Strontioborite, which was first described in 1960 and later discredited by the then named Commission on New Minerals and Mineral Names of the International Mineralogical Association (IMA CNMMN), has been re-investigated (electron microprobe, single-crystal and powder X-ray diffraction, crystal structure determination and IR spectroscopy) on two specimens, including the holotype, and revalidated by the IMA Commission on New Minerals, Nomenclature and Classification (CNMNC). Strontioborite is known only at the Chelkar salt dome (North Caspian Region, Western Kazakhstan), in halite rocks with bischofite, magnesite, anhydrite, halurgite, boracite, ginorite and celestine. It forms colourless lamellar, scaly or tabular crystals up to 2 mm across. The chemical composition (wt.%, H2O is calculated for (OH)4 = 4 H apfu, according to structural data; holotype/neotype) is: CaO 1.42/0.27, SrO 23.10/23.79, B2O3 67.37/67.57, H2O 8.73/8.72, total 100.62/100.37. The empirical formulae [calculated based on 15 O apfu = O11(OH)4 pfu] of the holotype and neotype specimens are Sr0.92Ca0.10B7.98O11(OH)4 and Sr0.95Ca0.02B8.02O11(OH)4, respectively. The idealised formula is Sr[B8O11(OH)4]. Strontioborite is monoclinic, space group P21, a = 7.6192(3), b = 8.1867(2), c = 9.9164(3) Å, β = 108.357(4)°, V = 587.07(3) Å3 and Z = 2. The strongest reflections of the powder X-ray diffraction pattern [d,Å(I)(hkl)] are: 7.22(100)(100), 5.409(61)(110), 4.090(64)(020), 3.300(48)(210), 2.121(30)($\bar{1}$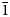 24) and 2.043(37)(040, 024, $\bar{2}$
24) and 2.043(37)(040, 024, $\bar{2}$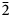 24). The crystal structure, solved from single-crystal X-ray diffraction data (R = 0.0372), is based upon the (100) layers of polymerised B–O–OH polyanions [B8O11(OH)4]2– and Sr-centred nine-fold polyhedra SrO6(OH)3. The B–O–OH polyanion is the cluster of three tetrahedra and three triangles; these clusters are decorated by the [B2O2(OH)3] pyro-group consisting of two triangles. The layers are linked via vertices of Sr-centred polyhedra, which share seven vertices with B-centred polyhedra of one layer and two vertices with B-centred polyhedra of the adjacent layer, and by the system of H bonds. The crystal chemistry of strontioborite is discussed in comparison with other natural and synthetic borates.
24). The crystal structure, solved from single-crystal X-ray diffraction data (R = 0.0372), is based upon the (100) layers of polymerised B–O–OH polyanions [B8O11(OH)4]2– and Sr-centred nine-fold polyhedra SrO6(OH)3. The B–O–OH polyanion is the cluster of three tetrahedra and three triangles; these clusters are decorated by the [B2O2(OH)3] pyro-group consisting of two triangles. The layers are linked via vertices of Sr-centred polyhedra, which share seven vertices with B-centred polyhedra of one layer and two vertices with B-centred polyhedra of the adjacent layer, and by the system of H bonds. The crystal chemistry of strontioborite is discussed in comparison with other natural and synthetic borates.