Introduction
Adolescence, the ages marking the transition from childhood to adulthood (i.e. from ~12 to 18 years old), is a key developmental period for the emergence of psychiatric disorders (Kessler et al., Reference Kessler, Berglund, Demler, Jin, Merikangas and Walters2005). Fear disorders, such as social anxiety disorder and specific phobia, and distress disorders, such as depressive disorders and generalized anxiety disorder (GAD), often emerge during late childhood to mid-adolescence (Kessler et al., Reference Kessler, Berglund, Demler, Jin, Merikangas and Walters2005; Lewinsohn, Clarke, Seeley, & Rohde, Reference Lewinsohn, Clarke, Seeley and Rohde1994). The transition to adolescence also exacerbates preexisting behavioral disorders, like oppositional defiant disorder (ODD) and conduct disorder (CD) (Moffitt, Reference Moffitt1993). Childhood and adolescent psychiatric disorders are associated with severe functional impairment and significant economic burden (Copeland, Wolke, Shanahan, & Jane Costello, Reference Copeland, Wolke, Shanahan and Jane Costello2015). Despite this major public health concern, there is still no consensus on the core mechanisms of dysfunction in youth.
The (un)predictability of threat is an important characteristic that impacts attention, defensive motivation, and subjective distress (Grillon, Baas, Lissek, Smith, & Milstein, Reference Grillon, Baas, Lissek, Smith and Milstein2004; Shankman, Robison-Andrew, Nelson, Altman, & Campbell, Reference Shankman, Robison-Andrew, Nelson, Altman and Campbell2011). Sensitivity to unpredictable threat has been identified as a potential core mechanism of psychiatric disorders in adults (Carleton, Reference Carleton2016; Grupe & Nitschke, Reference Grupe and Nitschke2013). Many investigations have examined the startle eyeblink reflex, an indicator of defense system activation, in anticipation of predictable and unpredictable threat. Psychiatric disorders, including panic disorder (PD) (Grillon et al., Reference Grillon, Lissek, Rabin, McDowell, Dvir and Pine2008), social phobia (Gorka, Lieberman, Shankman, & Phan, Reference Gorka, Lieberman, Shankman and Phan2017), depression (Robinson, Overstreet, Letkiewicz, & Grillon, Reference Robinson, Overstreet, Letkiewicz and Grillon2012), and alcohol use disorder (Gorka & Shankman, Reference Gorka and Shankman2017), have been characterized by heightened startle potentiation in anticipation of unpredictable, but not predictable, threat in adults. Behavioral disorders have not been examined in relation to startle potentiation in anticipation of unpredictable threat, but attention-deficit/hyperactivity disorder (ADHD) has been associated with attenuated startle potentiation to aversive and unpleasant stimuli in adults (Conzelmann et al., Reference Conzelmann, Woidich, Mucha, Weyers, Jacob, Lesch and Pauli2011). Research is needed in adolescents, who are in a developmental period of increased risk, and with a broader range of psychiatric disorders.
Individual differences in startle potentiation in anticipation of threat might also index vulnerability for psychiatric disorders. Research has indicated that adolescent offspring of parents with anxiety disorders (Grillon, Dierker, & Merikangas, Reference Grillon, Dierker and Merikangas1998) and adult offspring and adolescent grandchildren of individuals with depression (Grillon et al., Reference Grillon, Warner, Hille, Merikangas, Bruder, Tenke and Weissman2005) demonstrate heightened startle potentiation in a threatening context. Conversely, adolescent offspring of parents with alcoholism demonstrate impaired startle habituation (Grillon, Dierker, & Merikangas, Reference Grillon, Dierker and Merikangas1997). Moreover, one study in adults found that heightened startle potentiation in anticipation of unpredictable threat was associated with family history (i.e. familial risk) of PD (Nelson et al., Reference Nelson, McGowan, Sarapas, Robison-Andrew, Altman, Campbell and Shankman2013). The relationship between startle potentiation in anticipation of unpredictable threat and family history has never been examined in youth.
Attentional biases have also been implicated in multiple psychiatric disorders (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, Reference Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg and van IJzendoorn2007). For example, fear and distress disorders are often associated with attentional biases toward threat (Bar-Haim et al., Reference Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg and van IJzendoorn2007) and sad stimuli (Peckham, McHugh, & Otto, Reference Peckham, McHugh and Otto2010), while behavioral (Cepeda, Cepeda, & Kramer, Reference Cepeda, Cepeda and Kramer2000) and substance use disorders (Heitmann, Jonker, Ostafin, & de Jong, Reference Heitmann, Jonker, Ostafin and de Jong2020) are frequently associated with attentional disengagement. Event-related potentials (ERPs) provide a neurobiological measure of attentional processing, and the P300 is one of the most widely studied indicators of attentional engagement. Distress and fear disorders in adults have largely been associated with an enhanced P300 (Clark, McFarlane, Weber, & Battersby, Reference Clark, McFarlane, Weber and Battersby1996; Enoch, White, Harris, Rohrbaugh, & Goldman, Reference Enoch, White, Harris, Rohrbaugh and Goldman2001), but depression has also been associated with an attenuated P300 (Diner, Holcomb, & Dykman, Reference Diner, Holcomb and Dykman1985). Behavioral and substance use disorders have been consistently associated with an attenuated P300 in adolescents (Patrick et al., Reference Patrick, Bernat, Malone, Iacono, Krueger and McGue2006).
The P300 can also be measured in response to the acoustic startle probe, and research has indicated that the probe P300 is suppressed in anticipation of predictable and unpredictable threat relative to no threat in adults (Nelson & Hajcak, Reference Nelson and Hajcak2017b). P300 suppression reflects increased attentional resources being directed to the threatening context, resulting in less attention being allocated towards the processing of the acoustic startle probe. Though, it is still unclear whether P300 suppression in anticipation of unpredictable threat is associated with psychiatric disorders and/or risk.
Factor analytic studies on the hierarchical structure of common psychiatric disorders have identified two high-order spectra: internalizing and externalizing (Krueger, Reference Krueger1999). Twin studies have suggested that these dimensions influence the risk for psychiatric disorders in offspring through the transmission of genetic risk factors (Kendler et al., Reference Kendler, Walters, Neale, Kessler, Heath and Eaves1995). It has been hypothesized that these latent spectra could reveal fundamental neurobiological mechanisms shared across multiple psychiatric disorders (Watson, Reference Watson2005). However, no study has examined neurobiological sensitivity to unpredictable threat in relation to the internalizing and externalizing spectra.
The present study involved a comprehensive evaluation of neurobiological sensitivity to unpredictable threat as a potential mechanism of dysfunction for both the internalizing and externalizing spectra in adolescents. The sample consisted of 15-year-old adolescents and their biological mothers. Both the adolescent and biological mother completed semi-structured diagnostic interviews about the lifetime history of internalizing and externalizing disorders, and a confirmatory factor analysis was used to model internalizing and externalizing spectra. Adolescents also completed a no (N), predictable (P), and unpredictable (U) threat (NPU-threat) task while the startle reflex and probe P300 were recorded. We hypothesized that the adolescent internalizing spectrum would be positively associated with adolescent sensitivity to unpredictable threat, but the adolescent externalizing spectrum would be negatively associated with adolescent sensitivity to unpredictable threat. We hypothesized a similar relationship for the maternal internalizing and externalizing spectra and adolescent sensitivity to unpredictable threat. Finally, to examine whether the association between sensitivity to unpredictable threat and maternal psychopathology is attributable to offspring who have already developed psychopathology, we tested whether maternal internalizing and externalizing spectra were associated with adolescent sensitivity to unpredictable threat independent of adolescent psychopathology.
Methods
Participants
The sample was obtained from a longitudinal investigation of risk for psychopathology in an unselected community sample of youth from Long Island, NY (Klein & Finsaas, Reference Klein and Finsaas2017). Participants (N = 599) were recruited using a commercial mailing list of families with a 3-year-old child living in a 20-mile radius of Stony Brook University. Following the age 3 assessment, families were invited to participate in follow-up assessments when their child was approximately age 6, 9, 12, and 15. In the age 6 assessment, 50 children from underrepresented minority groups were added to increase the diversity of the sample (final N = 609). Informed consent was obtained from parents and assent was obtained from participants. Study procedures were approved by the Stony Brook University Institutional Review Board, and families were compensated for their participation.
Measures
Schedule for affective disorders and schizophrenia for school-age children: present and lifetime version (K-SADS-PL)
The K-SADS-PL (Kaufman et al., Reference Kaufman, Birmaher, Brent, Rao, Flynn, Moreci and Ryan1997) was administered at the age 9, 12 and 15 assessments, and diagnoses were combined across assessments to obtain lifetime diagnoses through the age 15 assessment. At age 9, lifetime history was assessed, and each subsequent wave (i.e. age 12 and 15) covered the time period since the last assessment. Interviews were conducted by advanced clinical psychology graduate students and a master's level clinician, with supervision conducted by a child psychiatrist and clinical psychologist.
Structured clinical interview for DSM-IV non-patient version (SCID–NP)
The SCID-NP (First, Spitzer, Gibbon, & Williams, Reference First, Spitzer, Gibbon and Williams2007) was administered to the biological mother at the initial and age 9 assessments. Diagnoses were combined across assessments to obtain lifetime maternal diagnoses through the age 9 assessment. Interviews were conducted by two master's or doctoral level clinical psychology students.
Stimuli
Stimuli were administered using PSYLAB (Contact Precision Instruments, London, United Kingdom). Acoustic startle probes were 40-ms duration, 103-dB bursts of white noise with near-instantaneous rise time presented binaurally through headphones. The aversive stimulus was a 1-s duration, 85-dB female scream played through the computer speakers approximately 24 in. in front of the participant. The scream sound was paired with a black and white image of a fearful female face (NimStim image 01_FE_O) (Tottenham et al., Reference Tottenham, Tanaka, Leon, McCarry, Nurse, Hare and Nelson2009).
Procedure
NPU-threat task
In a previous analysis using the same sample and NPU-threat task (Ferry, Beatty, Klein, & Nelson, Reference Ferry, Beatty, Klein and Nelsonunder review), adolescents demonstrated startle potentiation in anticipation of predictable and unpredictable threat and probe P300 suppression in anticipation of predictable threat. The present study utilized the same sample and NPU-threat task but included maternal and adolescent lifetime psychopathology measures; thus, the hypotheses tested in the present paper are orthogonal to Ferry et al. (Reference Ferry, Beatty, Klein and Nelsonunder review). The choices for processing parameters and quantification of startle potentiation and probe P300 suppression are identical across papers.
The NPU-threat task was a modified variant of the original paradigm (Schmitz & Grillon, Reference Schmitz and Grillon2012). The task included three within-subject conditions: no scream, predictable scream, and unpredictable scream. Text at the bottom of the screen informed participants of the current condition by displaying ‘no scream’, ‘scream at 1’, or ‘scream at any time’. Each condition lasted 60 s, during which a 5-s visual countdown (i.e. the cue) was presented four times. The cue began at number 5 and counted down sequentially to number 1, each number remained on the screen for a duration of 1 s. The interstimulus interval (i.e. the time between countdowns during the 60-s condition) ranged from 9 to 17 s, during which only the text describing the condition was on the screen. In the no threat condition, no screaming face stimulus was delivered. In the predictable threat condition, the screaming face stimulus was presented each time the countdown reached 1. In the unpredictable threat condition, the screaming face stimulus could be administered at any time (i.e. during the countdown or interstimulus interval). Startle probes were presented both during the countdown (2 to 4 s following countdown onset) and interstimulus interval (5 to 14 s following interstimulus interval onset). The time intervals between startle probes ranged from 4.7 s to 29.8 s, with an average of 12.1 s. The time intervals between screaming face stimulus onset and subsequent startle probes were always greater than 10 s to ensure that probes were not affected by prior screaming face stimuli. There were 6 trials (4 for baseline startle) for each condition and cue pair, including the no threat interstimulus interval (N ISI), no threat countdown (N CD), predictable threat interstimulus interval (P ISI), predictable threat countdown (P CD), unpredictable threat interstimulus interval (U ISI), unpredictable threat countdown (U CD). Across the P condition, the aversive stimulus was delivered at the end of every countdown (6 times). Across the U condition, the aversive stimulus was delivered during half of the interstimulus interval and half of the countdown trials (total of 6 times). Therefore, the total number of aversive stimuli presentations was matched between the predictable and unpredictable conditions.
EMG recording and data reduction
Startle eye blink electromyography (EMG) was recorded using the ActiveTwo system (BioSemi, Amsterdam, Netherlands) and measured from two 4-mm sintered Ag/AgCl electrodes placed over the orbicularis oculi muscle beneath the right eye. EMG activity was sampled at 2048 Hz and filtered between 28 and 512 Hz. Offline, EMG activity was rectified in a 200-ms window, beginning 50-ms before the onset of the startle probe, and a FIR filter with a band pass of 28–40 Hz was applied to the rectified data to smooth out sharp peaks. Peak amplitude of the startle reflex was determined in the 20- to 150-ms time frame following the startle probe onset relative to baseline (i.e. 50-ms preceding startle probe onset). Blinks were scored as nonresponses if EMG activity during the 20- to 150-ms post-probe time frame did not produce a blink peak that was visually differentiated from baseline activity. Blinks were scored as missing if the baseline period was contaminated with noise, movement artifact, or if a spontaneous or voluntary blink began before the minimal onset latency and thus interfered with the probe-elicited blink response. The present study examined blink magnitude (i.e. averages include values of 0 for nonresponse trials).
Startle potentiation during the predictable threat condition was quantified as the difference in the startle reflex between the predictable threat countdown and the no threat countdown. Startle potentiation during the unpredictable threat condition was quantified as the difference in the startle reflex between the average of the unpredictable threat countdown and interstimulus interval and the average of the no threat countdown and interstimulus interval. Startle potentiation scores were calculated using raw data.
EEG recording and data reduction
Continuous EEG was recorded using an elastic cap with 34 sintered Ag/AgCl electrodes placed according to the 10/20 system. Electrooculogram (EOG) was recorded using four additional facial electrodes: two placed approximately 1 cm outside of the right and left eyes and two placed approximately 1 cm above and below the right eye. Data were recorded using the ActiveTwo system. The EEG was digitized with a sampling rate of 2048 Hz using a low-pass fifth order sinc filter with a half-power cutoff of 417 Hz. A common mode sense active electrode producing a monopolar (nondifferential) channel was used as a recording reference.
EEG data were analyzed using BrainVision Analyzer 2 (Brain Products, Gilching, Germany). Data were referenced offline to the average of left and right mastoids, band-pass filtered (0.1 to 30 Hz), and corrected for eye movement artifacts (Gratton, Coles, & Donchin, Reference Gratton, Coles and Donchin1983). A semiautomatic procedure was employed to detect and reject artifacts. The criteria applied were a voltage step of more than 50 μV between sample points, a voltage difference of 300 μV within a trial, and a maximum voltage difference of less than 0.5 μV within 100-ms intervals. These intervals were rejected from individual channels in each trial. Visual inspection of the data was then conducted to detect and reject the remaining artifacts. A current source density (CSD) transform (order of splines = 5; maximal degree of Legendre polynomials = 10; λ smoothing parameter = 10−5) was applied to compute an estimate of the surface Laplacian based on the EEG voltage across the scalp electrodes.
Startle probe-locked epochs were extracted with a duration of 1200 ms, including a 200-ms prestimulus and 1000-ms post-stimulus interval. The 200-ms prestimulus interval was used as the baseline. Separate grand averages were conducted for each condition and cue. The probe P300 was scored as the average activity at Pz between 260 and 320-ms, where the maximal activity occurred.
Probe P300 suppression during the predictable threat condition was quantified as the difference between the average of the no threat countdown and interstimulus interval and the average of the predictable threat countdown and interstimulus interval. Probe P300 suppression during the unpredictable threat condition was quantified as the difference between the average of the no threat countdown and interstimulus interval and the average of the unpredictable threat countdown and interstimulus interval. Less positive values indicated greater probe P300 suppression.
Data analysis
The models included 523 adolescents (46.5% female) who had diagnostic interview data from at least one assessment (i.e. age 9, 12, and/or 15) and 592 biological mothers who had diagnostic interview data from study entry (i.e. age 3) and/or the age 9 assessment. Internalizing and externalizing latent variables were examined via CFA using Mplus 8.5. A weighted least squares estimator (WLSMV) was used because it is a robust estimator and is well suited for modeling dichotomous variables, such as our diagnoses (Muthén, du Toit, & Spisic, Reference Muthén, du Toit and Spisic1997). Two separate CFAs were conducted to test correlated-factors models of (1) adolescent internalizing-externalizing spectra and (2) maternal internalizing-externalizing spectra. Our models were based on the widely used two-factor internalizing and externalizing solution (Krueger, Reference Krueger1999). For adolescent data, lifetime diagnoses of depressive disorders [major depressive disorder (MDD) and dysthymia], GAD, phobias (agoraphobia, social phobia, and specific phobia), and separation anxiety disorder were parameterized to load on internalizing, and diagnoses of ADHD and disruptive behavior disorders (CD and ODD) were parameterized to load on externalizing. PD was not included in the model due to insufficient cases. For maternal data, lifetime diagnoses of depressive disorders (MDD and dysthymia), GAD, phobias (agoraphobia, social phobia, and specific phobia), and PD were parameterized to load on internalizing, and diagnoses of alcohol, cannabis, and other drug (opiate, cocaine, hallucinogen, sedative, and stimulant) use disorders were parameterized to load on externalizing. All factor variances were freely estimated and the first loading was set to one for each factor.
After testing our a priori two-factor CFAs in adolescent and maternal data, we extracted factor scores for each of the four latent factors (i.e. internalizing and externalizing factor scores, for adolescent and maternal data). The factor scores were continuous variables representing each individual's standing on the internalizing and externalizing factors. For example, higher internalizing factor scores indicated greater internalizing psychopathology. We used the resulting factor scores as continuous variables in all subsequent analyses. Figure 1 shows factor loadings of the adolescent and maternal diagnoses on internalizing and externalizing dimensions.

Fig. 1. Two-factor models of adolescent and maternal psychopathology. Standardized factor loadings are indicated by single-headed arrows and correlations between dimensions are indicated by double-headed arrows (all significant at p < 0.005).
Startle and ERP
Startle and ERP data were assessed for quality separately in adolescents and mothers. A total of 395 adolescents completed the NPU-threat task. For startle analyses, 21 adolescents were excluded from data analyses due to artifacts in greater than 50% of trials, poor signal, or equipment failure (n = 17), or failure to complete the task (n = 4), resulting in a final sample of 374 adolescents. For ERP analyses, 9 adolescents were excluded from data analyses due to artifacts in greater than 50% of trials, poor signal, or equipment failure (n = 5), or failure to finish the task (n = 4), resulting in a final sample of 386 adolescents.
The potential impact of adolescent psychiatric medication use was examined via a Predictability (predictable threat v. unpredictable threat) × Medication (yes v. no) mixed-measures analysis of variance (ANOVA), with predictability as the within-subject factor and medication as the between-subjects factor.
The relationship between adolescent internalizing and externalizing spectra and sensitivity to threat was examined via mixed-measures analyses of covariance (ANCOVA), with predictability as the within-subject factor and adolescent internalizing and externalizing factor scores included as continuous covariates. Separate ANCOVAs were conducted for the startle reflex and probe P300. Identical analyses were conducted for maternal internalizing and externalizing spectra and adolescent sensitivity to threat. If there were interactions from the ANCOVA, post-hoc Pearson correlations were conducted using internalizing and externalizing factor score residuals (i.e. continuous internalizing factor scores regressed on externalizing factor scores and vice versa) and startle reflex or P300 suppression residuals (i.e. unpredictable threat regressed on predictable and vice versa). If there were only main effects from the ANCOVA, then post-hoc Pearson correlations were conducted between internalizing and externalizing factor score residuals and startle reflex or P300 suppression to general threat (i.e. averaged across predictable and unpredictable threat conditions). All ANCOVA analyses were conducted in IBM SPSS Statistics, Version 26.0 (Armonk, NY, USA).
Results
Demographics
Table 1 displays adolescent and maternal diagnoses and adolescent medication use. The sample was on average 15.22 years-old (s.d. = 0.38), 53.2% male, and included 89.6% Caucasian, 7.1% African American, 2.8% Asian, 0.3% Native American, and 0.3% other race; 11.6% of participants reported Hispanic ethnicity.
Table 1. Participant demographics and clinical characteristics

a ADHD, attention-deficit/hyperactivity disorder.
b Seven adolescents were on 2 or more medications.
c SSRI, selective serotonin reuptake inhibitor and SNRI, serotonin-norepinephrine reuptake inhibitor.
d ‘Other’ medications included atypical antipsychotic (n = 4), atypical antidepressants (n = 1), tricyclic antidepressants (n = 1), serotonin modulators (n = 1).
Models
Model fit was evaluated in adolescents and mothers using several fit indices: the comparative fit index (CFI; Bentler, Reference Bentler2006), Tucker-Lewis index (TLI; Tucker and Lewis, Reference Tucker and Lewis1973), and root mean-square error of approximation (RMSEA; Steiger, Reference Steiger1990). CFI/TLI values of above 0.95 and RMSEA values of below 0.06 are generally used to indicate very good model fit (Hu & Bentler, Reference Hu and Bentler1999). Model 1, a correlated two-factor model for adolescent psychopathology (see Fig. 1 – left), provided good fit to the data [CFI = 0.982, TLI = 0.967, RMSEA = 0.025 (90% CI 0.000–0.061)]. Model 2, a correlated two-factor model for maternal psychopathology (see Fig. 1 – right), also provided good fit to the data [CFI = 1.000, TLI = 1.000, RMSEA = 0.000 (90% CI 0.000–0.033)] (Hu & Bentler, Reference Hu and Bentler1999). See Fig. 1 for standardized factor loadings (all significant at p < 0.005).
Startle reflex
Analyses of adolescent medication use and the startle reflex indicated a Predictability × Medication interaction, F(1, 372) = 7.11, p = 0.008, η p2 = 0.02. Follow-up analyses indicated that adolescents who were taking psychiatric medication had lower startle potentiation to unpredictable threat relative to those who were not taking medication, F(1, 372) = 5.84, p = 0.02, η p2 = 0.02, but there were no differences in startle potentiation to predictable threat, F(1, 372) = 0.21, p = 0.65, η p2 = 0.00. Therefore, adolescent medication use was used as a covariate in all subsequent analyses involving startle potentiation.
Analyses of adolescent psychopathology and startle potentiation indicated a Predictability × Adolescent Internalizing interaction, F(1, 370) = 5.78, p = 0.02, η p2 = 0.02. Table 2 displays follow-up partial correlations (controlling for adolescent medication use). The adolescent internalizing spectrum was positively associated with startle potentiation to unpredictable threat but was not associated with startle potentiation to predictable threat.
Table 2. Correlations between adolescent and maternal internalizing and externalizing factor score residuals and adolescents' startle reflex and probe P300 residuals to predictable and unpredictable threat

a All correlations with the startle reflex controlled for adolescent medication use.
b General threat indicates the average of predictable and unpredictable threat conditions.
Analyses of maternal psychopathology and adolescent startle potentiation indicated a Predictability × Maternal Internalizing, F(1, 370) = 6.57, p = 0.01, η p2 = 0.02, and a Predictability × Maternal Externalizing interaction, F(1, 370) = 6.45, p = 0.01, η p2 = 0.02. As shown in Table 2, the maternal internalizing spectrum was positively associated with adolescent startle potentiation to unpredictable threat but was not associated with startle potentiation to predictable threat. In addition, the maternal externalizing spectrum was negatively associated with adolescent startle potentiation to unpredictable threat and positively associated with adolescent startle potentiation to predictable threat.
When adolescent and maternal internalizing and externalizing factor scores were included as simultaneous independent variables in the same model, results again indicated Predictability × Adolescent Internalizing, F(1, 368) = 4.93, p = 0.03, η p2 = 0.01, Predictability × Maternal Internalizing, F(1, 368) = 5.33, p = 0.02, η p2 = 0.01, and Predictability × Maternal Externalizing interactions, F(1, 368) = 6.22, p = 0.01, η p2 = 0.01.
Probe P300
Analyses of adolescent psychopathology and the probe P300 indicated Predictability × Adolescent Internalizing, F(1, 383) = 6.42, p = 0.01, η p2 = 0.02, and Predictability × Adolescent Externalizing interactions, F(1, 383) = 5.78, p = 0.02, η p2 = 0.02. As shown in Table 2, the adolescent internalizing spectrum was positively associated with P300 suppression to unpredictable threat. In contrast, the adolescent externalizing spectrum was negatively associated with P300 suppression to unpredictable threat. Adolescent internalizing and externalizing spectra were not associated with probe P300 suppression to the predictable threat.
Analyses of maternal psychopathology and adolescent probe P300 indicated the main effects of Maternal Internalizing, F(1, 383) = 4.21, p = 0.04, η p2 = 0.01, and Maternal Externalizing, F(1, 383) = 12.40, p < 0.001, η p2 = 0.03. As shown in Table 2, the maternal internalizing spectrum was positively associated with adolescent P300 suppression to general threat at a trend level. In contrast, the maternal externalizing spectrum was negatively associated with adolescent P300 suppression to general threat.
When adolescent and maternal internalizing and externalizing factor scores were included as simultaneous independent variables, results again indicated Predictability × Adolescent Internalizing, F(1, 381) = 4.88, p = 0.03, η p2 = 0.01, and Predictability × Adolescent Externalizing interactions, F(1, 381) = 6.97, p = 0.01, η p2 = 0.02, and main effects of Maternal Internalizing, F(1, 381) = 3.90, p = 0.049, η p2 = 0.01, and Maternal Externalizing, F(1, 381) = 11.84, p = 0.001, η p2 = 0.03.
See online Supplementary Materials for additional analyses containing adolescent and maternal psychopathology and adolescent probe P300 suppression using different quantification methods for the predictable threat condition (i.e. quantified as the difference between the no threat countdown and predictable threat countdown and the no threat interstimulus interval and predictable threat interstimulus interval.
Discussion
The present study indicates that adolescent neurobiological sensitivity to unpredictable threat is associated with both personal and maternal history of internalizing and externalizing spectra (Fig. 2). The adolescent internalizing spectrum was positively associated with defensive motivation to unpredictable threat. In addition, the adolescent internalizing and externalizing spectra were associated with heightened and attenuated, respectively, attentional engagement to unpredictable threat. The maternal internalizing and externalizing spectra also demonstrated opposite relationships (heightened and attenuated, respectively) with their adolescent offspring's defensive motivation to unpredictable threat and attentional engagement to the general threat. Further, the maternal internalizing and externalizing spectra were independently related to adolescent defensive motivation and attentional engagement to threat. These findings indicate that the association between maternal psychopathology and adolescent sensitivity to unpredictable threat is not attributable to adolescent lifetime psychopathology.
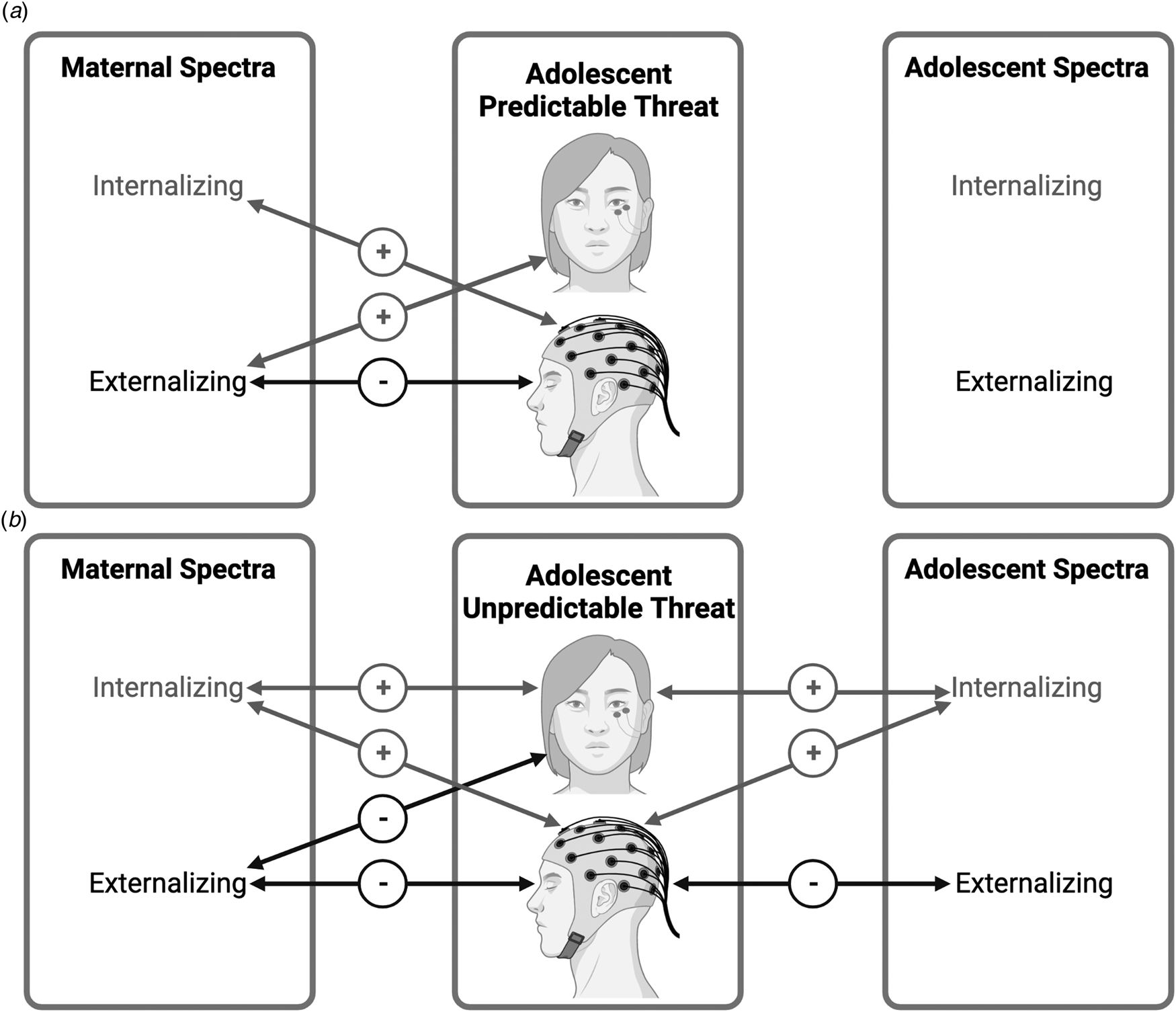
Fig. 2. Maternal and adolescent psychopathology spectra and adolescent psychophysiological reactivity to predictable and unpredictable threat. + Indicates a positive association between the psychopathology spectra and startle potentiation (top) or P300 suppression (bottom) to (a) predictable and (b) unpredictable threat. - Indicates a negative association between the psychopathology spectra and startle potentiation or P300 suppression to (a) predictable and (b) unpredictable threat. Created with BioRender.com.
Similar to the adult literature (Gorka et al., Reference Gorka, Lieberman, Shankman and Phan2017; Grillon et al., Reference Grillon, Lissek, Rabin, McDowell, Dvir and Pine2008), startle potentiation in anticipation of unpredictable threat was related to lifetime history of internalizing disorders in adolescents. One previous investigation found that greater social phobia symptoms in youth were associated with heightened startle potentiation to unpredictable threat (Nelson & Hajcak, Reference Nelson and Hajcak2017a). The present study extends this literature and indicates that adolescent defensive motivation to unpredictable threat is associated with the higher-order internalizing spectrum. Adolescence is a period marked by increased stress due to physical maturation and environmental and social changes (Crone & Dahl, Reference Crone and Dahl2012). Sensitivity to unpredictable threat might represent a key factor that interacts with such stress and other factors to lead to the development of psychiatric disorders.
Also similar to the adult literature (Nelson et al., Reference Nelson, McGowan, Sarapas, Robison-Andrew, Altman, Campbell and Shankman2013), adolescent startle potentiation in anticipation of unpredictable threat was associated with maternal psychopathology. Our findings indicate that familial risk for internalizing and externalizing spectra is characterized by distinct and opposite profiles of sensitivity to unpredictability. These novel results suggest that defensive motivation in anticipation of unpredictable threat is associated with risk for the internalizing and externalizing spectra during a critical developmental period. Identifying individuals who fall on either extreme of the sensitivity to unpredictability spectrum (i.e. heightened or attenuated) may be useful in prioritizing youth in greatest need of preventative efforts, thus supporting initiatives for transdiagnostic staging models.
There is a robust body of literature indicating that attentional engagement with threat is an important component of defensive responding (Lang, Reference Lang1995). Our results provide novel evidence that aberrant processing of threat in adolescents with internalizing and externalizing psychopathology (indexed by probe P300 suppression) may also be specific to unpredictable threat. In contrast, the maternal internalizing and externalizing spectra were differentially associated with adolescent attentional engagement to threat, irrespective of predictability. These results are consistent with research demonstrating that children of parents with internalizing psychopathology demonstrate attentional biases toward threat and negative stimuli (Kujawa et al., Reference Kujawa, Torpey, Kim, Hajcak, Rose, Gotlib and Klein2011). Adolescent abnormalities in attentional engagement to unpredictable threat v. general threat might represent mechanisms that distinguish between personal versus familial lifetime history of psychopathology.
Overall, the present study is the first to demonstrate that personal history of, and familial risk for, the internalizing and externalizing spectra may have unique profiles in terms of neural measures of both defensive motivation and attentional engagement to unpredictable threat. These findings have important implications for identifying and understanding mechanisms of risk for psychiatric disorders. Extant research on neurobiological risk factors has primarily focused on diagnostic groups, though known vulnerability factors tend to operate via multifinality (Cicchetti & Rogosch, Reference Cicchetti and Rogosch1996). This study facilitates more efficient identification of risk factors for psychiatric disorders by applying latent variable modeling techniques and examining sensitivity to unpredictability as a mechanism shared across higher-order spectra of psychiatric disorders.
This work is consistent with the recent Hierarchical Taxonomy of Psychopathology (HiTOP) system, which addresses diagnostic comorbidity by directly modeling the aspects of psychopathology (e.g. clinical signs, symptoms and diagnoses) that systematically co-occur (Kotov et al., Reference Kotov, Krueger, Watson, Achenbach, Althoff, Bagby and Zimmerman2017). The present study used the HiTOP framework to link clinical phenotypes with measures of neurobiological systems that are targeted by the National Institute of Mental Health's (NIMH) Research Domain Criteria (RDoC) initiative. RDoC seeks to identify biobehavioral domains (i.e. negative valence) that are common across several disorders and then relate these dimensions to specific biological processes at varying units of analysis (e.g. genes, cells, neural circuits, behaviors) (Insel et al., Reference Insel, Cuthbert, Garvey, Heinssen, Pine, Quinn and Wang2010; Sanislow et al., Reference Sanislow, Pine, Quinn, Kozak, Garvey, Heinssen and Cuthbert2010). Consistent with the aims of the RDoC initiative, we discovered that sensitivity to unpredictable threat (i.e. ‘potential threat’) is a transdiagnostic risk marker for psychopathology. Results from the present study provide substantial evidence that a proposed RDoC domain (‘potential threat’) is associated with risk for high-order dimensions of psychopathology. These findings support initiatives to integrate neurobiological and dimensional psychopathology constructs to advance the field of clinical neuroscience (Latzman, DeYoung, & HiTOP Neurobiological Foundations Workgroup, Reference Latzman and DeYoung2020).
The present study had some limitations that should be taken into consideration. First, maternal and adolescent internalizing and externalizing spectra included a few different diagnoses. ADHD, aggression/antisocial behavioral, and separation anxiety disorders were not assessed in adults. This was consistent with diagnostic practice with adults at the time that assessments were conducted (e.g. the DSM-IV version of the SCID). Moreover, there were not a sufficient number of adolescent PD or substance use disorder cases to warrant inclusion in the internalizing and externalizing spectra, respectively. Larger studies with a more diverse range of psychopathology are needed to more adequately measure the internalizing and externalizing spectra in adolescents and their mothers. Additionally, familial risk was only examined in mothers. Future studies should extend this to fathers and siblings. Startle potentiation (during the countdown) and P300 suppression (during the countdown and interstimulus interval) were quantified differently for the predictable threat conditions; these decisions were based on established task effects from previous investigations (Correa, Li, Nelson, & Shankman, Reference Correa, Li, Nelson and Shankman2022; Ferry & Nelson, Reference Ferry and Nelson2020; MacNamara & Barley, Reference MacNamara and Barley2018; Nelson & Hajcak, Reference Nelson and Hajcak2017b; Nelson, Hajcak, & Shankman, Reference Nelson, Hajcak and Shankman2015), including one investigation that used the same sample as the present study (Ferry et al., Reference Ferry, Beatty, Klein and Nelsonunder review). Future investigations should further investigate why P300 suppression occurs across both the countdown and interstimulus interval phases of the predictable threat condition considering this is inconsistent with the threat contingencies of the NPU-threat task. Further, the effect sizes were small, but this is common in research examining the relationship between variables with no common method variance (Paulus & Thompson, Reference Paulus and Thompson2019). Finally, the present study used a combination of longitudinal and cross-sectional elements. Specifically, maternal lifetime psychopathology was measured when offspring were 9 years-old – prior to the evaluation of adolescent sensitivity to unpredictable threat, which was measured when offspring were 15 years-old. Despite this, sensitivity to the unpredictable threat was not measured when the offspring were 9 years-old. Future studies should consider the examination of sensitivity to threat across critical developmental periods, like adolescence. Lastly, future research should determine whether sensitivity to unpredictable threat in youth interacts with both developmental and environmental factors and triggers the onset of future psychiatric disorders.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291722002434.
Location of work and address for reprints
Clare C. Beatty, Department of Psychology, Stony Brook University, Stony Brook, NY, 11794-2500. Phone: (631) 632-7843; Email: [email protected].
Financial support
Support for this research was provided through the National Institute of Mental Health grant R01 MH069942 awarded to D.N.K. and K01 MH107808 awarded to B.D.N.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.







