ability to substantially raise endogenous adenosine levels by using the hypothermia mouse model (Xiao et al., Reference Xiao, Gavrilova, Liu, Lewicki, Reitman and Jacobson2023). These discrepancies between in vitro and in vivo studies could be also explained by the fact that A2AR can form heteromers with other GPCRs, including CB1R (Carriba et al., Reference Carriba, Ortiz, Patkar, Justinova, Stroik, Themann, Müller, Woods, Hope, Ciruela, Casadó, Canela, Lluis, Goldberg, Moratalla, Franco and Ferré2007; Ferré et al., Reference Ferré, Lluís, Justinova, Quiroz, Orru, Navarro, Canela, Franco and Goldberg2010; Aso et al., Reference Aso, Fernández-Dueñas, López-Cano, Taura, Watanabe, Ferrer, Luján and Ciruela2019), in physiological conditions different from that obtained in heterologous expression systems. The assembly of A2AR-containing heteromers leads to changes in the agonist recognition, signalling, and trafficking, which might result in different A2AR activity in the presence of CBD.
Although we evaluated the effects of CBD in cultured cells expressing A2AR, these results could be relevant for many disorders in which A2AR activity increases. For example, in certain inflammatory processes and cardiovascular diseases, but also in pathological conditions that affect the central nervous system, such as Alzheimer’s disease, Parkinson’s disease, attention deficit hyperactivity disorder, fragile X syndrome, depression, or anxiety (Domenici et al., Reference Domenici, Ferrante, Martire, Chiodi, Pepponi, Tebano and Popoli2019). A2ARs, which are widely expressed both in neurons and glia, are mainly found in the dorsal and ventral striatum and other nuclei of the basal ganglia, where they play a key role in the control of voluntary movements, as well as in motivational, emotional and cognitive processes (Sebastião and Ribeiro, Reference Sebastião and Ribeiro2009). In this way, A2ARs are involved in regulating the release of neurotransmitters and contribute to the homeostatic control of synaptic transmission and brain function (Sebastião and Ribeiro, Reference Sebastião and Ribeiro2009). In general, our results are consistent with the positive effects reported for CBD in various brain disorders that can be associated with an exacerbated A2AR function, where CBD would tone down A2AR hyperactivity.
Overall, the present study provides evidence on the ability of CBD to negatively modulate A2AR signalling. The CBD-mediated negative modulation of A2AR function is restricted to the receptor-effector coupling and does not interfere with the binding of the orthosteric ligand. Accordingly, we provide a new and genuine pharmacological way to modulate the adenosinergic system in pathological conditions in which A2AR function is increased.
Acknowledgements
We thank Centres de Recerca de Catalunya Programme/Generalitat de Catalunya for IDIBELL institutional support and Maria de Maeztu MDM-2017-0729 to Institut de Neurociencies, Universitat de Barcelona. We are also grateful to the CannaLatan network members (CYTED-Programa Iberoamericano de Ciencia y Tecnología para el Desarrollo) for the fruitful discussion about the results.
Author contribution
EA, VFD, and FC conceived and designed the study and wrote the manuscript. NSF performed all the experiments and contributed to the manuscript preparation. FC and AC designed the cDNA constructs used in this study. LGA, LIS, and JA cloned and validated cDNA constructs. KAJ provided the fluorescent selective A2AR antagonist.
Financial support
This study was supported by Ministerio de Ciencia, Innovación y Universidades–Agencia Estatal de Investigación-FEDER funds/European Regional Development Fund – ‘a way to build Europe’ grant RTI2018-097773-A-I00 to EA and PID2020-118511RB-I00 to FC. Founded by MCIN/AEI /10.13039/501100011033 ‘ESF Investing in your future’ grant PRE2018–084480 to JA, grant PRE2019-088153 to NSF and grant FPU19/03142 to LGA. Also supported by ‘Acció instrumental de formació de científics i tecnòlegs’ (SLT017/20/000114) of the Departament de Salut de la Generalitat de Catalunya to LIS. The study was also supported by the Luxembourg Institute of Health (LIH), Luxembourg National Research Fund (INTER/FNRS grants 20/15084569 to AC) and the National Institute of Diabetes and Digestive and Kidney Diseases NIDDK Intramural Research Program (ZIADK031117 to KAJ).
Competing interests
None of the authors declare any conflict of interest.
Ethical standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on experimentation with cells and DNA constructs.





