Book contents
- Frontmatter
- Contents
- List of Contributors
- Preface
- Chapter 1 The mediastinum
- Chapter 2 Imaging of the mediastinum
- Chapter 3 Inflammatory diseases of the mediastinum
- Chapter 4 The thymus gland
- Chapter 5 Pathology of non-neoplastic conditions of the thymus
- Chapter 6 Low-grade and intermediate-grade malignant epithelial tumors of the thymus: thymomas
- Chapter 7 High-grade malignant epithelial tumors of the thymus: primary thymic carcinomas
- Chapter 8 Neuroendocrine carcinomas of the thymus
- Chapter 9 Germ cell tumors of the mediastinum
- Chapter 10 Parathyroid lesions, paragangliomas, thyroid tumors, and pleomorphic adenomas of the mediastinum
- Chapter 11 Hematopoietic neoplasms of the mediastinum
- Chapter 12 Cystic lesions of the mediastinum
- Chapter 13 Mesenchymal tumors of the mediastinum
- Chapter 14 Tell me what you need, so I’ll know what to say
- Chapter 15 Clinical pathology of disorders of the mediastinum
- Chapter 16 Surgical pathology of the heart
- Chapter 17 Morphologic alterations of serous membranes of the mediastinum in reactive and neoplastic settings
- Index
- References
Chapter 5 - Pathology of non-neoplastic conditions of the thymus
Published online by Cambridge University Press: 05 February 2015
- Frontmatter
- Contents
- List of Contributors
- Preface
- Chapter 1 The mediastinum
- Chapter 2 Imaging of the mediastinum
- Chapter 3 Inflammatory diseases of the mediastinum
- Chapter 4 The thymus gland
- Chapter 5 Pathology of non-neoplastic conditions of the thymus
- Chapter 6 Low-grade and intermediate-grade malignant epithelial tumors of the thymus: thymomas
- Chapter 7 High-grade malignant epithelial tumors of the thymus: primary thymic carcinomas
- Chapter 8 Neuroendocrine carcinomas of the thymus
- Chapter 9 Germ cell tumors of the mediastinum
- Chapter 10 Parathyroid lesions, paragangliomas, thyroid tumors, and pleomorphic adenomas of the mediastinum
- Chapter 11 Hematopoietic neoplasms of the mediastinum
- Chapter 12 Cystic lesions of the mediastinum
- Chapter 13 Mesenchymal tumors of the mediastinum
- Chapter 14 Tell me what you need, so I’ll know what to say
- Chapter 15 Clinical pathology of disorders of the mediastinum
- Chapter 16 Surgical pathology of the heart
- Chapter 17 Morphologic alterations of serous membranes of the mediastinum in reactive and neoplastic settings
- Index
- References
Summary
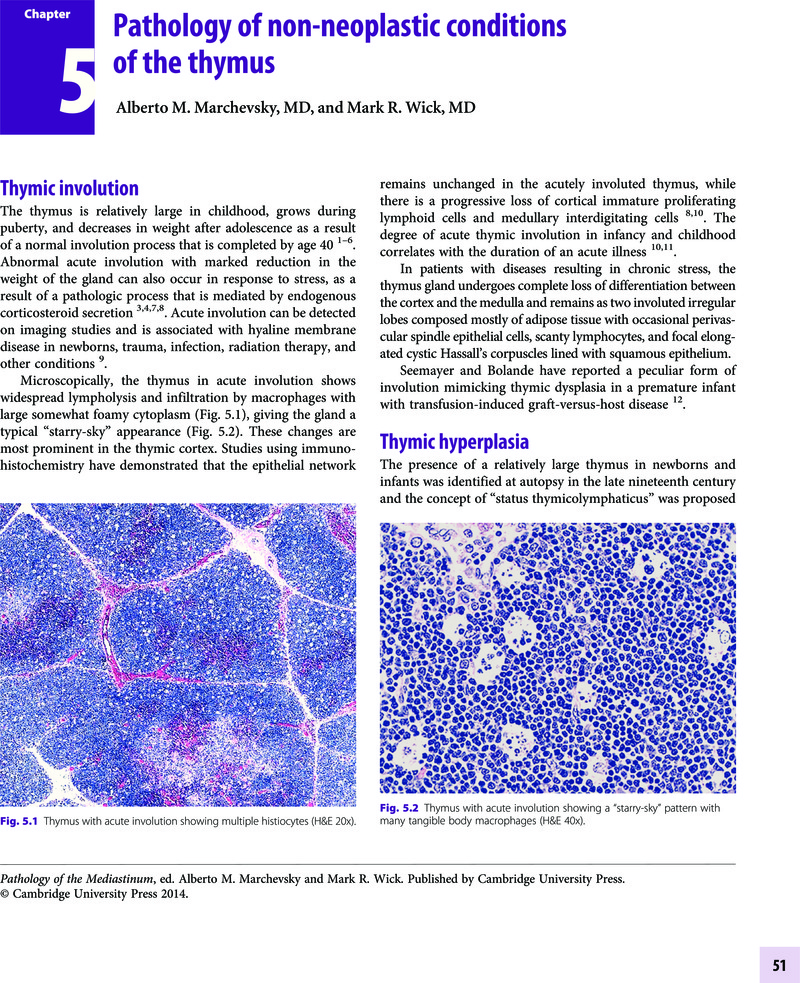
- Type
- Chapter
- Information
- Pathology of the Mediastinum , pp. 51 - 64Publisher: Cambridge University PressPrint publication year: 2000

