INTRODUCTION
Tuberculosis (TB) poses a global public health threat and remains one of the major causes of death from infectious diseases. The WHO estimated that 9·4 million people worldwide suffered from TB in 2010, representing 140/100 000 person-years (p-yr) [1]. The TB burden in Taiwan was approximately 69·8/100 000 p-yr in 2008 (94% for pulmonary TB and 6% for extrapulmonary TB) [2]. With an ageing or deteriorating immune system, the causative microorganism Mycobacterium tuberculosis can reactivate and cause severe and prolonged pneumonia, pulmonary scarring, and wasting. Risk factors for TB are often caused by impaired host immunity or increased exposure to TB bacteria. Immunocompromised patients, such as those with HIV infection, autoimmune diseases, and end-stage renal disease (ESRD) requiring haemodialysis, have an increased risk of TB [Reference Antonucci3].
The increased risk of TB in ESRD patients has been the subject of attention for several decades. The incidence of TB is higher in ESRD patients than in the general population because of impaired host immunity [Reference Sen4, Reference Hussein, Mooij and Roujouleh5]. A previous investigation reported increased incidence rates (6·9- to 52·5-fold) of TB in patients with ESRD and also receiving dialysis compared to the general population [Reference Hussein, Mooij and Roujouleh5]. Some previous studies have described a higher incidence of TB in ESRD patients [Reference Sen4, Reference Chou6–Reference Lee8]. However, those results might not be generalizable because they were a case report [Reference Kostov and Petrov7], a case-control study [Reference Chou6], used a small-sized sample from a university hospital [Reference Sen4], or evaluated self-reported TB disease and previous TB contact, which might thus be subject to recall bias [Reference Lee8]. The previous studies also had no control group, or did not observe the incidence of ESRD cases and follow them long enough to investigate the ensuing risks of TB.
ESRD and TB have both have large effects on public health. Taiwan has the highest incidence and prevalence rates of ESRD in the world [9]. The incidence of ESRD in Taiwan was 361/million population, while similarly high rates were found in the USA and Japan, at 369 and 288, respectively, in 2010. The prevalence of ESRD was 2584/million in 2010 in Taiwan, and 2260 and 1870, respectively, in Japan and the USA. The increased risk for TB in ESRD patients, therefore, warrants further detailed investigation. The present nationwide population-based cohort study included 4131 ESRD patients receiving dialysis and 16 558 controls to evaluate the risk of subsequent TB.
METHODS
Data sources
National Health Insurance (NHI) is a mandatory universal health insurance programme, offering comprehensive medical care coverage to all Taiwanese residents. Up to 99% of the residents in Taiwan (∼23 million people) have been enrolled in the NHI programme since 1996. The NHI sample files, which are constructed and managed by the National Health Research Institute (NHRI), consist of comprehensive use and enrolment information for a randomly selected sample of 1 000 000 NHI beneficiaries, representing about 5% of all enrollees in Taiwan in 2000. A multistage stratified systematic sampling design was used. There were no statistically significant differences in sex or age between the sample group and all enrollees. Comprehensive healthcare data included the enrolment files, the claims data, the catastrophic illness files, and the registry for drug prescriptions.
All information allowing a specific patient to be identified was encrypted. The confidentiality of the data abides by the data regulations of the Bureau of National Health Insurance. This study was approved by the NHRI. The Institutional Review Board of National Yang Ming University also approved this study (IRB no. 1000130).
Study population
A retrospective cohort study was conducted from 1 January 1998 to 31 December 2009, based on ambulatory care and inpatient discharge records. All the newly diagnosed ESRD patients receiving dialysis from 1 January 1998 to 31 December 2008 were selected as the study cohort. All patients were follow-up until 31 December 2009. ESRD patients needing dialysis can apply for a catastrophic illness record in Taiwan. Records of a patient's catastrophic illness are entered on the insurance card, enabling patients with such illnesses to be treated without having to pay a co-payment upon presentation of their insurance card. ESRD patients needing dialysis can apply for a catastrophic illness record in Taiwan. From 1998 to 2009, 5073 patients received more than 10 dialysis treatments in 4 weeks and there were 761 (15%) patients without a catastrophic illness record. Most patients without catastrophic illness record received continual dialysis for less than 6 months, and were therefore not considered to be representative ESRD patients. We therefore required ESRD patients in our study to hold a catastrophic illness record. A different coding system ‘A code’ was implemented for outpatient clinics and departments in Taiwan before 2000. The ‘A code’ system was developed by the NHI in Taiwan and was based on the International Classification of Diseases, Ninth Revision (ICD-9) [10]. After 2000, all outpatient clinics and departments were required by the NHI to use the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). ESRD was defined by a compatible ICD-9-CM code (585) or A code (A350) and the patient holding a catastrophic illness record on the insurance card. Patients aged <20 years, and those who had TB diagnosed before or within 30 days of incidence cases of ESRD, were excluded [Reference Li11]. A total of 4131 ESRD patients (including 3901 haemodialysis patients and 230 peritoneal dialysis patients) receiving dialysis were included in the analyses (Fig. 1).
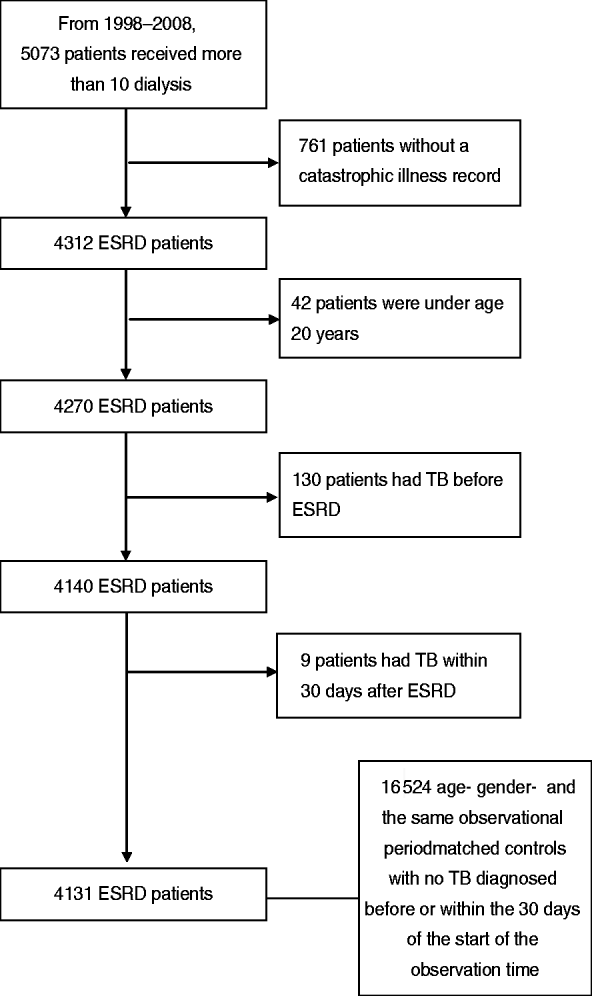
Fig. 1. Patient selection flow chart. ESRD, End-stage renal disease; TB, tuberculosis.
Comorbidities were defined based on the claims data with at least five ambulatory visits. These included diabetes mellitus (ICD-9-CM code 250 or A code A181), hypertension (ICD-9-CM codes 401-405 or A code A26), silicosis (ICD-9-CM codes 501-504 or A code A326), chronic obstructive pulmonary disease (COPD) (ICD-9-CM codes 490-492, 496 or A codes A323, A325), connective tissue diseases (ICD-9-CM codes 710, 714, or A codes A430, A431), malignancy (ICD-9-CM codes 140-239 or A codes A08-A14), HIV infection (ICD-9-CM codes 042, 043, V08) and renal or other organ transplants (V42).
Control group
Patients without an ESRD record were used as the control group. Within each age/sex grouping, four controls were selected at random from the database in the same observational period. Similar to ESRD patients, those with TB diagnosed before or within 30 days of the start of the observation time were excluded. A total of 16524 patients served as a comparison group.
Incidence of TB disease
The incidence of TB disease was defined by compatible ICD-9-CM codes (010-018) or the A code (A02), and the prescriptions of at least two anti-TB medications for more than 28 days. A previous study also used the same criteria to identify TB [Reference Wu12]. The TB incidence rate was calculated as the number of TB patients divided by the observed person-years.
TB risk analysis
The two cohorts were followed until the development of TB, death, or the end of 2009. Each patient was followed from incidence care for ESRD up to a maximum of 12 years. Incidence rates (IRs)/100 000 p-yr and incidence rate ratios (IRRs) of TB were evaluated.
Statistical analysis
The incidence of TB in ESRD patients and controls was compared using IRs and IRRs. Cumulative incidence analyses were performed using the Kaplan–Meier method, and the differences between the curves were tested using the log-rank test. The Cox proportional hazards model was used to calculate the hazard ratio (HR) and 95% confidence interval (CI) to determine if having ESRD and receiving dialysis is a risk factor for development of TB. Variables in this model included age, sex, hypertension, silicosis, COPD, connective tissue disease, and malignancy. The software SAS 9.2 (SAS Institute Inc., Cary, NC, USA) was used to link the data; Stata v. 10 (Stata Corporation, USA) was used to perform the statistical analyses.
RESULTS
Demographic data
Between 1998 and 2009, the evaluated patients consisted of 4131 ESRD patients receiving dialysis and 16 524 age- and gender-matched controls. The mean duration of follow-up in the ESRD patients was 4·93 years; in the controls it was 4·99 years. The observation periods were 21 620 p-yr in the ESRD cases and 87 668 p-yr in the controls. Individual matching resulted in comparable distributions of age and sex between the ESRD patient and control groups. Table 1 shows the characteristics of the study samples with other comorbidity data, including diabetes mellitus, hypertension, silicosis, COPD, connective tissue disease, malignancy, and HIV infection. ESRD patients receiving dialysis had a higher prevalence of diabetes mellitus and hypertension than the controls.
Table 1. Baseline characteristics of ESRD patients and age- and gender-matched controls
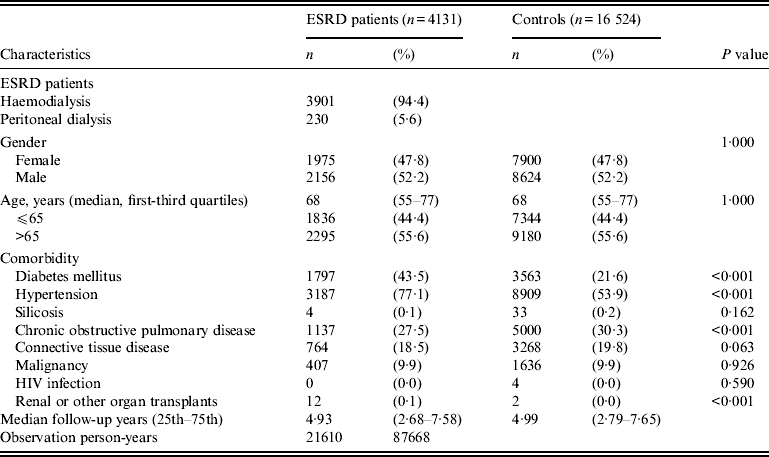
ESRD, End-stage renal disease.
Incidence rates for TB
As shown in Table 2, the ESRD patients receiving dialysis were associated with a significantly higher risk of pulmonary TB compared to controls (IRR 2·34, 95% CI 1·82–2·99). In analysis, stratified by age and sex, comparing ESRD patients receiving dialysis to the control group, there was an association between TB and ESRD. The IR of TB after ESRD and receiving dialysis was 486/100 000 p-yr, which was significantly higher than the IR of TB of 208/100 000 p-yr in the control group. The average interval from onset of ESRD to diagnosis of TB was 2·54 years (95% CI 2·05–3·02).
Table 2 Characteristics of TB cases in ESRD patients and controls
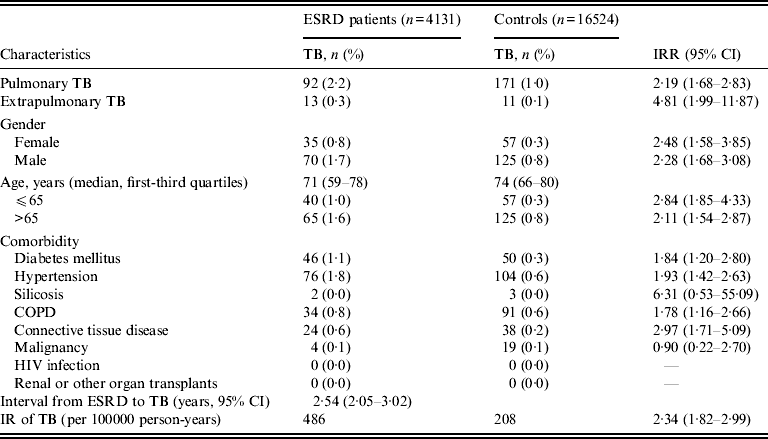
ESRD, End-stage renal disease; TB, tuberculosis; CI, confidence interval; COPD, Chronic obstructive pulmonary disease IR, incidence rate; IRR, incidence rate ratio.
Table 3 shows the risk of TB according to year of follow-up for ESRD and control patients. Compared to the control group, the IRRs of the ESRD with dialysis cohort were 4·13 (95% CI 2·56–6·68), 2·12 (95% CI 1·36–3·27), 1·35 (95% CI 0·68–2·52), 1·73 (95% CI 0·88–3·26), and 2·06 (95% CI 0·33–9·63) within 1 year, 1–2 years, 3–4 years, 5–8 years, and 9–12 years, respectively. The ESRD/dialysis cohort experienced significantly higher risk of TB within the first 2 years of occurrence.
Table 3 Temporal association of tuberculosis incidences in ESRD patients and controls
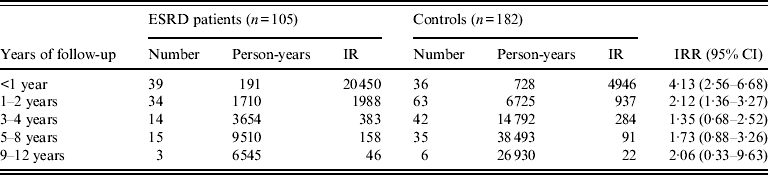
ESRD, End-stage renal disease; CI, confidence interval; IR, incidence rate (per 100 000 person-years); IRR, incidence rate ratio.
Cumulative incidence and relative risks of TB
Figure 2 shows the Kaplan–Meier estimates of the cumulative incidences of TB for ESRD patients and controls. The cumulative incidence of TB in ESRD patients was significantly higher than that of the controls. As shown in Table 4, the Cox proportional hazards analysis identified ESRD (HR 2·40, 95% CI 1·87–3·08), age >65 years (HR 2·41, 95% CI 1·83–3·17), male sex (HR 1·94, 95% CI 1·51–2·49) diabetes mellitus (HR 1·36, 95% CI 1·05–1·76), silicosis (HR 7·70, 95% CI 3·15–18·83) and COPD (HR 1·61, 95% CI 1·26–2·06) as independent risk factors for TB. In the ESRD patients, compared to haemodialysis patients, peritoneal dialysis patients had no significantly higher risk of TB disease (HR 0·43, 95% CI 0·10–1·73).
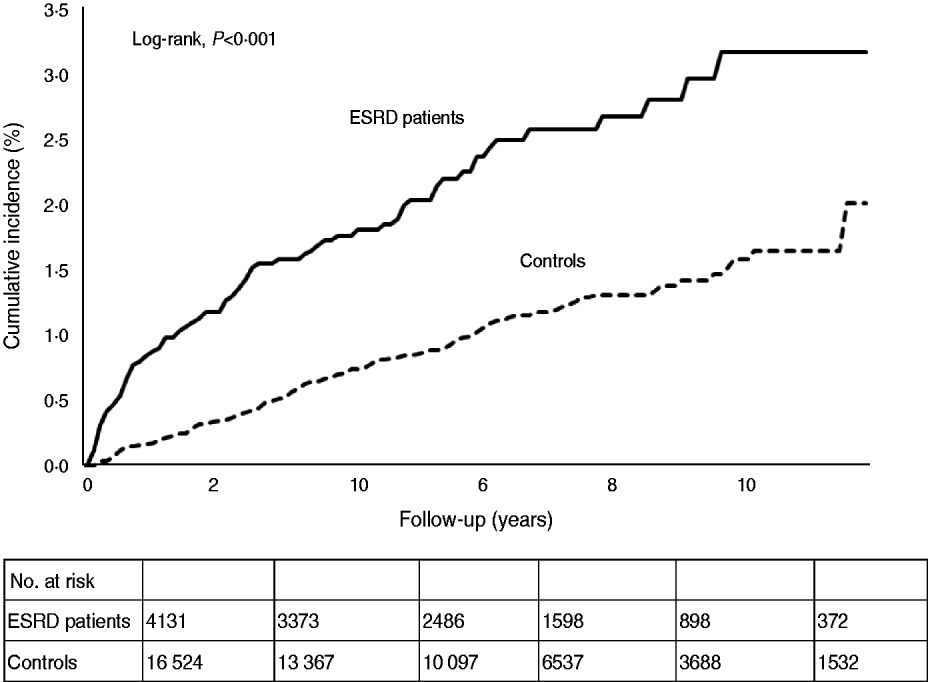
Fig. 2. Cumulative incidences of tuberculosis for the end-stage renal disease and control cohorts.
Table 4 Multivariate analysis for the prediction of tuberculosis development
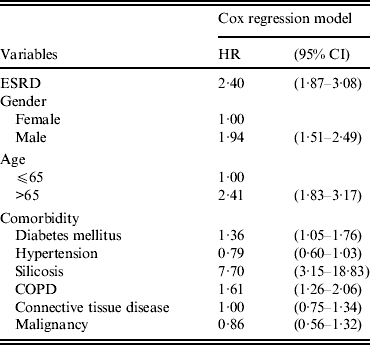
ESRD, End-stage renal disease; HR, hazard ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease.
DISCUSSION
This is a nationwide population-based cohort study to investigate the risk of TB following onset of ESRD. Taiwan has a relatively low TB incidence rate of about 69·8/100 000 p-yr [2]; thus, a relatively large sample size is needed to obtain sufficient statistical power. Most of the previous studies were case reports and hospital-based, with limited numbers of observations, possible selection bias, and a lack of a control group. This study's population-based data includes an age- and gender-matched control group, which provides improved external validation than the previous investigations. Use of NHI claims data enabled the temporal observation of TB following onset of ESRD and receiving of dialysis.
Diagnosis of TB in Taiwan is based on clinical suspicion, followed by chest X-rays, and confirmation using sputum smear and culture. Treatment of TB is similar to common practices in Western countries [13]. Patients receive at least two drugs for the treatment of TB. Patients with latent TB infection were not treated by medications during our study [2]; thus, all selected patients had a confirmed diagnosis of TB using the ICD-9-CM definition and at least two anti-TB medications. By law, TB is a notifiable disease in Taiwan. The Taiwan Centers for Disease Control (CDC) mandate that all patients diagnosed with TB are reported. The incidence rate of TB disease in those aged ⩾55 years for the period between 2002 and 2008 was obtained from released TB statistics from the CDC, Taiwan. The expected rate of TB was 219/100 000 p-yr, which is similar to the TB incidence rate of 208/100 000 p-yr in the control group. The overall TB incidence rate is relatively close to that reported by the CDC in Taiwan [2]. For ESRD diagnosis, both diagnostic codes and the Catastrophic Illness Registry were used. Therefore, ESRD and TB data should be valid.
There is a lengthy time period for entering the cohort. Each patient has a different follow-up time. To verify the relationship between ESRD and TB, we stratified the ESRD patients to different cohort periods (1998–2002, 1998–2004, 1998–2006). All patients in the same cohort had the same duration of follow-up (7 years for 1998–2002, 5 years for 1998–2004, and 3 years for 1998–2006). The HRs for TB were 3·23 (95% CI 2·13–4·89) for the 1998–2002 cohort, 3·03 (95% CI 2·09–4·40) for the 1998–2004 cohort, and 3·62 (95% CI 2·55–5·13) for the 1998–2006 cohort. Patients with ESRD were associated with an increased risk of TB in the different cohort periods under observation. The results remained robust despite stratifying the patients into different cohort periods with the same duration of follow-up. Alternatively, to verify the effects of medical advancement on the relationship between ESRD and TB, we identified two cohort periods (1998–2002 and 2003–2006) and followed them for 3 years. The HR of TB was 4·22 (95% CI 2·41–7·36) for the 1998–2002 cohort, and 2·71 (95% CI 1·86–3·94) for the 2003–2006 cohort. Thus, a significant relationship was observed to exist between ESRD and TB. Future study is necessary to examine subjects entering the cohort at a later time because they would be subject to different medical treatments (which might affect immunity differentially) than those entered earlier.
Study observations, including increased rates of TB in ESRD/dialysis patients compared to controls, had a crude IRR of 2·34 (95% CI 1·82–2·99). These results were consistent with previous studies that reported a higher risk of TB in ESRD patients [Reference Sen4, Reference Hussein, Mooij and Roujouleh5]. After adjusting for possible confounders, the multivariate analysis also revealed having ESRD and receiving dialysis to be a significant risk factor for TB (HR 2·40, 95% CI 1·87–3·08). Increased TB in ESRD patients is biologically plausible. Impairment of the immune system, and three times weekly attendance to hospital for dialysis, might increase the risk of TB. Several previous studies identified a high frequency of TB in the first year of dialysis [Reference Hussein, Mooij and Roujouleh5, Reference Chou6, Reference Cengiz14–Reference Chia16], which was attributed to the patients’ seriously impaired host defence mechanism during the initial dialysis period. In the present study, the mean time between ESRD and TB diagnosis was 2·54 ± 0·48 years. Compared to controls, the ESRD cohort had a significantly higher risk of TB within 1 year (IRR 4·13, 95% CI 2·56–6·68), and 1–2 years (IRR 2·12, 95% CI 1·36–3·27), of ESRD occurrence. ESRD patients should, thus, be monitored more carefully, especially within 2 years of initiating dialysis.
ESRD patients treated with haemodialysis or peritoneal dialysis had a potentially increased probability of TB [Reference Dobler, McDonald and Marks17, Reference Gursu18]. However, no statistically significant difference of TB was found in haemodialysis and peritoneal dialysis patients in our study, which is consistent with a previous study [Reference Li11]. In addition, a statistically significant relationship between ESRD and TB was observed with and without the exclusion of peritoneal dialysis patients (HR 2·45, 95% CI 1·90–3·16 vs. HR 2·40, 95% CI 1·87–3·08). Haemodialysis (94%) is the main treatment modality for patients with ESRD in Taiwan. Therefore, our results are not generalizable to locations, such as Hong Kong, where peritoneal dialysis is the main modality. In addition, compared to the control group, a higher proportion of extrapulmonary TB was noted in ESRD patients (12·4% vs. 6%), with an IRR of 4·81 (95% CI 1·99–11·87). These results are compatible with previous reports that the incidence of TB and the frequency of extrapulmonary involvement are high in dialysis patients [Reference Sen4, Reference Abdelrahman, Sinha and Karkar19].
HIV infection causes an increased risk of TB. According to the WHO 2010 annual report, 15% of TB patients were HIV positive. However, the HIV infection rate in TB patients in Taiwan was only 0·6% in 2007 [2]. In our study, we identified only four patients with HIV infection in the control patients and no patients with HIV infection in the ESRD patients; however, none developed TB during the observation period. In addition, patients with renal or other organ transplants (and accompanying iatrogenic immunosuppression) have an increased risk of TB. There were only 12 patients with renal or other organ transplants in the ESRD patients, and two patients with organ transplants were identified in the control group in our study; however, none of them developed TB during the observation period. Thus, HIV infection, and renal or other organ transplants were not considered to be important factors in our data analysis.
The present study suffers from a few limitations that should also be considered when analysing its results. First, the study population was in Taiwan, where the annual incidence of TB is abouut 62/100 000 p-yr. The development of TB could be affected by demographic and socioeconomic factors; therefore TB incidence in ESRD patients might differ in other countries. Second, observations were retrospective, collected based on diagnostic code and prescription history. Although TB cases diagnosed before ESRD onset were excluded, it was not possible to differentiate newly diagnosed TB cases caused by endogenous reactivation or by recent transmission of TB. Third, ESRD patients receiving dialysis usually received greater medical attention, by way of frequent examinations, which might contribute to a higher rate of notified TB cases in this group. However, Taiwanese visit medical physicians more frequently than those residing in most Western countries possibly due to the compulsory NHI system. The frequency of hospital visits is 29 times/year for people aged 68 years, which is the average age of the control group in this study. The control group is therefore expected to have received appropriate medical attention compared to ESRD patients. In addition, TB symptoms are not usually overlooked; thus overestimation in ESRD patients should occur minimally. Fourth, tobacco smoking is strongly associated with TB [Reference Lin, Ezzati and Murray20–Reference Lin22]. The study database did not include smoking history; therefore, the analyses were unable to include adjustment for smoking as a contributory factor. A previous report described that 40% of men, and less than 5% of women, smoked in 2001 in Taiwan. Using this statistic, it was possible to compare the risk of TB in men and women to evaluate the possible effects of smoking on TB [Reference Lin22]. After stratifying by sex, the results remained robust. The HR for TB was significantly higher in men (HR 2·29, 95% CI 1·68–3·11) and women (HR 2·63, 95% CI 1·69–4·10) ESRD patients. Further research, including more detailed smoking history, could further advance knowledge on this subject.
The major strength of the present study is its nationwide population-based setting, which includes a relatively large number of ESRD patients, and enabled observation of the temporal relationship between onset of ESRD and subsequent TB. The findings provide epidemiological evidence that ESRD/dialysis patients are at risk for subsequent TB. Although we do not know if these patients had reactivated TB from a latent infection primarily within the first 2 years, acquired TB de novo subsequent to visiting hospitals more frequently, or for other unknown reasons, all ESRD patients are recommended to have TB screening using a sputum smear and culture and an X-ray chest examination within 2 years. Until this occurs, patients should be monitored for unusual presentation and non-specific symptoms, such as fever, anorexia, weight loss, and night sweats, on an ongoing basis.
ACKNOWLEDGEMENTS
This study was supported by the Taiwan Ministry of Education, through its ‘Aim of the Top University Plan’, and by Taiwan's National Science Council, through grant no. 99-2314-B-254-001; and grant no. 99TPECH03 from the Taipei City Hospital, Taiwan.
DECLARATION OF INTEREST
None.








