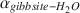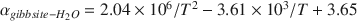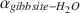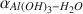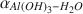Oxygen-isotope data were obtained for synthetic aluminum-hydroxide phases precipitated over 65–125 mo and have been compared to results from similar experiments conducted for 3–56 mo. The Al(OH)3 polymorphs, gibbsite, nordstrandite, and bayerite, were synthesized, but gibbsite was dominant in most samples, and commonly the only phase present. Using pure gibbsite samples, the following oxygen-isotope fractionation factors, 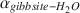 , were obtained: 1.0167 ± 0.0003 (9 ± 1°C), 1.0147 ± 0.0007 (24 ± 2°C), 1.0120 ± 0.0003 (51 ± 2°C). These values, and the associated equation for an oxygen-isotope geothermometer for the interval 0–60°C 103ln
, were obtained: 1.0167 ± 0.0003 (9 ± 1°C), 1.0147 ± 0.0007 (24 ± 2°C), 1.0120 ± 0.0003 (51 ± 2°C). These values, and the associated equation for an oxygen-isotope geothermometer for the interval 0–60°C 103ln 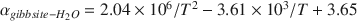 (T in K), are not significantly different from those obtained from experiments of much shorter duration. These results, and the good agreement with
(T in K), are not significantly different from those obtained from experiments of much shorter duration. These results, and the good agreement with 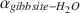 values obtained for well-constrained natural systems, suggest that the experimentally determined fractionation factors describe equilibrium conditions for gibbsite that has precipitated directly from solution.
values obtained for well-constrained natural systems, suggest that the experimentally determined fractionation factors describe equilibrium conditions for gibbsite that has precipitated directly from solution.
As also proposed by others using a modified-increment calculation, our synthesis experiments suggest that 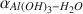 is polymorph-dependent at low temperatures and that a significant temperature-dependent trend exists in the values of
is polymorph-dependent at low temperatures and that a significant temperature-dependent trend exists in the values of 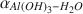 . However, previously calculated fractionation factors obtained using the modified-increment method are higher than those obtained from the experiments, with this discrepancy becoming larger as temperature decreases.
. However, previously calculated fractionation factors obtained using the modified-increment method are higher than those obtained from the experiments, with this discrepancy becoming larger as temperature decreases.