Two major species were identified in Fe-treated montmorillonite: monomeric or dimeric hydroxoaqua cations ${\rm{Fe}}({\rm{OH}})_x^{(3 - x) + }$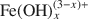 (form I), and polymeric structures with edge-shared Fe(O,OH)6 (form II). These species have different electron spectra (absorption maximum is 29,600 cm−1 in form I, and 26,000 and 28,000 cm−1 in form II), chemical and thermal stability, and electrochemical behavior. Form I behaves as a partly exchangeable cation in interaction with Cu2+ from Cu-trien solution and Ni2+ from Ni-EDTA, that can be used for selective quantitative analysis. On heating above the dehydration temperature (∼100–150°C) montmorillonite with Fe3+ in form I is converted to a mica-like structure and Fe3+ ions are fixed more strongly in the montmorillonite structure. Form II behaves similarly to hydrous ferric oxides, but its thermal crystallization to hematite is postponed to ∼500–600°C. The Fe3+ cations in the interlayer space are much less thermally stable than Al pillars in pillared interlayered clays (PILCs). Form I is more active in oxidative dehydrogenation of propane, while form II is the active species in sorption of As and the non-specific combustion of propane. To produce only form II by the treatment of montmorillonite with Fe3+, its load must be kept below ∼20 wt.%; otherwise the usual hydrous ferric oxides are formed.
(form I), and polymeric structures with edge-shared Fe(O,OH)6 (form II). These species have different electron spectra (absorption maximum is 29,600 cm−1 in form I, and 26,000 and 28,000 cm−1 in form II), chemical and thermal stability, and electrochemical behavior. Form I behaves as a partly exchangeable cation in interaction with Cu2+ from Cu-trien solution and Ni2+ from Ni-EDTA, that can be used for selective quantitative analysis. On heating above the dehydration temperature (∼100–150°C) montmorillonite with Fe3+ in form I is converted to a mica-like structure and Fe3+ ions are fixed more strongly in the montmorillonite structure. Form II behaves similarly to hydrous ferric oxides, but its thermal crystallization to hematite is postponed to ∼500–600°C. The Fe3+ cations in the interlayer space are much less thermally stable than Al pillars in pillared interlayered clays (PILCs). Form I is more active in oxidative dehydrogenation of propane, while form II is the active species in sorption of As and the non-specific combustion of propane. To produce only form II by the treatment of montmorillonite with Fe3+, its load must be kept below ∼20 wt.%; otherwise the usual hydrous ferric oxides are formed.