The structure of antimonian dussertite, 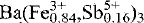 (AsO4)2(OH,H2O)6, has been refined in space group R3̄m with a 7.410(3), c 17.484(4) Å, Z = 3, to R = 3.2 % and Rw = 3.7 % using 377 observed reflections with I ≥ 3 σ(I). The structure is of the alunite-type and consists of sheets of corner-sharing (Fe3+,Sb5+)O6 octahedra parallel to (0001). The substitution of Sb5+ for Fe3+, and not for As5+, is unambiguously demonstrated not only by the structure refinement but also by electron microprobe analyses and crystal-chemical considerations. The icosahedrally coordinated Ba cations occupy cavities between pairs of octahedral sheets and are surrounded by six O atoms from the AsO4 tetrahedra and six O atoms from the (Fe3+,Sb5+)O6 octahedra. The mean bond lengths for the various coordination polyhedra are As-O 1.684(3) Å, (Fe,Sb)-O 2.004(1) Å, and Ba-O 2.872(2) Å. A hydrogen-bonding network is modelled using bond-valence calculations. The dussertite sample investigated is the first member of the crandallite group found to contain substantial Sb.
(AsO4)2(OH,H2O)6, has been refined in space group R3̄m with a 7.410(3), c 17.484(4) Å, Z = 3, to R = 3.2 % and Rw = 3.7 % using 377 observed reflections with I ≥ 3 σ(I). The structure is of the alunite-type and consists of sheets of corner-sharing (Fe3+,Sb5+)O6 octahedra parallel to (0001). The substitution of Sb5+ for Fe3+, and not for As5+, is unambiguously demonstrated not only by the structure refinement but also by electron microprobe analyses and crystal-chemical considerations. The icosahedrally coordinated Ba cations occupy cavities between pairs of octahedral sheets and are surrounded by six O atoms from the AsO4 tetrahedra and six O atoms from the (Fe3+,Sb5+)O6 octahedra. The mean bond lengths for the various coordination polyhedra are As-O 1.684(3) Å, (Fe,Sb)-O 2.004(1) Å, and Ba-O 2.872(2) Å. A hydrogen-bonding network is modelled using bond-valence calculations. The dussertite sample investigated is the first member of the crandallite group found to contain substantial Sb.

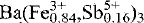 (AsO
(AsO