An α-particle passing through a gas loses velocity because it gives up energy to the gaseous atoms. If we can calculate the average energy transferred to an atom, the stopping power of the gas follows immediately. The earlier theories, by Thomson and Darwin, took account of only the close collisions, in which the α-particle actually passes through the atom. Bohr, however, in 1913, took into account the transfers of energy to atoms at distances from the track considerably larger than atomic dimensions. His calculation was purely classical, and dealt with an atomic, model in which the electrons were capable of simple harmonic motions. The result was in very satisfactory agreement with experiment. For atoms containing each a single electron, with mass μ, charge ε, and natural period ω, Bohr's formula is
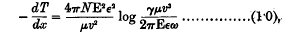
where − dT/dx is the rate of loss of energy by an α-particle whose charge is E, and velocity v; N is the number of atoms per c.c.; and γ = 1·123.