Introduction
Haliclona Grant, 1841 is the most speciose genus of the phylum Porifera, with more than 440 valid species (de Voogd et al., Reference de Voogd, Alvarez, Boury-Esnault, Carballo, Díaz, Dohrmann, Downey, Hajdu, Hooper, Kelly, Klautau, Manconi, Morrow, Pisera, Ríos, Rützler, Schönberg, Vacelet and van Soest2022) distributed in seven subgenera, namely H. (Haliclona) Grant, 1841, H. (Reniera) Schmidt, 1862, H. (Gellius) Gray, 1867, H. (Halichoclona) de Laubenfels, 1932, H. (Rhizoniera) Griessinger, 1971, H. (Soestella) de Weerdt, Reference de Weerdt2000 and H. (Flagellia) van Soest, 2017. At the same time, it is recognized as one of the hardest targets for taxonomic studies in sponges, given the great simplicity of its spicular component (non-ornamented, most have only oxeas/strongyles, few with microscleres) and great intraspecific variability in skeleton anatomy, which are the basis of its classification (de Weerdt, Reference de Weerdt1985, Reference de Weerdt1986, Reference de Weerdt2000). In turn, the molecular systematics studies based on cox1 mtDNA, 18S rDNA and 28S rDNA (Redmond et al., Reference Redmond, Raleigh, van Soest, Kelly, Travers, Bradshaw, Vartia, Stephens and McCormack2011, Reference Redmond, Morrow, Thacker, Diaz, Boury-Esnault, Cardenas, Hajdu, Lobo-Hajdu, Picton, Pomponi, Kayal and Collins2013) highlighted the polyphyly of Haliclona and several other genera and families in Haplosclerida, questioning the phylogenetic value of these traditional morphological characters for the systematics of the order Haplosclerida.
Nevertheless, advances regarding the search for new morphological data that could reconcile morphology and molecular systematics is still incipient. For this purpose, ultrastructural data of choanocytes looks promising (Pozdnyakov & Karpov, Reference Pozdnyakov and Karpov2016; Pozdnyakov et al., Reference Pozdnyakov, Sokolova, Ereskovsky A and Karpov2018) but more information from a wider number of taxa still needs to be gathered in order to create any meaningful systematics framework. Thus, until we reach that milestone, it is still necessary to organize the biodiversity of Haplosclerida within the currently accepted classification, in spite of the poly- or paraphyletic status of the higher taxa considered.
The knowledge on Haliclona spp. along the Brazilian coast remains deficient, with 15 species recorded, nine of them from coastal waters of north-eastern Brazil, but none from Fernando de Noronha Archipelago or Rocas Atoll (Muricy et al., Reference Muricy, Lopes, Hajdu, Carvalho, Moraes, Klautau, Menegola and Pinheiro2011, Reference Muricy, Esteves, Monteiro, Rodrigues and Albano2015; Sandes et al., Reference Sandes, Bispo and Pinheiro2014; Bispo et al., Reference Bispo, Correia and Hajdu2016). However, the record of an undescribed species of Haliclona from Rocas Atoll (Moraes et al., Reference Moraes, Vilanova and Muricy2003, Reference Moraes, Ventura, Klautau, Hajdu, Muricy, Alves and Castro2006; Moraes, Reference Moraes2011) and the number of new species of Haliclona recently described from Brazil (five spp.) is clearly indicative of the underestimated richness of this group in these areas. Further taxonomic investigation of museum vouchers from Fernando de Noronha, Rocas Atoll and Ceará State identified as ‘Haliclona sp.’ or ‘Chalinula sp.’ resulted in the discovery of two new species of Haliclona. In this paper, we aim to provide a formal description of both.
Materials and methods
Study area
Fernando de Noronha Archipelago and Rocas Atoll are the only emerged portions of the seamounts in the Fernando de Noronha Chain, which extends east-west and reaches areas close to the continental shelf of north-eastern Brazil (South-western Atlantic) (Almeida, Reference Almeida2015). Fernando de Noronha Archipelago lies in the easternmost seamount of this chain, encompassing 19 islands distributed in about 18 km2, where sandy beaches, rocky shores, submarine caves and tidal pools occur (Moraes, Reference Moraes2011; Almeida, Reference Almeida2015). In turn, Rocas Atoll is the only atoll in the South Atlantic. It has 7.5 km2 in area and is located 145 km west of Fernando de Noronha (Almeida, Reference Almeida2015). Rocas is unique in the world for the predominance of coralline algae as the main reef builder, but developing typical atoll structures such as reef front, reef flat (with channels, sand cays and pools) and lagoon (Kikuchi & Leão, Reference Kikuchi and Leão1997). Along the Ceará State coast, we sampled the Mundaú reef, which is a ferruginous sandstone (beachrock) reef that emerges during low tides (Matthews-Cascon & Lotufo, Reference Matthews-Cascon and Lotufo2006).
Sampling and taxonomic procedures
Materials were collected by scuba diving or snorkelling at several localities (Table 1) along Fernando de Noronha Archipelago (Pernambuco State), Rocas Atoll (Rio Grande do Norte state) and Mundaú reef (Ceará State) (Figure 1). Samples from oceanic islands were obtained during expeditions conducted between 1999 and 2003, while those from Ceará were collected in 2014.
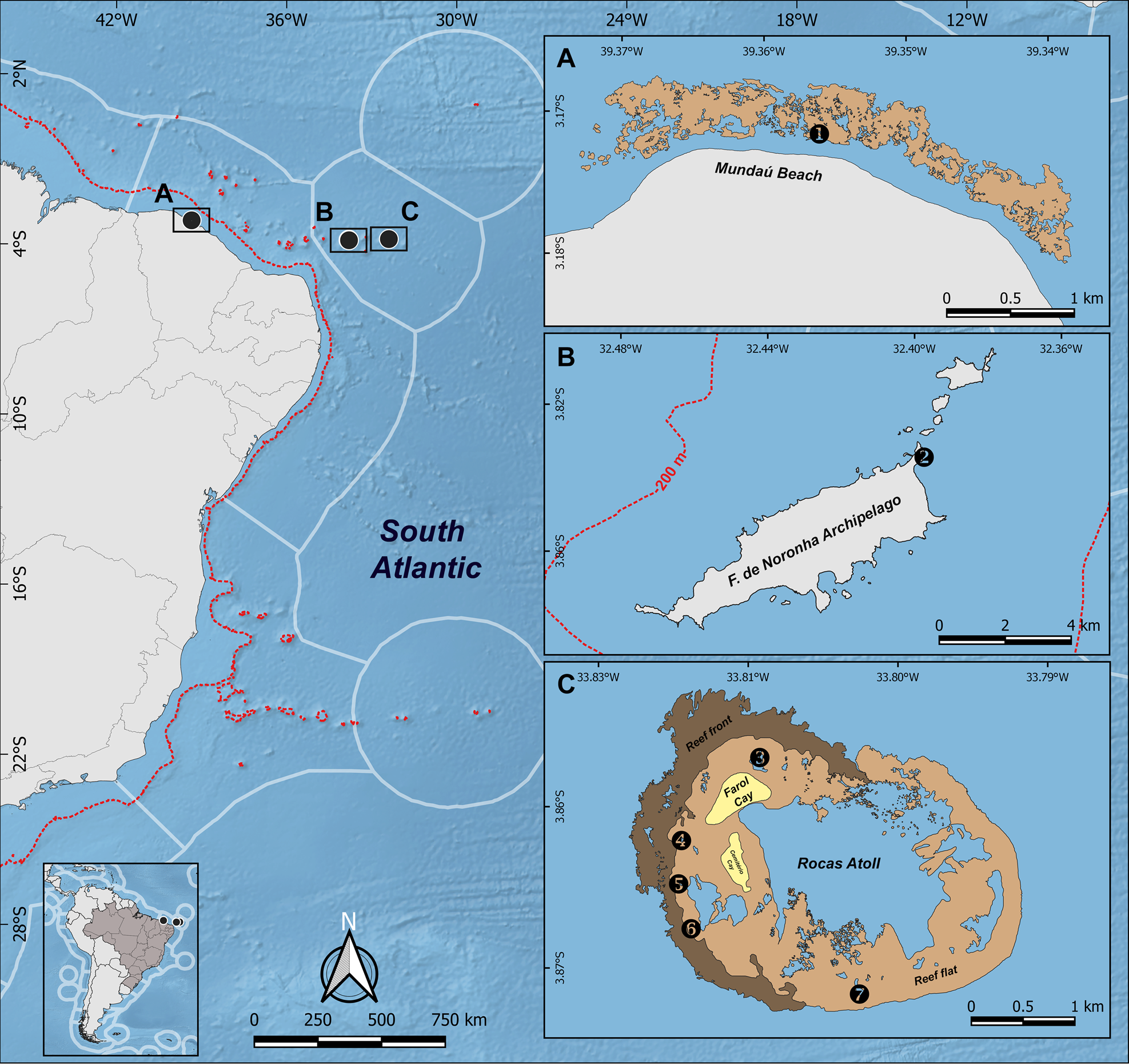
Fig. 1. Map indicating sites where Haliclona (Halichoclona) insularis sp. nov. and Haliclona (Soestella) moraesi sp. nov. were collected. (A) Detail of part of Ceará State coast showing the Mundaú Reef (1). Detail of Fernando de Noronha Archipelago, showing the Buraco da Raquel locality (2). (C) Detail of Rocas Atoll, showing the (3) Farol tide pool, (4) Garoupinha tide pool, (5) Cemiteriozinho tide pool, (6) Mapas tide pool and (7) Âncoras tide pool. Red dashed lines in the map indicate the 200 m isobath. Inset A is inserted in the North-eastern Brazil Ecoregion. Insets B and C in the Fernando de Noronha and Atol das Rocas Ecoregion.
Table 1. List of collecting sites of Haliclona (Halichoclona) insularis sp. nov. and Haliclona (Soestella) moraesi sp. nov. Ecoregions names based on Spalding et al. (Reference Spalding, Fox, Allen, Davidson, Ferdaña, Finlayson, Halpern, Jorge, Lombana, Lourie, Martin, McManus, Molnar, Recchia and Robertson2007)

Specimens were deposited in the Porifera Collection of Museu Nacional of the Universidade Federal do Rio de Janeiro (MNRJ – Rio de Janeiro, Brazil). Comparative material was obtained from Museo di Storia Naturale Giacomo Doria (MSNG – Genoa, Italy). This publication and nomenclatural acts were registered in ZooBank: urn:lsid:zoobank.org:pub:8756F0E4-9E43-42F3-8A9F-18A34FFE4C72. For taxonomic descriptions we proceeded with the preparation of thick sections, and dissociated spicules for light (LM) and electron microscopy (SEM), following the methods described in Hajdu et al. (Reference Hajdu, Peixinho and Fernandez2011). SEM micrographs were obtained using a JEOL 6390 LV of the Departamento de Invertebrados of Museu Nacional/UFRJ. Spicule dimensions were based on the measurements of 30 spicules per specimen, and expressed as minimum–mean–maximum for length × width. DNA was extracted and polymerase chain reactions were attempted as described in Supplementary File 1.
Results
Systematics
Class DEMOSPONGIAE Sollas, 1885
Order HAPLOSCLERIDA Topsent, 1928
Family CHALINIDAE Gray, 1867
Genus Haliclona Grant, 1841
Definition
Chalinidae with unispicular secondary lines (de Weerdt, Reference de Weerdt, Hooper and van Soest2002).
Subgenus Haliclona (Halichoclona) de Laubenfels, 1932
Definition
Chalinidae with a choanosomal skeleton consisting of a subisotropic, somewhat confused reticulation, commonly intercepted by many choanosomal spaces. Ectosomal skeleton of the same structure as the choanosome, usually very loosely overlaying the choanosome, from which it may be separated by extensive subectosomal spaces. Spongin absent or very scarce, at the nodes of the spicules. Megascleres usually acerate or hastate oxeas. Microscleres, if present, microxeas or sigmas. Sponges commonly relatively crisp and brittle, only slightly compressible (de Weerdt, Reference de Weerdt, Hooper and van Soest2002).
Haliclona (Halichoclona) insularis sp. nov.
urn:lsid:zoobank.org:act:B1B128DF-69CB-41F6-A261-276B787817E4
(Figures 2A–C & 3A–J; Table 2)

Fig. 2. Morphological variability of Haliclona (Halichoclona) insularis sp. nov. in situ. (A–B) Completely beige specimens, with great development of fistular outgrowths. (A) MNRJ 6655, holotype (yellow sponge unidentified). (C–E) Specimens with combination of red and beige colour on the surface, and also with fistular outgrowth. (C) Notice the white interior. (D) MNRJ 6284. (E) MNRJ 6665. White arrows indicate fistular outgrowths.

Fig. 3. Skeletal architecture and oxeas of Haliclona (Halichoclona) insularis sp. nov. (A–C, holotype) MNRJ 6655. (D–F, paratype) MNRJ 2906. (G–I, paratype) MNRJ 7898. From left to right: ectosome, choanosome and choanosome in detail. (J) Oxeas, from MNRJ 6251. (A–I) light microscopy images. (J) SEM images.
Table 2. Spicule measurements of Haliclona (Halichoclona) insularis sp. nov.

Material examined
Holotype: MNRJ 6655 – Âncoras tide pool (Rocas Atoll, Rio Grande do Norte State), 0.5–4 m depth, coll. E. Hajdu, M. Oliveira & U. Pinheiro, 31 August 2002. Paratypes: MNRJ 2906 – Âncoras tide pool (Rocas Atoll, Rio Grande do Norte State), 0.5–4 m depth, coll. F. Moraes, 24 November 1999; MNRJ 7898 – Buraco da Raquel (Fernando de Noronha Archipelago, Pernambuco State), 2 m depth, coll. F. Moraes, 18 November 2003; Additional material examined: MNRJ 6251 – Cemiteriozinho tide pool (Rocas Atoll, Rio Grande do Norte State), 0.5–4 m depth, coll. E. Hajdu, M. Oliveira & U. Pinheiro, 22 August 2002; MNRJ 6284 – Mapas tide pool (Rocas Atoll, Rio Grande do Norte State), Rocas Atoll (RN), ~5 m depth, coll. E. Hajdu, M. Oliveira & U. Pinheiro, 23 August 2002; MNRJ 6398, 6946 – Mapas tide pool (Rocas Atoll, Rio Grande do Norte State), ~5 m depth, coll. E. Hajdu, M. Oliveira & U. Pinheiro, 29 August 2002; MNRJ 6665 – Âncoras tide pool (Rocas Atoll, Rio Grande do Norte State), 0.5–4 m depth, coll. E. Hajdu, M. Oliveira & U. Pinheiro, 1 September 2002.
Comparative material examined
Adocia perforata Pulitzer-Finali, 1986: MSNG 47706 – holotype (a fragment deposited on MNRJ 19916), La Parguera (Puerto Rico), 0.5–1.0 m depth, coll. G. Pulitzer-Finali, 18 May 1964.
Diagnosis
The species is distinguished from other Haliclona spp. in the Tropical Western Atlantic by the combination of surface with light to deep red colour intermixed with white to beige areas (occasionally completely beige), white to beige choanosome, small fistular outgrowths, fragile consistency and blunt oxeas (90–175 × 1–5 μm) with irregular tips.
Description
Irregularly massive to cushion-shaped (Figure 2), ~2–5 cm in thickness/height. Small fistular outgrowths project from the surface up to 6 mm high, but these are mostly lost in preserved specimens. Oscula circular, 0.4–1 cm in diameter, and flush with the surface. Surface smooth, uneven, punctate, but also easily detached from the choanosome in most parts. Consistency in preserved specimens, very fragile, brittle, not resilient, resembling a soft ‘crumb-of-bread’. Most collected specimens, now completely fragmented in small pieces. Colour alive beige (Figure 2A, B) or a combination of an ectosome with light to deeply red coloured areas intermixed with white to beige coloured areas (Figure 2C–E), choanosome always white to beige.
Skeleton
Ectosome a dense, regular, tangential, isodictyal reticulation of delicate, unispicular, three- or four-sided meshes (Figure 3A, D, G); reticulation denser and confused in parts of the ectosome, or with wide circular meshes in other parts, presumably associated to inhalant areas on the surface. Choanosome, a dense, isodictyal to isotropic reticulation of uni- to paucispicular (1–3 spicules) triangular meshes, with additional spicules in confusion (Figure 3B, C, E, F, H, I). Choanosomal spaces also present, 70‒780 μm in diameter, subectosomal spaces not observed (Figure 3B, E, H). Spongin scarce, found in small amounts at the nodes of the reticulation.
Spicules
Oxeas, slightly curved, with long, blunt and mostly irregular tips (Figure 3J), 90–132–175 × 1.0–2.5–5.0 μm (Table 2).
Ecology and distribution
Collected in shallow waters of Brazilian oceanic islands, in reefs or rocky shores, in cryptic microhabitats of areas highly influenced by tidal movement, whose bottom is mostly composed of calcareous algae and rocks. This species is provisionally endemic from the Fernando de Noronha and Atol das Rocas Ecoregion. In Fernando de Noronha Archipelago, it was only found at Buraco da Raquel, in the main island, a locality with similar structure to the reef pools at Rocas Atoll (Moraes, Reference Moraes2011).
Etymology
The name insularis refers to this species' distribution, seemingly limited to oceanic islands in the Brazilian EEZ.
Remarks
There are another seven species of Haliclona (Halichoclona) in the TWA, namely: H. (Halich.) albifragilis (Hechtel, 1965); H. (Halich.) dura Sandes et al., 2014; H. (Halich.) lernerae Campos et al., 2005; H. (Halich.) magnifica de Weerdt et al., 1991; H. (Halich.) pulitzerfinalii van Soest & Hooper, 2020 (originally described as Adocia perforata Pulitzer-Finali, 1986); H. (Halich.) vansoesti de Weerdt et al., 1999 and H. (Halich.) stoneae de Weerdt, 2000. The species that approaches the new species the most by the combination of two or more colours are H. (Halich.) pulitzerfinalii and H. (Halich.) vansoesti.
Haliclona (Halich.) pulitzerfinalii (as A. perforata) was considered by de Weerdt (Reference de Weerdt2000: 64) as incertae sedis, since ‘It has a skeleton not unlike the subgenus Halichoclona … but it is denser, approaching Xestospongia’. In the World Porifera Database - WPD (de Voogd et al., Reference de Voogd, Alvarez, Boury-Esnault, Carballo, Díaz, Dohrmann, Downey, Hajdu, Hooper, Kelly, Klautau, Manconi, Morrow, Pisera, Ríos, Rützler, Schönberg, Vacelet and van Soest2022), this species is assigned to subgenus Halichoclona, although this decision is apparently not based on a published source. Nevertheless, we had the opportunity to examine a fragment of the holotype, and found its skeletal architecture to agree with the WPD assignment. The species fits best in the current definition of H. (Halichoclona) than in Xestospongia, mainly because of its subisotropic skeleton with small oxeas (110–115 μm), as opposed to oxeas usually longer than 200 μm in Xestospongia, and usually arranged in pauci- to multispicular longitudinal tracts with wide-spaced roundish meshes [sensu Systema Porifera (Desqueyroux-Faúndez & Valentine, Reference Desqueyroux-Faúndez and Valentine2002)].
When comparing H. (Halich.) insularis sp. nov. and H. (Halich.) pulitzerfinalii, a striking distinction stems from their colour alive. It is red/beige externally, and white/beige internally in H. (Halich.) insularis sp. nov.; but dull violaceus externally, and ‘tan-cream’ (beige) internally, in H. (Halich.) pulitzerfinalii. In addition, our reexamination of the holotype made us confident that both species are distinct since H. (Halich.) pulitzerfinalii has a firm consistency, instead of the fragile, crumb-of-bread like consistency of the new species. Also, H. (Halich.) insularis sp. nov. has longer oxeas (90–175 μm) than H. (Halich.) pulitzerfinalii (100–115 μm). The common small fistular outgrowths are also characteristic of the new species.
Another species in the TWA approaching the new species regarding the presence of a two-colour combination is H. (Halich.) vansoesti. The new species is, nevertheless, easily distinguished from H. (Halich.) vansoesti by the coloured surface combined with a beige choanosome, in contrast with the semi-transparent ectosome and more diverse colour possibilities in the choanosome (lilac, pink or beige) of the latter. Moreover, the oxeas in H. (Halich.) insularis sp. nov. are shorter and thinner than those of H. (Halich.) vansoesti (90–175 × 1–5 μm in the former vs 120–222 × 3.6–12 μm in the latter).
Subgenus Haliclona (Soestella) de Weerdt, 2000
Definition
Chalinidae with a subanisotropic choanosomal skeleton consisting of ill-defined paucispicular primary lines, irregularly connected by paucispicular secondary lines. There is a slight but consistent tendency of the spicules to form rounded meshes. Ectosomal skeleton a discontinuous, tangential, rather open reticulation, due to many rounded meshes framed by spicules in lines of 2–5 spicules thick. Spongin always present at the nodes of spicules, but never abundant. Oxeas usually slender. Microscleres, if present, sigmas, toxas or raphides (de Weerdt, Reference de Weerdt, Hooper and van Soest2002).
Haliclona (Soestella) moraesi sp. nov.
urn:lsid:zoobank.org:act:776B11E4-32A4-4F68-9261-62784F8725B9
(Figures 4A–D, 5A–F, 6A, B, Table 3)

Fig. 4. Morphology in situ of Haliclona (Soestella) moraesi sp. nov. (A–B) Specimens from Rocas Atoll. (C–D) Specimens from Ceará coast. (C) MNRJ 17989, paratype. (D) MNRJ 17794.
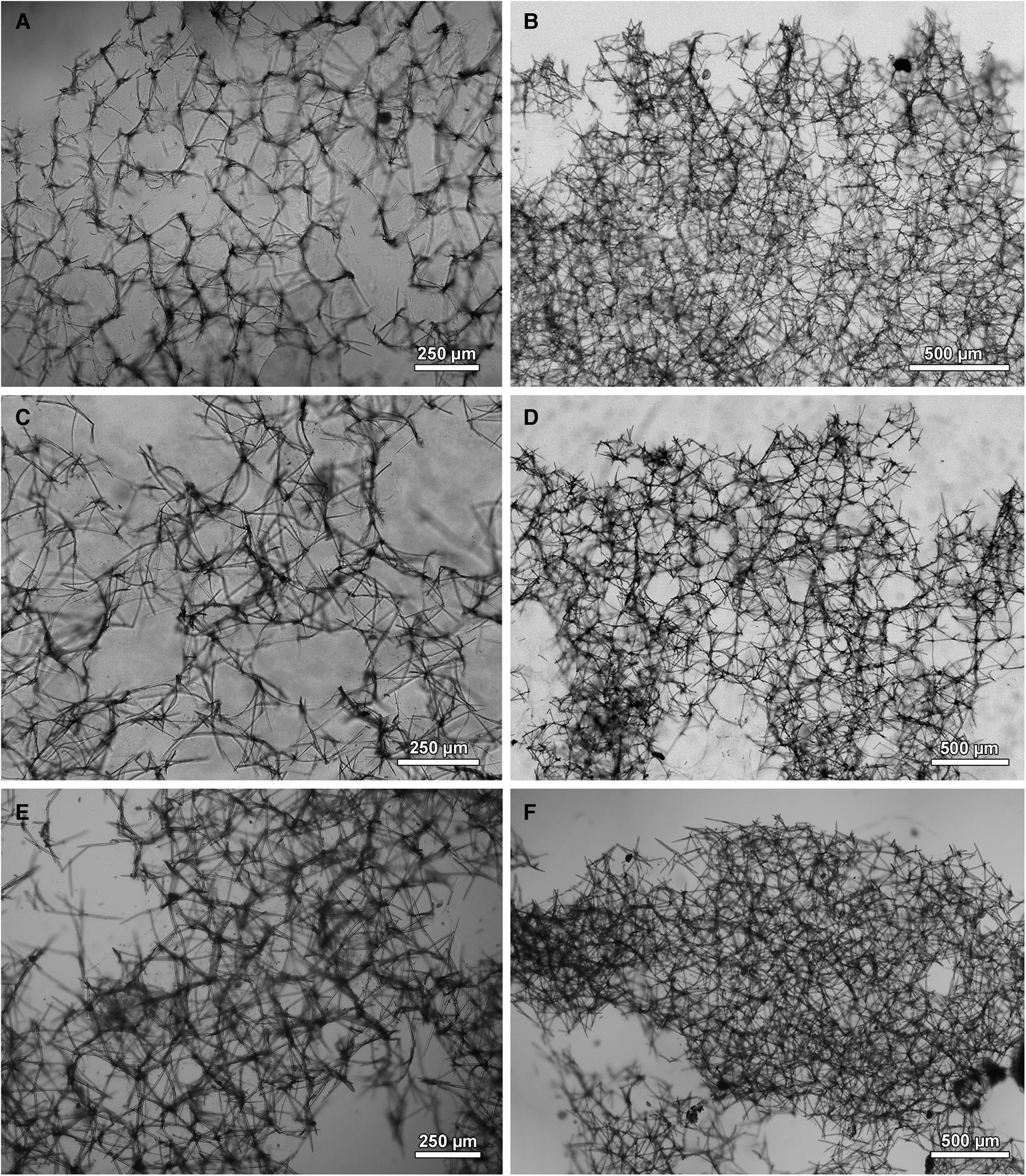
Fig. 5. Skeletal architecture of Haliclona (Soestella) moraesi sp. nov. (A–B) MNRJ 2950, holotype. (C–D) MNRJ 6246, paratype. (E–F) MNRJ 6252. Left column: ectosome. Right column: choanosome.

Fig. 6. Variability in spicules geometry in Haliclona (Soestella) moraesi sp. nov. (A, D1) MNRJ 2950, holotype. (B, D2) MNRJ 6249, paratype. (C) MNRJ 17989, paratype. (A, B, D1, D2) SEM images. (C) light microscopy images.
Table 3. Spicules measurements of Haliclona (Soestella) moraesi sp. nov.

Haliclona sp. Moraes, Reference Moraes2011: 171–173.
Material examined
Holotype: MNRJ 2950 – Âncoras tide pool (Rocas Atoll, Rio Grande do Norte State), 0.5–4 m depth, coll. F. Moraes, 26 November 1999. Paratypes: MNRJ 2916 – Farol tide pool (Rocas Atoll, Rio Grande do Norte State), 1–2 m depth, coll. F. Moraes, 22 November 1999; MNRJ 6246, 6249 – Cemiteriozinho tide pool (Rocas Atoll, Rio Grande do Norte State), 0.5–4 m depth, coll. E. Hajdu, M. Ventura & U. Pinheiro, 22 August 2002; MNRJ 6283 – Garoupinha tide pool (Rocas Atoll, Rio Grande do Norte State), 0.5–3 m depth, coll. E. Hajdu, M. Ventura & U. Pinheiro, 22 August 2002; MNRJ 17989 – Mundaú (Trairi, Ceará State), 1 m depth, coll. A. Bispo, 31 March 2014. Additional material examined: MNRJ 2195 – Âncoras tide pool (Rocas Atoll, Rio Grande do Norte State,), 0.5–4 m depth, coll. G. Muricy, 5 March 1999; MNRJ 2941 – Farol tide pool (Rocas Atoll, Rio Grande do Norte State), 1–2 m depth, coll. E. Vilanova, 20 November 1999; MNRJ 6250, 6252 – Cemiteriozinho tide pool (Rocas Atoll, Rio Grande do Norte State), 0.5–4 m depth, coll. E. Hajdu, M. Ventura & U. Pinheiro, 22 August 2002; MNRJ 6275 – Cemiteriozinho tide pool (Rocas Atoll, Rio Grande do Norte State), 0.5–4 m depth, coll. E. Hajdu, M. Ventura & U. Pinheiro, 22 August 2002; MNRJ 6625 – Tidal pools (Rocas Atoll, Rio Grande do Norte State), 0.5 m depth, coll. E. Hajdu, M. Ventura & U. Pinheiro, 28 August 2002; MNRJ 17774 – Mundaú (Trairi, Ceará State), 1 m depth, coll. A. Bispo, 29 March 2014; MNRJ 17275 – Mundaú (Trairi, Ceará State), 1 m depth, coll. E. Hajdu, 29 March 2014; MNRJ 17794 – Mundaú (Trairi, Ceará State), 1 m depth, coll. A. Bispo, 31 March 2014.
Diagnosis
The only Western Atlantic species of Haliclona with the combination of thickly encrusting to cushion-shaped habit, purple or light pink colour, a dense (sub)isotropic skeleton with rounded meshes, and without microscleres.
Description
Cushion-shaped to thickly encrusting sponge (Figure 4), up to 1.3 cm thick. Oscula circular, 1.0–3.2 mm in diameter, flush with the surface, or in slightly elevated tubular projections up to 8 mm high. Surface smooth, even and punctate. Consistency soft and fragile in preserved specimens from Rocas Atoll, but slightly firm and brittle in specimens from Ceará. Colour alive in most specimens is purple (Figure 4A–C), a single specimen was light pink (Figure 4D), turning beige in ethanol.
Skeleton
Ectosome a dense, tangential reticulation, mostly composed of unispicular tracts, creating isotropic to isodictyal meshes, sometimes rounded, with many spicules in confusion (Figure 5A, C, E); occasional paucispicular tracts (2–5 spicules across) present. Choanosome a dense, (sub)isotropic reticulation, generally confused, with paucispicular primary lines (2–7 spicules across) inconsistently present (Figure 5B, D, F). Rounded meshes also present in the choanosome, but not always prominent. Wide choanosomal and subectosomal spaces usually present, 500–2000 μm. Spongin scarce, but present at the nodes of the reticulation.
Spicules
Oxeas, slightly curved, with long, blunt, sometimes irregular tips (Figure 6), 88–148–218 × 1.5–4.1–12 μm (Table 3).
Ecology and distribution
Not very common, found generally in sciophilous areas, in depths between 0 and 3 m (Moraes, Reference Moraes2011). This species is so far only known from Rocas Atoll (3°S), in the Fernando de Noronha and Atol das Rocas Ecoregion and from the Ceará coast.
Etymology
This species is named after Dr Fernando Coreixas de Moraes, who first described the species in his book entitled ‘Esponjas das Ilhas Oceânicas Brasileiras’ (Moraes, Reference Moraes2011), and in honour of his great contribution to our knowledge of the sponges from Brazilian oceanic islands.
Remarks
Haliclona (Soestella) moraesi sp. nov. is assigned to this subgenus due to the tendency of its skeleton to form rounded meshes. It is distinct from other congeners by the combination of thickly encrusting to cushion-shaped habit, pink or purple colour, and a dense (sub)isotropic skeleton with rounded meshes.
Specimens from Rocas Atoll have smaller and thinner oxeas in comparison to specimens from Ceará, which results in the firmer consistency and denser aspect of the skeleton in those from the continental coast. However, pending genetic corroboration, we consider this to be within an acceptable intraspecific variability given the distinct environmental conditions of coastal and oceanic waters. Previous studies in the Colombian Caribbean associated such kinds of variation in spicule dimensions to the greater input of dissolved silica from river plumes in areas closer to the continent (Zea, Reference Zea1987).
In comparison with additional western Atlantic H. (Soestella) spp., only H. (S.) walentinae approaches the new species in colour, since both are purple (Diaz et al., Reference Diaz, Thacker, Rützler, Piantoni, Custódio, Lôbo-Hajdu, Hajdu and Muricy2007). However, the new species is set apart from H. (S.) walentinae by the latter's two-colour combination of purple exterior and tan interior, thinly encrusting habit (1–2 mm thickness), resilient consistency, surface with canals converging to the oscula, and filamentous cyanobacteria enveloping the skeletal tracts (Diaz et al., Reference Diaz, Thacker, Rützler, Piantoni, Custódio, Lôbo-Hajdu, Hajdu and Muricy2007). The remaining H. (Soestella) spp. in the Western Atlantic are all distinct from the new species by habit and/or spiculation (see Bispo et al., Reference Bispo, Correia and Hajdu2016: Table).
Other Haliclona spp. with similar colour to H. (S.) moraesi sp. nov. are H. (Halicl.) lilacea, H. (Re.) implexiformis, H. (Re.) portroyalensis, H. (Re.) tubifera, H. (Halich.) magnifica de Weerdt et al., 1991, H. (Halich.) pulitzerfinalii, H. (Halich.) stoneae de Weerdt, Reference de Weerdt2000 and H. (Halich.) vansoesti. They are all distinguished from the new species by several features of habit, spicules and skeleton, the latter implicit in their currently distinct subgeneric assignments.
The new species is distinguished from H. (Halicl.) lilacea due to the latter's thinly encrusting habit, inconspicuous oscula, conulose surface, and occurrence of ascending multispicular tracts. Conversely, H. (Re.) implexiformis, H. (Re.) portroyalensis and H. (Re.) tubifera are distinguished by their delicate, isodictyal, and unispicular renieroid architecture (de Weerdt, Reference de Weerdt2000). In addition, H. (Re.) portroyalensis has toxas (Jackson et al., Reference Jackson, de Weerdt and Webber2006), and H. (Re.) tubifera has a very distinctive shape, with oscular tubes elevated up to 5 cm, and many thin proliferations branching from the sponge body (de Weerdt, Reference de Weerdt2000). None of these features has been observed in H. (S.) moraesi sp. nov. On the other hand, H. (Halich.) magnifica develops thick-walled tubes and fistular projections in the surface (de Weerdt, Reference de Weerdt2000), which are not present in H. (S.) moraesi sp. nov. The larger oxeas (286–359 μm) and the presence of sigmas in H. (Halich.) stonae also set it apart from the new species (de Weerdt, Reference de Weerdt2000). Lastly, the presence of oscules on top of short tubular projections with thin and translucent walls in addition to the translucent, whitish and detachable ectosome of H. (Halich.) vansoesti (de Weerdt, Reference de Weerdt2000; Muricy et al., Reference Muricy, Esteves, Monteiro, Rodrigues and Albano2015) clearly sets this species apart from H. (S.) moraesi sp. nov., which can also develop tubular projections, but never with translucent walls. Still, the ectosome in the new species is neither translucent, nor detachable.
Discussion
The description of two new species of Haliclona expands the number of species of this genus in the Brazilian coast to 17. They also fill the knowledge gap for the family Chalinidae in Fernando de Noronha and Rocas Atoll, from where only H. (S.) moraesi (as Haliclona sp. in Moraes, Reference Moraes2011) was previously known. It is important to highlight that these species were deposited in collections between 1999 and 2014, being a clear example of the Linnean shortfall (Lomolino, Reference Lomolino, Lomolino and Lawrence2004) and the taxonomic impediment (Wheeler et al., Reference Wheeler, Raven and Wilson2004). Formal description for these collections were delayed by 15 years, after properly training taxonomists in Haplosclerida systematics (e.g. Bispo et al., Reference Bispo, Correia and Hajdu2016; Rocha et al., Reference Rocha, Moraes, Salani and Hajdu2021; present study). Surely, these shortfalls limit our knowledge on basic biodiversity data, such as local checklists, species distribution and patterns of endemism. Ultimately, they hamper conservation planning (Cardoso et al., Reference Cardoso, Erwin, Borges and New2011) considering that marine sponges are one of the main benthic biological resources in the Brazilian Exclusive Economic Zone (Lavrado & Ignacio, Reference Lavrado and Ignacio2006), especially in the contexts of global warming and increasing environmental impacts of anthropogenic sources.
Although we succeeded in extracting genomic DNA from both H. (Halich.) insularis sp. nov. and H. (S.) moraesi sp. nov., all the attempts to amplify COI and 28S genes from the material studied were unsuccessful in spite of running different protocols (including the addition of additives such as BSA, modification of annealing temperature, increasing of denaturation temperature, etc.) (Supplementary File 1). Thus, description of the new species resorts solely on morphology. Nevertheless, both species are morphologically very distinct from the remaining Haliclona spp. occurring in the Tropical Western Atlantic (Bispo et al., Reference Bispo, Correia and Hajdu2016: Table 2) so we decided not to postpone their formal description any further. The solidity of the proposition of these two species can be confirmed with molecular approaches in the future, especially after the design of new and more specific primers and the collection of fresh material from where high-quality DNA for downstream application could be obtained.
The variability of the shape and size of oxeas in H. (S.) moraesi sp. nov. in coastal vs oceanic waters points to the need for a cautious interpretation of these features for taxonomic purposes in Haplosclerida, especially when studying populations living under different environmental conditions. As already stressed in the Remarks section for this species, the smaller sizes and more irregular tips in specimens from Rocas Atoll are likely related to smaller amounts of dissolved silica available on oceanic waters in comparison to the continental coast (Zea, Reference Zea1987).
Regarding species distribution, H. (Halich.) insularis sp. nov. occurrence is restricted to Rocas Atoll and Fernando de Noronha. In turn, the distribution of H. (S.) moraesi sp. nov. is intriguing, as it is found in Rocas Atoll and in coastal waters along Ceará State. It is well-known that oceanic islands in north-east Brazil are part of the Fernando de Noronha Chain, a group of seamounts geographically close to the continental slope (Almeida, Reference Almeida2015). These seamounts likely acted as a refuge during the Last Glacial Maximum (LGM) (Souza et al., Reference Souza, Dias Júnior, Galetti, Machado, Pichorim and Molina2015; Peluso et al., Reference Peluso, Tascheri, Nunes, Castro, Pires and Zilberberg2018), where the sea level lowered about 130 m and most of the continental shelf along the Brazilian coast emerged above sea level resulting in a great reduction of available habitats (Ludt & Rocha, Reference Ludt and Rocha2015). Study on the population genetics of the stony coral Mussismilia hispida (Peluso et al., Reference Peluso, Tascheri, Nunes, Castro, Pires and Zilberberg2018) revealed that genetic diversity in Fernando de Noronha and Rocas Atoll are comparable to that observed in coastal areas. This contradicts island biogeography theory, and reinforces the hypothesis of population expansion in oceanic islands during the LGM, making these islands the main source of migrants to the coast (Souza et al., Reference Souza, Dias Júnior, Galetti, Machado, Pichorim and Molina2015; Peluso et al., Reference Peluso, Tascheri, Nunes, Castro, Pires and Zilberberg2018). Whether this is the case of H. (S.) moraesi sp. nov. remains to be investigated through population genetics studies. In time, a population genetic study of the Chondrilla nucula complex (Klautau et al., Reference Klautau, Russo, Lazoski, Boury-Esnault, Thorpe and Solé-Cava1999) showed an overall similar pattern to that found in M. hispida (Peluso et al., Reference Peluso, Tascheri, Nunes, Castro, Pires and Zilberberg2018), with populations of ‘Chondrilla D’ from Fernando de Noronha and central portions of the Brazilian coast (down to Arraial do Cabo) showing higher genetic diversity than those in its southern limit. Nevertheless, in the case of C. nucula, migration scenarios were not tested or hypothesized by the authors at that time.
Despite the polyphyly identified by molecular markers, the use of genera and subgenera in Chalinidae still has practical utility, considering that species of the same (sub)genus share among themselves a morphospace that is useful in the delimitation of new species. There are few examples of species where the diagnostic characteristics of one (sub)genus overlap with those of others. For example, some species of Chalinula Schmidt, 1868, such as C. molitba (de Laubenfels, 1949), have great variability in their skeletal architecture and can be easily confused with Haliclona (Reniera) (de Weerdt, Reference de Weerdt2000). Also, some species of H. (Reniera) may have multispicular bundles, such as H. (Re.) tubifera (George & Wilson, 1919) and H. (Re.) urceolus (Rathke & Vahl, 1806) (de Weerdt, Reference de Weerdt1986, Reference de Weerdt2000), which may lead to confusion with Cladocroce Topsent, 1982. Attention should be taken with H. (Soestella), especially because the development of rounded meshes is not always prominent, e.g. H. (S.) caerulea, H. (S.) piscaderaensis (van Soest, 1980), H. (S.) vermeuleni de Weerdt, Reference de Weerdt2000 and H. (S.) walentinae Diaz et al., Reference Diaz, Thacker, Rützler, Piantoni, Custódio, Lôbo-Hajdu, Hajdu and Muricy2007 (de Weerdt, Reference de Weerdt2000; Diaz et al., Reference Diaz, Thacker, Rützler, Piantoni, Custódio, Lôbo-Hajdu, Hajdu and Muricy2007).
Thus, proposition of new H. (Soestella) species should provide comparisons with congeners sharing a similar external morphology regardless of subgenus. While proposition of new H. (Reniera) species should also consider similar species of Chalinula and Cladocroce and vice versa. It is noteworthy that the usefulness of diagnostic characters of Chalinidae (sub)genera for species delimitation can still be tested using an integrative taxonomic approach, using morphology and molecular data, which will allow a better assessment of the morphological variability limits of each species.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315423000140.
Acknowledgements
Dr Giuliano Doria (MSNG) is thanked for providing access to the type of Adocia perforata Pulitzer-Finali, 1986. We also acknowledge Prof. Dr Guilherme Muricy, Prof. Dr Ulisses Pinheiro, Drs Mariana Carvalho, Camille Leal, Fernando Moraes and Sula Salani, as well as Maira V. de Oliveira for collecting or assistance during collections of studied materials. We are grateful to the anonymous reviewer whose comments and suggestions were important for the improvement of the manuscript.
Financial support
AB is grateful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the provision of a doctoral scholarship and to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for his postdoctoral fellowship (PDJ 152552/2022-7). EH is grateful to CAPES (CIMAR 23038.001427/2014-15), CNPq (Universal, 478978/2001-4; PNPD, 560308/2010-8; Productivity, 308811/2013-5, 308303/2017-2), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (CNE 102.292/2013, 202.624/2019; Apoio Emergencial ao Museu Nacional 200.124/2019).











