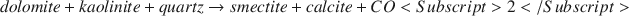Despite substantial scientific research efforts, accurate determination of the petrophysical effects of clay minerals on reservoir sands remains problematic. Diagenetic clays such as smectite and illite are of particular interest because of the pronounced effects these clays can have on reservoir quality. Here, results are reported from an experimental study based on the hydrothermal growth of smectite in synthetic sands. The sands contained quartz, dolomite and kaolinite, and were reacted at 175–200°C, for 19–45 d. The hydrothermal reaction can be written as follows:$$dolomite + kaolinite + quartz \to smectite + calcite + CO<Subscript>2</Subscript>$$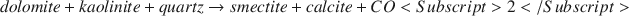
X-ray diffraction (XRD), electron microprobe (EMP) and electron diffraction (ED) analysis show that the synthetic Mg-rich smectite formed is saponite, with a cation exchange capacity (CEC) of about 100 meq/100 g. After reaction, brine permeability reductions of up to 98% were observed from the growth of less than 5% smectite. Scanning electron microscopy (SEM) observations of critical-point-dried reacted samples show that the clay behaves as a pervasive microporous cement with a complex pore-bridging texture affecting most of the available pore space. Morphologically, the clay is similar to naturally occurring diagenetic smectite from Gulf Coast sandstone reservoirs. The delicate clay texture collapses during air-drying and forms pore-lining masses. This phenomenon is similar to that observed for air-dried reservoir samples which contain dispersed diagenetic clays. An air-dried sample, then resaturated with brine, showed a marked increase in permeability. This increase is associated with the irreversible collapse of the clay texture. The experimental results indicate that the growth of diagenetic clay can severely reduce formation permeability, even at very low clay contents. The results also demonstrate the utility of hydrothermal experimental petrophysics for investigating the effects of diagenesis on rock properties.