Paragenetic, textural, and chemical characteristics of columbite-tantalite minerals are examined as steps towards identifying the metallogenetic processes of their host granitoids. Columbite-tantalite-bearing granitoids of the Eastern Desert province of Egypt can be categorized into: (i) metaluminous alkali granites; (ii) peraluminous Li-albite granites; and (iii) metasomatized biotite and/or muscovite granite (i.e. apogranites).
Columbite of the alkali granite is of FeNb2O6 composition and associated with annite. The low F and Li contents of the associated mica precludes the important role of these volatile elements during the late stage of evolution of the alkali granites, thus delaying fractionation of Mn over Fe and Ta over Nb.
Compositionally, columbite-tantalite of the Li-albite granites is constrained between MnNb2O6 and MnTa2O6 (the Ta/(Nb+Ta)atom. ratio ranges between 0.10 and 0.80). This low to high ratio and the association of columbite-tantalite with topaz, fluorite and lithian micas (in the series zinnwaldite-white mica) indicate a higher solubility for Ta-fluoride complex compounds and their more stabilized state at lower temperatures in Li- and F-rich sodic melts. The columbite-tantalite commonly exhibits a mottled or patchy zoned texture with the rims consistently higher in Ta than the cores, reflecting the later effect of a corrosive supercritical vapour phase.
The columbites of metasomatized granites range in composition between FeNb2O6 and MnNb2O6. They are characterized by high Ti and U, and low Ta contents (the Ta/(Nb+Ta)atom. ranges between 0.01 and 0.15), indicating deposition from alkaline (K+ Na+ -rich), and relatively high-temperature interacting fluids. However, the Mn-enriched columbites are commonly encountered in the apical parts of the apogranites and formed in response to high μKF and μLiF required for stabilizing the associated Li-siderophyllite or zinnwaldite. Columbites of the apogranites commonly exhibit progressive (either normal or reverse) zoning which can be attributed to the disequilibrium conditions (e.g. sudden change in the pH) between the growing crystal and the solutions.

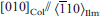 , which can be explained in terms of preservation of the oxygen close packing between the ilmenite and columbite structures. The interfaces between any two of the three different phases are coherent but show lattice strain contrast and sometimes dislocations because of their different unit-cell dimensions. On the basis of textural observations, titanohematite is supposed to exsolve first, followed by columbite-tantalite at temperatures below 500°C. The addition of MnO to the Fe
, which can be explained in terms of preservation of the oxygen close packing between the ilmenite and columbite structures. The interfaces between any two of the three different phases are coherent but show lattice strain contrast and sometimes dislocations because of their different unit-cell dimensions. On the basis of textural observations, titanohematite is supposed to exsolve first, followed by columbite-tantalite at temperatures below 500°C. The addition of MnO to the Fe