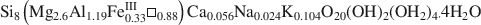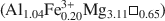The chemical composition of hydrothermal chlorite was determined by means of more than 200 electron microprobe analyses (EMPA) in almost all of the 70 chlorite-bearing samples taken from 5 boreholes in a study of the active geothermal system of Los Humeros, Mexico. Bulk rock composition of 6 different volcanic lithologies, as well as available in situ temperatures and chemical compositions of chlorite, were analyzed by principal component analysis (PCA) in order to test the dependence of chlorite composition on physicochemical parameters. The results show that chlorite minerals display a wide range of chemical compositions in this hydrothermal system that reflect the particular conditions of crystallization episodes: The Na + K + 2Ca values are low (from 0 to 0.6) and they show no correlation patterns with octahedral vacancies (¤) in chlorite, indicating that compositional variations are not due to the intergrowth of smectite and/or illite. The octahedral occupancy of most chlorite is relatively high (from 11.3 to 11.95), especially that from a high-temperature range, as is the case of metamorphic chlorite. The octahedral occupancy seems not to be related to other chemical variables of chlorite from the G3, G4 and G5 lithologic units, suggesting that the lack of complete occupancy is not dependent on “contamination” by other silicates (such as quartz). Cationic substitution in tetrahedral sites in chlorite is small and via a Tschermak exchange (MgVISiIV ↔ AlVIAlIV). To preserve a charge balance in the structure, an octahedral substitution of R2+ by Al3+ accompanies the Tschermak exchange. The chemical composition of hydrothermal chlorite is very similar to that of metamorphic chlorite but slightly different from equivalent phases found in diagenetic environments. In hydrothermal chlorite the SiVI, AlVI and ¤ decrease, whereas the AlIV and Fe2+ contents increase with the degree of alteration and depth, the same way as in chlorite formed in diagenetic high-temperature environments. The ferrous iron content, in general, increases with depth and temperature; however, whole-rock chemistry affects the iron distribution in chlorite of Los Humeros. Changes in the oxygen fugacity of fluids at depth also affect the iron distribution in chlorite, XFe = Fe/(Fe + Mg), which ranges from 0.30 to 0.38 in oxidizing conditions and from 0.39 to 0.60 in reducing conditions. Finally, the chemical composition of chlorite in Los Humeros appears to change with temperature, but the correlations of ¤ and AlIV with temperature are more variable than in another nearby active geothermal system located in Los Azufres, Mexico. This implies that geothermometers based on chlorite composition and empirically calibrated in some geothermal systems cannot be generalized and it is necessary to consider other physicochemical variables.