Phenotype of cultured ocular epithelial transplants has been shown to affect clinical success rates following transplantation to the cornea. The purpose of this study was to evaluate the relationship between cell nucleus morphometry and phenotype in three types of cultured epithelial cells. This study provides knowledge for the development of a non-invasive method of determining the phenotype of cultured epithelium before transplantation. Cultured human conjunctival epithelial cells (HCjE), human epidermal keratinocytes (HEK), and human retinal pigment epithelial cells (HRPE) were analyzed by quantitative immunofluorescence. Assessments of nucleus morphometry and nucleus-to-cytoplasm ratio (N/C ratio) were performed using ImageJ. Spearman’s correlation coefficient was employed for statistical analysis. Levels of the proliferation marker PCNA in HCjE, HEK, and HRPE correlated positively with nuclear area. Nuclear area correlated significantly with levels of the undifferentiated cell marker ABCG2 in HCjE. Bmi1 levels, but not p63α levels, correlated significantly with nuclear area in HEK. The N/C ratio did not correlate significantly with any of the immunomarkers in HCjE (ABCG2, CK7, and PCNA) and HRPE (PCNA). In HEK, however, the N/C ratio was negatively correlated with levels of the undifferentiated cell marker CK14 and positively correlated with Bmi1 expression. The size of the nuclear area correlated positively with proliferation markers in all three epithelia. Morphometric indicators of phenotype in cultured epithelia can be identified using ImageJ. Conversely, the N/C ratio did not show a uniform relationship with phenotype in HCjE, HEK, or HRPE. N/C ratio therefore, may not be a useful morphometric marker for in vitro assessment of phenotype in these three epithelia.

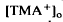 , which was maximal in amplitude in the most distal portion of the space surrounding photoreceptors, the subretinal space. The light-evoked decrease in
, which was maximal in amplitude in the most distal portion of the space surrounding photoreceptors, the subretinal space. The light-evoked decrease in 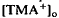 was considerably slower and of a different overall time course than the light-evoked decrease in
was considerably slower and of a different overall time course than the light-evoked decrease in 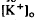 , also recorded in the subretinal space.
, also recorded in the subretinal space. 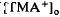 decreased to a peak at 38 s after the onset of illumination, then slowly recovered towards the baseline, and transiently increased following the offset of illumination. It resembled the light-evoked
decreased to a peak at 38 s after the onset of illumination, then slowly recovered towards the baseline, and transiently increased following the offset of illumination. It resembled the light-evoked 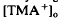 decreases previously recorded in the
decreases previously recorded in the 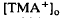 decrease is considered to originate from a light-evoked increase in the volume of the subretinal space (or subretinal hydration). A mathematical model accounting for
decrease is considered to originate from a light-evoked increase in the volume of the subretinal space (or subretinal hydration). A mathematical model accounting for 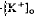 diffusion predicted that the volume increase underlying the response was 63% on average and could be as large as 95% and last for minutes. The estimated volume increase was then used to examine its effect on K
diffusion predicted that the volume increase underlying the response was 63% on average and could be as large as 95% and last for minutes. The estimated volume increase was then used to examine its effect on K