Significant outcomes
-
Leveraging data that reflect patterns (quantity, frequency, and timing) of prenatal alcohol and tobacco exposure (PAE, PTE) data strengthened ability to statistically detect brain signatures of PAE and PTE in a small cohort of 6-year-olds.
-
Examining PAE, PTE and their interaction yielded three distinct profiles of cortical and subcortical alterations, suggesting teratogenicity of co-exposures is not as simple as being additive or synergistic and rather is highly complex.
-
Community levels of PAE and PTE in a non-clinical sample of 6-year-olds are detectable throughout the brain.
Considerations
-
Research on PAE, PTE and their interaction that includes a range of different QFT features will require larger sample sizes, or targeted neural circuitries and not whole brain, to avoid false-negative findings.
-
Given the dynamic trajectories of brain volumes over time, with significant increases in cortical thickness at ages 5–6 years, and then subsequent thinning when approaching pubertal maturation, it is possible that the outcomes of PAE and PTE are also dynamic, and alterations in brain developmental trajectories following teratogen exposures may overlap. This may result in no observable group differences at certain ages, including around 6 years of age, as in the case in this small study that served as a pilot for a larger study.
Introduction
Prenatal alcohol exposure (PAE) can produce enduring alterations on the developing human brain, which can lead to challenges in a range of physical, behavioural, neurological and mental health. Significant individual variability is observed in the range of these outcomes, theoretically in part, due to differing patterns of PAE and presence/absence of tobacco exposure (PTE) (Cook et al., Reference Cook, Green, Lilley, Anderson, Baldwin, Chudley, Conry, LeBlanc, Loock, Lutke and Mallon2016; McLachlan et al., Reference McLachlan, Paolozza, Kully-Martens, Portales-Casamar, Pavlidis, Andrew, Hanlon-Dearman, Loock, McFarlane, Nikkel and Pei2017). Animal models clearly demonstrate alcohol as a teratogen, with variable PAE effects on brain and cognitive outcomes that depend on quantity, frequency and timing (QFT) of PAE (Sulik, Reference Sulik, Johnston, Daft, Russell, Dehart, Opitz and Reynolds1986). While foetal alcohol spectrum disorder (FASD) is preventable, PAE is in the leading known cause of intellectual and developmental disability (May et al., Reference May, Blankenship, Marais, Gossage, Kalberg, Barnard, De Vries, Robinson, Adnams, Buckley and Manning2013; Popova et al., Reference Popova, Lange, Shield, Mihic, Chudley, Mukherjee, Bekmuradov and Rehm2016; Roozen et al., Reference Roozen, Peters, Kok, Townend, Nijhuis and Curfs2016; Lange et al., Reference Lange, Probst, Gmel, Rehm, Burd and Popova2017), with an estimated 428 comorbidities (i.e. conduct disorder and receptive language disorder; Popova et al., Reference Popova, Lange, Shield, Mihic, Chudley, Mukherjee, Bekmuradov and Rehm2016), making it a major global health concern.
There are several key gaps in FASD human literature. First, most published studies examining the impact of PAE on the human brain have relied on retrospective designs for acquisition of PAE patterns, introducing caregiver/parent recall bias and error, and poor to no data on QFT. Second, much work has focused on clinical samples recruited for known or strongly suspected with heavy PAE, or completely unexposed participants as a comparison group. Thus, FASD literature often excludes community patterns of PAE, particularly regarding mild to moderate PAE. Third, prenatal tobacco exposure (PTE) can also cause deleterious effects on brain and cognitive development (El Marroun et al., Reference El Marroun, Schmidt, Franken, Jaddoe, Hofman, Van Der Lugt, Verhulst, Tiemeier and White2014; Wiebe et al., Reference Wiebe, Clark, De Jong, Chevalier, Espy and Wakschlag2015; El Marroun et al., Reference El Marroun, Tiemeier, Franken, Jaddoe, van der Lugt, Verhulst, Lahey and White2016), as shown in animal studies indicating an interaction between PAE and PTE, (Bhattacharya et al., Reference Bhattacharya, Fujihashi, Majrashi, Bloemer, Bhattacharya, Buabeid, Escobar, Moore, Suppiramaniam and Dhanasekaran2020); however, there is a paucity of human studies that examine this interaction (Odendaal et al., Reference Odendaal, Kruger and Botha2020). Fourth, conducting magnetic resonance imaging (MRI) on young children aged 6 years is challenging due to excessive movement that results in motion artefacts interfering in usable scans; thus, this age range is limited in FASD human brain imaging literature for informing early identification of teratogenic alterations in brain structure.
Historically, the prevalence of FASD in the Cape region of South Africa was much higher than other global regions, with an estimate between 13.6% and 20.9% in a high-risk community sample of South African children in the first grade (May et al., Reference May, Blankenship, Marais, Gossage, Kalberg, Barnard, De Vries, Robinson, Adnams, Buckley and Manning2013), compared to conservative estimates of 1%–5% in the USA (May et al., Reference May, Chambers, Kalberg, Zellner, Feldman, Buckley, Kopald, Hasken, Xu, Honerkamp-Smith and Taras2018). This historical high prevalence reflects the history of South Africa, where there was systematic oppression of black farmworkers, including provision of alcohol in lieu of wages. Ongoing contributing factors to this risk may include prevailing socio-economic disadvantages, psychological distress and depression, exposure to traumatic stressors, and intimate partner violence (Tomlinson et al., Reference Tomlinson, O’Connor, Le Roux, Stewart, Mbewu, Harwood and Rotheram-Borus2014; Stein et al., Reference Stein, Koen, Donald, Adnams, Koopowitz, Lund, Marais, Myers, Roos, Sorsdahl and Stern2015; Koen et al., Reference Koen, Brittain, Donald, Barnett, Koopowitz, Maré, Zar and Stein2016; Donald et al., Reference Donald, Hoogenhout, du Plooy, Wedderburn, Nhapi, Barnett, Hoffman, Malcolm-Smith, Zar and Stein2018).
A longitudinal community birth cohort, the Prenatal Alcohol, Sudden Infant Death Syndrome and Stillbirth (PASS) Network in this region, allows prospective investigation of the impact of a range of prenatal alcohol and potential co-occurring tobacco exposures. Working with PASS, the present study assessed cortical and subcortical brain region sizes among 6 year olds whose parents participated in prospective PAE and PTE data collection with PASS birth cohort and subsequent associations with: (1) PAE, PTE, and their interaction; and (2) patterns of exposure (QFT). No other brain imaging study has examined the relationship between PAE, PTE and brain volumes of children all aged 6 years. However, based on extant literature contrasting cortical volumes in FASD and neurotypical children (Nuñez et al., Reference Nunez, Roussotte and Sowell2011), and in cross-sectional subcortical effects of PAE in older children versus neurotypical (Inkelis et al., Reference Inkelis, Moore, Bischoff-Grethe and Riley2020), it is hypothesised that significant interactions will occur in frontal and basal ganglia regions early enough in development that it is detectable by the age of 6 years.
Materials and methods
Study design and participants
This research was a small substudy embedded in the Safe Passage Study within the PASS Network, Western Cape, South Africa, designed to serve as a pilot for a larger neuroimaging study. This was a unique international community-based prospective birth cohort study investigating the role of PAE in the risk for sudden infant death syndrome (SIDS), stillbirth, foetal alcohol syndrome (FAS) and FASD, funded by the National Institute of Child Health and Human Development (NICHD), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), and the National Institute on Deafness and Other Communication Disorders (NIDCD).
As part of the Safe Passage Study of the PASS Network at Stellenbosch University, South Africa, N = 7,060 pregnant people were recruited from Bishop Lavis and Belhar residential areas between August 2007 and January 2015. These sites were selected on account of a historical reputation for having a high prevalence of PAE and SIDS and the need to include populations where the marked ethnic and socio-economic disparities in SIDS remain understudied. Recruitment of pregnant women for the Safe Passage Study occurred between 6 weeks of gestation and the day admitted for delivery. Methods and timelines are described in full elsewhere (Dukes et al., Reference Dukes, Burd, Elliott, Fifer, Folkerth, Hankins, Hereld, Hoffman, Myers, Odendaal and Signore2014).
The Safe Passage Study used a modified Timeline Follow-Back (TLFB) (Dukes et al., Reference Dukes, Tripp, Petersen, Robinson, Odendaal, Elliott, Willinger, Hereld, Raffo, Kinney and Groenewald2017), a validated method to capture alcohol exposure with a high level of detail, at the time around conception until the last day that the pregnant person reported drinking. A validation study done by Himes et al. (Reference Himes, Dukes, Tripp, Petersen, Raffo, Burd, Odendaal, Elliott, Hereld, Signore and Willinger2015) using a subset of Safe Passage Study pregnant participants indicated strong concordance between parental reports using this approach and meconium biomarkers of alcohol exposure. The modified TLFB was administered at the recruitment interview (approximately 6 weeks GA), again at three different prenatal visits (20–24 weeks, 28–32 weeks and 34+ weeks), and at 1-month post-delivery. Exposure information was collected at the recruitment interview to capture substance exposure at the time around conception (15 days before and after the last menstrual period) and for 30 days prior to the participant’s last reported drinking day. In subsequent interviews, if the participant reported consumption since their previous visit, the reference period consisted of the 30 days prior to the last drinking day. Peri-conception (2 weeks prior and 2 weeks following the last menstrual period) alcohol intake information was also collected. Detailed information was obtained to standardise and calculate the total grams of alcohol consumed on each drinking day or episode, and detailed information regarding the type(s) of alcoholic beverage consumed was collected: whether the drink was frozen or included ice; number, and size of containers; the number of persons sharing; and interval of ingestion were collected for each drinking day.
For the purposes of this pilot study, 80 mothers with varying levels and timing of exposure in pregnancy and who had initially indicated that they would be willing to participate in future studies were approached when their children (born as participants in the Safe Passage Study) were approximately 6 years old and invited to participate in the pilot study. All potential participants would necessarily have fulfilled the inclusion criteria of the Safe Passage Study, and these include
-
1. Pregnant parent was able to give consent;
-
2. Pregnant parent was at least 16 years of age at the time of consent;
-
3. Pregnant parent and child can speak Afrikaans and/or English. This criterion was not considered to unfairly exclude many research candidates: Afrikaans and English are the main spoken languages in the study region; thus, this criterion was included purely for practical reasons.
Additional exclusion criteria for children participating in the present study were as follows:
-
1. History of traumatic brain injury with loss of consciousness exceeding 10 min;
-
2. Presence of a major medical or central nervous system disorder;
-
3. Prenatal exposure to drugs (aside from tobacco and alcohol);
-
4. Implant (e.g. metal shunt) or medical condition that posed a risk during scanning.
Of the 80 randomly selected participants invited, 72 birthing parents agreed to participate. From the 72 parent participants, 51 of their children completed the scan.
Calculating QFT of PAE and PTE
The quantity of PAE was measured using the total amount of standard drinks consumed during pregnancy and the average number of drinks per drinking day. The frequency of PAE was measured using the total number of days with binge consumption (more than four drinks per sitting). Timing of PAE identified the total amount of drinks consumed per trimester.
The quantity of PTE was measured using the total amount of cigarettes smoked throughout pregnancy. The timing of PTE was measured using the total amount of cigarettes smoked per trimester. There was no measure of frequency of PTE.
Image acquisition
Scanning occurred at the Cape Universities Brain Imaging Center (CUBIC) located at Tygerberg Hospital, Cape Town. Whole-brain T1-weighted images were acquired for each participant using a 3-Tesla Siemens Allegra scanner. Scan parameters were as follows: repetition time (TR) = 2530 ms; echo time (TE) = 6.53 ms; flip angle = 7°; field of view = 224x168 mm2; and voxel = 1x1x1 mm. Children were familiarised with the MRI environment during a session in a mock scanner on the same day prior to the actual scanning session.
Image processing
FreeSurfer’s v5.3 recon-all pipeline was used to perform volumetric segmentation. This pipeline involve motion correction and averaging (Reuter et al., Reference Reuter, Rosas and Fischl2010) of multiple volumetric T1-weighted images (when more than one was available), removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Ségonne et al., Reference Ségonne, Pacheco and Fischl2007), automated Talairach transformation, segmentation of the subcortical white matter and deep grey matter volumetric structures (including hippocampus, amygdala, caudate, putamen and ventricles) (Fischl & Dale, Reference Fischl and Dale2000; Fischl et al., Reference Fischl, Salat, van der Kouwe, Makris, Ségonne, Quinn and Dale2004a), intensity normalisation (Sled et al., Reference Sled, Zijdenbos and Evans1998), tessellation of the grey matter to white matter boundary, automated topology correction (Fischl et al., Reference Fischl, Salat, Busa, Albert, Dieterich, Haselgrove, Van Der Kouwe, Killiany, Kennedy, Klaveness and Montillo2002; Ségonne et al., Reference Ségonne, Pacheco and Fischl2007), and surface deformation following intensity gradients to optimally place the grey/white and grey/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Dale & Sereno, Reference Dale and Sereno1993; Dale et al., Reference Dale, Fischl and Sereno1999; Fischl & Dale, Reference Fischl and Dale2000). Once the cortical models are complete, a number of deformable procedures can be performed for further data processing and analysis including surface inflation (Fischl et al., Reference Fischl, Sereno, Tootell and Dale1999a), registration to a spherical atlas which is based on individual cortical folding patterns to match cortical geometry across subjects (Fischl et al., Reference Fischl, Sereno and Dale1999b), parcellation of the cerebral cortex into units with respect to gyral and sulcal structure (Fischl et al., Reference Fischl, Van Der Kouwe, Destrieux, Halgren, Ségonne, Salat, Busa, Seidman, Goldstein, Kennedy and Caviness2004b; Desikan et al., Reference Desikan, Ségonne, Fischl, Quinn, Dickerson, Blacker, Buckner, Dale, Maguire, Hyman and Albert2006), and creation of a variety of surface-based data including maps of curvature and sulcal depth. Whole-brain metrics for cortical and subcortical volume Regions of Interest (ROI) were extracted using the Desikan–Killiany atlas in tabular format. FreeSurfer’s subcortical pipeline segments each voxel into one of approximately 27 ROIs. These include the following bilateral structures: cerebral white matter, cerebral cortex, lateral ventricles, inferior lateral ventricles, cerebellum white matter, cerebellum cortex, thalamus, caudate, putamen, pallidum, hippocampus, amygdala, and accumbens area as well as midline and specialised labels such as: lesions, vessels, 3rd ventricle, 4thventricle, brain stem and cerebrospinal fluid. These ROIs include the caudate of the left and right hemisphere, putamen of the left hemisphere, anterior cincular cortex and the cerebellum of the right hemisphere. The Deskian–Killiany atlas was used to parcellate the cortical surface into 68 ROIs. Cortical volume, thickness and surface area metrics were calculated for each ROI by hemisphere.
Statistical analyses
All statistical analyses were performed in CRAN R v4.1. Linear regression was run in R with the lm() package to identify associations between structural brain metrics and prenatal exposure (PAE and PTE) as well as the PAE x PTE interaction. The regression model included age (months) and biological sex, and intracranial volume as factors. The main effects and interactions were considered statistically significant at p < 0.05. The Benjamini–Hochberg false-discovery rate (FDR) correction (q < 0.05) was applied within each metric group (e.g. within cortical volume, cortical thickness, cortical area and subcortical volume, within each hemisphere).
Results
Demographics and patterns of PAE and PTE
Due to missing PTE information, four children with PAE were removed from the analysis. The final sample for analysis included 30 children with PAE and 16 control children without PAE (see Table 1). There was no significant difference in sex, household income, maternal age at birth or gestational age with PAE relative to the no-PAE control group (p’s > 0.20). Patterns of PTE were slightly different between PAE and no-PAE control group (Table 2), where a statistical trend was observed for larger proportion of participants exposed to PTE in PAE (70%) compared to no-PAE (56%, p = 0.08) and larger quantity of cigarettes per day in trimester 2 in PAE (mean = 4 cigs/day) compared to no-PAE (mean = 2 cigs/day) groups (p = 0.08). Within the PAE group, on average, the total number of alcoholic drinks consumed were highest in the first and second trimester and reduced in the third trimester. In contrast, PTE was consistent throughout pregnancy for both PAE and non-PAE groups.
Table 1. Descriptive characteristics of the participants

Group statistics presented as Mean ± STDEV (range). p-Values of t-tests demonstrate no significant between-group (Control vs. PAE) differences in demographic variables.
Note: n = 5 missing data points for monthly income.
Table 2. Details of PAE and PTE quantity, frequency, and timing

Group statistics presented as Mean ± STDEV, (range). p-Values of t-tests demonstrate significant between-group (Control vs. PAE) differences in patterns of PAE and PTE. Self-reported drinking and smoking were obtained at the recruitment interview, including up to three prenatal visits after recruitment, using a modified timeline follow-back interview for alcohol and tobacco use. Total grams of alcohol consumed per drink were calculated and converted into standard drinks using the NIAAA definition of one standard drink equals 14 g of pure alcohol. A binge episode was defined as the intake of ≥ 4 standard drinks on a given day.
Main and interactive effects of PAE and PTE on cortical ROIs
Main effects of PAE (Fig. 1) revealed larger cortical volumes (r. fusiform, l. postcentral) and larger cortical area (r. fusiform, r. pars orbitalis) compared to Control (uncorrected p’s < 0.01); however, none survived FDR correction for multiple comparisons (n = 68 cortical ROIs).

Fig. 1. Cortical brain outcomes: main effects of PAE.
Main effects of PTE (Fig. 2) were primarily associated with larger brain metrics [larger cortical volume (r. lateral occipital), larger cortical area (r. temporal pole) and thicker cortices (r. medial orbital frontal, parahippocampal, pars orbitalis, pars triangularis, transverse temporal; l. cuneus, entorhinal, isthmus cingulate, lateral occipital) (uncorrected p’s < 0.03], with the exception of smaller right paracentral volume and area (uncorrected p’s < 0.05); however, none survived FDR correction for multiple comparisons (n = 68 cortical ROIs).

Fig. 2. Cortical outcomes: main effects of PTE.
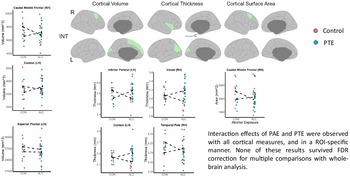
Interactive effects of PAE and PTE (Fig. 3) revealed a unique pattern of cortical alterations from what was observed for main effects of both. The interactions across seven cortical outcomes demonstrated that the relative difference between the group means for PTE compared to no-PTE was tended to be reversed between the PAE and no-PAE conditions (e.g. larger vs. smaller mean). This was observed in cortical volume (r. caudal middle frontal, l. cuneus and l. superior frontal), cortical thickness (l. interior parietal, l. cuneus, r. insula and r. temporal pole) and cortical area (r. caudal middle frontal) (uncorrected p’s < 0.05); however, none survived FDR correction for multiple comparisons (n = 68 cortical ROIs).

Fig. 3. Cortical outcomes: interactive effects of PAE plus PTE.
Main and interactive effects of PAE and PTE on subcortical ROIs
No associations between PAE and subcortical volumes were observed (p’s > 0.05) prior to FDR corrections. PTE was associated with decreased volume in the left putamen (uncorrected p = 0.03), which did not pass FDR corrections. PAE x PTE interactions were found in the anterior, central and posterior segments of the corpus callosum (uncorrected p’s < 0.03) but did not pass FDR correction, where differences between PTE and no-PTE were larger in no-PAE group compared to PAE in the central and mid-posterior corpus callosum (Fig. 4).

Fig. 4. Subcortical brain outcomes: main and interactive effects of PAE and PTE.
Associations of QFT on cortical and subcortical ROIs
ROIs that reached significance prior to FDR correction for either PAE main effect or the PAE x PTE interaction were further studied in relation to QFT of PAE. When characteristics of PAE patterns were examined in relation to cortical and subcortical brain outcomes, associations were observed between quantity and frequency of PAE (but not timing), and with cortical alterations only, and not subcortical (Fig. 5). Specifically, more quantity (total drinks) and greater frequency (frequency of binge episodes) were significantly related to larger volume and larger area of the fusiform on the right hemisphere (corrected p’s < 0.05). Frequency of binge episodes was also positively associated with greater surface area of the fusiform on the left hemisphere (p < 0.05).

Fig. 5. All brain associations with quantity and frequency of PAE.
Prenatal tobacco exposure
ROIs that reached significance prior to FDR correction for either PTE main effect or the PTE x PAE interaction were further studied in relation to quantity and timing of PTE (frequency data were not collected). Quantity of PTE was not associated with any cortical or subcortical brain outcomes. However, timing of PTE was associated with cortical, but not subcortical, brain outcomes (Fig. 6). Specifically, Presence of PTE in trimester 1 was significantly related to greater cortical thickness (r. pars orbitalis and l. lateral occipital) (corrected p’s < 0.05). Other findings relating to timing of PTE were found but did not survive FDR correction (thicker left entorhinal cortex with trimester 2 PTE; thinner right pars orbitalis cortex with trimester 3 PTE) (uncorrected p’s < 0.05).

Fig. 6. All brain associations with quantity and timing of PTE.
Discussion
Several key findings emerge from this pilot study: (1) leveraging QFT data significantly detected brain signatures of PAE and PTE at the age of 6 years (results passed correction for multiple comparisons after pre-screening ROIs for PAE or PTE main effects across the whole brain); (2) examining PAE, PTE and their interaction yielded three distinct profiles of cortical and subcortical alterations; and (3) community levels of PAE and PTE in a non-clinical sample of 6 year olds appears to be detectable primarily in cortical, but not as much within subcortical, brain regions. Together, these findings provide evidence towards the theory that specific combinations of prenatal substance exposures and their unique pattern of exposure result in complex profiles of teratogenic outcomes on the brain and are not simply additive in their teratogenic potential. The use of prospective QFT data extends our understanding of how important it may be to consider the pattern of exposures and presence/absence of co-exposures when examining brain alterations following prenatal alcohol and/or tobacco exposure.
Prior to correction for multiple testing, both PAE and PTE, as well as their interaction, were associated with a range of brain alterations. PAE was only associated with cortical and not subcortical alterations at this age range [e.g. larger cortical volume (r. fusiform and l. postcentral) and larger cortical area (r. fusiform and pars orbitalis)]. PTE was associated with both cortical and subcortical alterations, and interestingly, the brain regions did not overlap with those associated with PAE [e.g. larger cortical volume (r. lateral occipital and paracentral), altered cortical area (smaller r. paracentral and larger r. temporal pole), thicker cortices (r. medial orbital frontal, parahippocampal, pars orbitalis, pars triangularis, transverse temporal; l. cuneus, entorhinal, isthmus cingulate and lateral occipital) and decreased subcortical volume in the left putamen]. Furthermore, the profile of ROIs associated with the interactive effects of PAE with PTE exhibited a third profile that was completely distinct from PAE only alterations, had slight overlap with PTE only alterations (e.g. l. cuneus and r. temporal pole), and implicated many unique brain alterations that were not observed when examining PAE or PTE alone [e.g. altered cortical volume (r. caudal middle frontal, l. cuneus and superior frontal), altered cortical thickness (r. temporal pole and insula; l. cuneus and inferior parietal), altered cortical area (r. caudal middle frontal), and altered subcortical midline structures (three segments of the corpus callosum]. These three profiles of brain alterations suggest that the impact of PAE on brain structure at 6 years of age is unique from PTE, and their interactive effects (PAE + PTE) are likely complex and not simply additive or synergistic in teratogenic potential. Importantly, however, these findings of PAE, PTE and their interaction on brain metrics remain preliminary, as none survived correction for multiple comparisons. It is possible that results would have passed corrections for multiple comparisons with a higher-powered study (e.g. larger participant sample size, focused within clinical population known to demonstrate symptomology of FASD, examined only targeted neural circuitry rather than a whole-brain analysis in the present study). None the less, current statistical trends following analyses examining PAE interactions with PTE suggest that this interaction deserves further study in larger samples, and further studies should also consider the higher PTE doses that naturally co-occur with PAE, compared to mono-substance use of PTE or PAE only. PAE by PTE interactions were observed within the corticospinal tract among 2 year olds participating in a research study from a neighbouring community to the one current study’s participants reside in, further supporting the hypothesis that consideration of co-exposures is needed to advance our teratogenicity following often individualised patterns of prenatal exposures (Roos et al., Reference Roos, Fouche, Ipser, Narr, Woods, Zar, Stein and Donald2021; Subramoney et al., Reference Subramoney, Joshi, Wedderburn, Lee, Roos, Woods, Zar, Narr, Stein and Donald2022).
It is noteworthy that despite the small sample size of this pilot study, the total amount of standard drinks consumed during pregnancy and the average number of drinks per drinking day were positively associated with cortical volume in the right fusiform gyrus. This is in contrast to other neuroimaging studies, which have consistently demonstrated reduced cortical volume in children prenatally exposed to alcohol (Rajaprakash et al., Reference Rajaprakash, Chakravarty, Lerch and Rovet2014; Migliorini et al., Reference Migliorini, Moore, Glass, Infante, Tapert, Jones, Mattson and Riley2015). However, a study performed in neonates in an adjacent community recently found similar alterations in the fusiform following PAE (Roos et al., Reference Roos, Fouche, Ipser, Narr, Woods, Zar, Stein and Donald2021), corroborating teratogenic impact on the fusiform by PAE in early postnatal life up to 6 years old in the current study. The fusiform gyrus plays a role in high-level tasks related to visual processing, including processing of information about faces (Weiner & Grill-Spector, Reference Weiner and Grill-Spector2012; Rangarajan et al., Reference Rangarajan, Hermes, Foster, Weiner, Jacques, Grill-Spector and Parvizi2014). Face processing is a critically important perceptual ability; identifying and interpreting facial emotions have a critical role in social functioning, and children with FASDs may have difficulty doing so (Lindinger et al., Reference Lindinger, Jacobson, Dodge, Malcolm-Smith, Molteno, Meintjes and Jacobson2022). These findings together with the observation of similar size effects for both PAE and PTE are novel and deserve to be consolidated with more extensive work.
Several limitations deserve emphasis. Firstly, as described above, work on PAE and PTE that includes a range of different QFT features will require larger sample sizes to avoid false-negative findings. Secondly, we did not include as potential moderators of our findings, a range of poverty-related factors, such as maternal exposure to interpersonal violence, childhood exposure to community violence and food insecurity (Tomlinson et al., Reference Tomlinson, O’Connor, Le Roux, Stewart, Mbewu, Harwood and Rotheram-Borus2014; Stein et al., Reference Stein, Koen, Donald, Adnams, Koopowitz, Lund, Marais, Myers, Roos, Sorsdahl and Stern2015; Koen et al., Reference Koen, Brittain, Donald, Barnett, Koopowitz, Maré, Zar and Stein2016; Donald et al., Reference Donald, Hoogenhout, du Plooy, Wedderburn, Nhapi, Barnett, Hoffman, Malcolm-Smith, Zar and Stein2018; Gonzalez et al., Reference Gonzalez, Palmer, Uban, Jernigan, Thompson and Sowell2020; Uban et al., Reference Uban, Kan, Wozniak, Mattson, Coles and Sowell2020). Thirdly, given the focus on 6-year-olds, we are unable to determine whether the findings here are true null findings or simply underpowered. Given the dynamic trajectories of brain volumes over time, with significant increases in cortical thickness at ages 5–6 years, and then subsequent thinning when approaching pubertal maturation, it is possible that the outcomes of PAE and PTE are also likely dynamic, and alterations in brain developmental trajectories following teratogen exposures may overlap. This may result in no observable group differences at certain ages, including around 6 years of age, as in the case in this small study. Finally, exclusion criteria based on co-use of other substances other than alcohol and tobacco may have excluded women and their developing babies that may be most impacted by teratogens, and poly-use beyond the two most common ones (alcohol and tobacco) warrants more investigation to serve this likely high-risk subsample.
In conclusion, even in this small sample, significant positive associations of alcohol quantity with cortical brain volume and surface area of the right fusiform were observed. Larger samples and longitudinal examination will be needed to fully delineate the impact of PAE and PTE, as well as QFT characteristics on the developing brain, particularly when investigating naturally occurring patterns of PAE among the community, rather than solely focusing on very high levels of PAE found in clinical FASD studies. Given the large range of profiles of prenatal exposures that occur in the global population, research that evolves our understanding of the complexity of teratogenicity is greatly needed. This can be achieved by examining the entire range of: (1) postnatal developmental periods including neonates to young children; (2) combinations of prenatal co-exposures; (3) impact of widely variable patterns of exposures among developing babies and children; and (4) ecological contexts in which the prenatal exposures occur to understand interactions with larger sociodemographic and neighbourhood factors to inform future interventions.
Acknowledgements
The authors gratefully acknowledge the contribution of the personnel and investigators of the Safe Passage Study. The authors would especially like to acknowledge the families for their participation in this study.
Authors’ contributions
SJB designed the study and participated in data collection. KAU, EK, DJ, BM and SA assisted with data analysis and interpretation. KAU, DJ, SCB and DJS drafted the manuscript. LBK assisted with formatting the manuscript. All authors contributed to the final draft of the manuscript.
Financial support
The PASS Research Network was supported by the National Institute on Alcohol Abuse and Alcoholism, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Institute on Deafness and Other Communication Disorders through the Cooperative Agreement Mechanism (U01 HD055154, U01 HD045935, U01 HD055155, U01 HD045991 and U01 AA016501). This pilot study was funded by an ABMRF grant. KAU was supported by K01AA026889. Collaboration work and future research on this topic are funded with funds from the National Institute of Alcohol Abuse and Alcoholism (5R01AA025653-04, Sowell PI). This publication was made possible in part by a grant from Carnegie Corporation of New York, supporting the author D Jonker. The statements made and views expressed are solely the responsibility of the authors.
Conflicts of interest
None.
Ethical standards
Ethical approval for human subject research was obtained from the Human Research Ethics Committee of the Faculty of Health Sciences of Stellenbosch University (REF 248/2014). Informed consent and assent were obtained from parents/guardians and participants before enrolment.