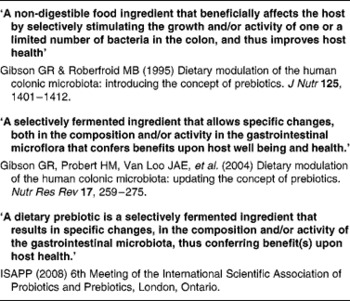
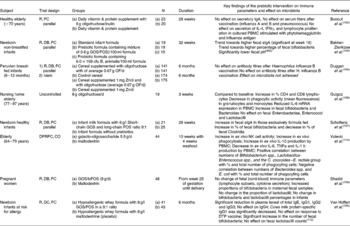
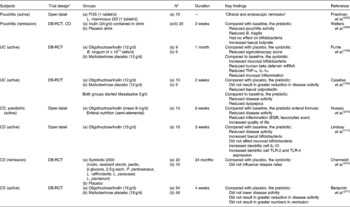
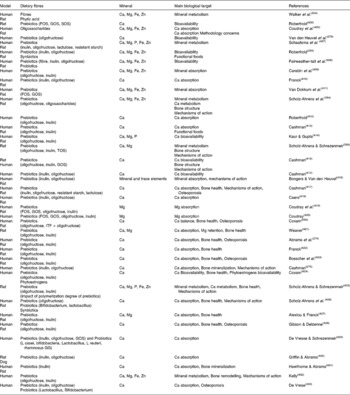
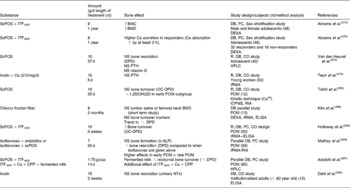
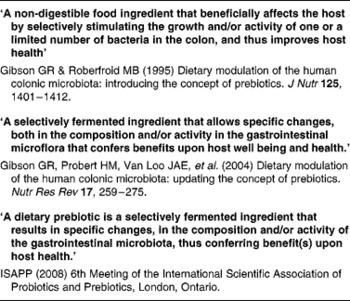
The main author of this section is Professor Roberfroid. In the 1980s, Japanese researchers(Reference Yazawa, Imai and Tamura1, Reference Mitsuoka, Hidaka and Eida2) had already demonstrated that specific non-digestible oligosaccharides (especially fructo-oligosaccharides) were selectively fermented by bifidobacteria and had the capacity, upon feeding, in stimulating their growth in human faeces. These observations were confirmed and further expanded by Gibson & Roberfroid(Reference Gibson and Roberfroid3) who introduced the concept of prebiotics and have recently published a review of the research which includes the most recent development(Reference Gibson, Probert and Van Loo4) (Table 1). During the last 15 years, this concept has attracted the interest of many academic as well as industrial scientists and it has become a popular research topic in nutrition and, more recently, in the biomedical fields.
Table 1 Developing definitions of the prebiotic concept
Early research in the mid-1990s on prebiotics has contributed towards the development and validation of new molecular biology-based methods resulting in easy-to-handle, sensitive, and highly specific methods to identify and quantify the large variety of micro-organisms composing the gut microbiota(Reference Suau, Bonnet and Sutren5–Reference Green, Brostoff and Hudspith16). The application of such methods has improved our knowledge of the gut microbiota composition in terms of variety, classification, identity and relative concentrations of genera or species of micro-organisms, as well as in terms of their properties and interactions/co-operations with each other and with intestinal epithelial cells. This has led the International Scientific Association for Probiotics and Prebiotics (ISAPP) (6th meeting in Ontario, Canada, November 2008) to propose the concept of ‘normobiosis’ to characterise a normal gut microbiota in which genera/species of micro-organisms with potential health benefits predominate in number over potentially harmful ones as opposed to ‘dysbiosis’ which characterises a gut microbiota in which one or a few potentially harmful genus(era)/species of micro-organisms are dominant, thus creating a disease-prone situation.
A large part of the research activity has concentrated and still does focus on the in vitro and in vivo abilities of selective modification in the composition of the complex gut microbiota, in particular research has focused on the selective stimulation of growth of mainly bifidobacteria, but also lactobacilli. In the future, it is likely that this may be expanded towards other genera, e.g. Eubacterium, Faecalibacterium and Roseburia. It has become clear that products, causing such a selective modification in gut microbiota's composition and/or activity(ies), could, in addition, either induce beneficial physiological effects not only in the colon but also within the whole body and/or contribute towards reducing the risk of miscellaneous intestinal and systemic pathologies. These effects are summarised in Table 2 and have been discussed, on a regular basis, at international conferences(Reference Roberfroid and Gibson17–Reference Roberfroid and Buddington19) and were, more recently, reviewed in a handbook(Reference Gibson and Roberfroid20). They are also topics for the present document.
Table 2 Summary of the main physiological and patho-physiological targets for prebiotic effects, i.e effects associated with a selective stimulation of growth and/or activity(ies) of one or a limited number of gut microorganisms

The intensive research of the past 15 years has contributed towards an improved understanding of the complexity of the gut microbiota. This includes the discovery of new phyla/genera, their relative concentration in the gut microbiota, the key role of diet in modulating its composition, the changes associated with ageing or chronic diseases and the individual character of gut microbiota composition. In addition, past research has given us insights into its roles in human physiology and miscellaneous pathophysiological conditions. The gut microbiota is thus now perceived as a key player in health and well-being with, as a principal condition, a composition in which potentially health-promoting dominant micro-organisms (especially the saccharolytic genera/species, e.g. bifidobacteria) are elevated and/or more active than the potentially harmful ones (especially the proteolytic/putrefactive genera/species)(Reference Gibson and Roberfroid3, Reference Cummings, Antoine and Azpiroz21), a situation known as ‘normobiotic’ or ‘eubiotic’. It is now well recognised that, within such a potentially health beneficial dominant microbiota, the genus Bifidobacterium plays an important role although future research may show different genera/species to also be important. Indeed, it has been hypothesised that increasing bifidobacteria in gut microbiota might improve health status and reduce disease risk.
As a result of discussions with both academic and industry experts (in the ILSI Europe Prebiotic Expert Group and Prebiotic Task force, respectively), the present document does not aim at proposing a new definition of a prebiotic nor at identifying which food components/ingredients/supplements classify as prebiotic but rather to validate and expand the original idea of the prebiotic concept, as
The selective stimulation of growth and/or activity(ies) of one or a limited number of microbial genus(era)/species in the gut microbiota that confer(s) health benefits to the host,
with ‘selectivity’ being the key condition that needs to be demonstrated, in vivo, in the complex human (animal) gut microbiota by applying the most relevant and validated methodology(ies) to quantify a wide variety of genera/species composing the gut microbiota;
‘activity(ies)’ meaning a metabolic profile(s), molecular signalling, prokaryote–eucaryote cell–cell interaction linked to one specific microbial genus/species or resulting from the coordinated activity of a limited number of microbial genus(era);
‘confer(s)’ referring to one or a limited number of selectively stimulated genus(era)/species in the gut microbiota.
In this concept, the use of ‘gut microbiota’ is limited to the application to food/feed components.
Moreover, it is implicit that ‘health benefit(s)’ must be linked/correlated, directly or indirectly, with the presence in relatively high concentrations and/or activity(ies) of one or a limited number of selectively stimulated micro-organisms in the gut microbiota. Indeed, such a conceptual approach emphasises the link between ‘selective stimulation of growth and/or activity(ies) of one or a limited number of specific bacteria genus/species’ and ‘health benefit(s)’. Consequently, only food components/ingredients/supplements for which both such a selective stimulation has been scientifically substantiated and health benefits have been evaluated are included in the review process. The expression ‘prebiotic effect(s)’ will be used to identify or refer to selective changes in gut microbiota composition as well as specific (patho-) physiological effects both in experimental and in human intervention studies. However, it must be kept in mind that to substantiate a ‘prebiotic’ effect will require the demonstration that such an effect is likely to be ‘causally’ linked to or at least correlated with selective change(s) in gut microbiota composition.
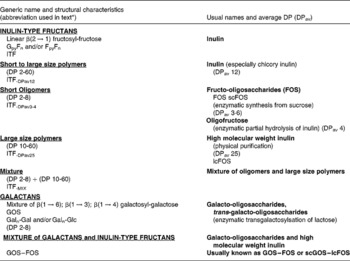
Currently and mostly for historical reasons, the majority of the scientific data (both experimental and human) on prebiotic effects have been obtained using food ingredients/supplements belonging to two chemical groups namely inulin-type fructans (ITF) and the galacto-oligosaccharides (GOS) (for more details on the chemistry, nomenclature and abbreviations used in the present review see Table 3). These have repeatedly demonstrated the capacity to selectively stimulate the growth of bifidobacteria and, in some cases, lactobacilli leading to a significant change in gut microbiota composition. Concurrently, most of the health benefits possibly associated with the prebiotic effects were discovered and demonstrated using the same food ingredients/supplements. This, by no means, precludes other products of demonstrating such prebiotic effects with the same or other health benefits. However, since the aim of the present review is, primarily, to expand and validate the prebiotic concept, it will neither emphasise nor identify which specific products can be classified as ‘prebiotic’. A precise list of potential candidates for such a classification would require a detailed review of all published studies using each potential candidate as well as the evaluation of their validity and their relevance. This was not the mandate given to the group of experts who collectively wrote the manuscript. For such a discussion, the reader should consult the different chapters in the recently published Handbook of Prebiotics (Reference Gibson and Roberfroid20). It is important to emphasise the fact that the prebiotic effect and the dietary fibre effect have two different attributes. Being resistant (partly or totally) to digestion and being fermented (at least the so-called soluble dietary fibres) both may concern gut microbiota composition and activity. What makes them different is the selectivity of the prebiotic effect as described earlier.
Table 3 Description and usual nomenclature of the main products with established prebiotic effect
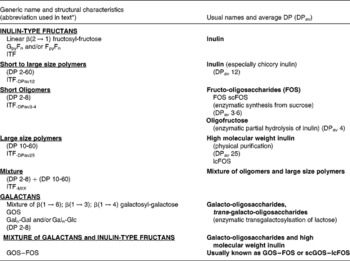
DP, degree of polymerisation; ITF, inulin-type fructans; lcFOS, long-chain fructo-oligosaccharides; GOS, galacto-oligosaccharides; Gal, galactose; Glc, glucose; scGOS, short-chain galacto-oligosaccharides.
* The abbreviations mentioned in this table will be used throughout the documents to identify the different compounds used in the studies.
In the concluding chapter, tentative answers to the above questions will be presented and discussed with the main objective to prospectively prioritise topics for further research in the field.
Prebiotic effects in the gut
Microbiota of the gastro-intestinal tract
The main authors of this section are Professor Gibson, Dr Hoyles and Dr McCartney and specifically Professor Rastall for the in vitro subsection.
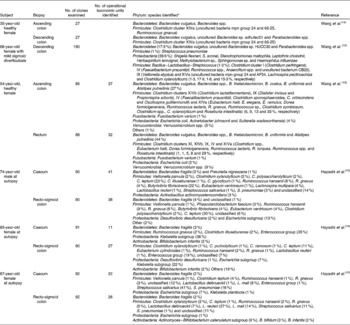
The microbiota of the human gastro-intestinal (GI) tract inhabits a complex ecosystem(Reference Wilson and Blitchington22). Factors such as pH, peristalsis, nutrient availability, oxidation–reduction potential within the tissue, age of host, host health, bacterial adhesion, bacterial co-operation, mucin secretions containing Igs, bacterial antagonism and transit time influence the numbers and diversity of bacteria present in the different regions of the GI tract(Reference Kerckhoffs, Samson, van Berge Henegouwen, Ouwehand and Vaughan23). Until 20 years ago, our knowledge of the GI microbiota relied upon cultivation-based methods and recovery of bacteria from faecal samples. However, with the advent of molecular techniques and their application to biopsy and faecal samples, our knowledge of the GI microbiota has increased dramatically(Reference Suau, Bonnet and Sutren5–Reference Green, Brostoff and Hudspith16). An understanding of the bacteria making up the GI microbiota is important due to its involvement in the development of the GI mucosal immune system, maintenance of a normal physiological environment and for providing essential nutrients(Reference O'Connor, Barrett, Fitzgerald and Tamine24).
The stomach
Although the bacterial load in the stomach is low in healthy adults (approximately 102 colony forming unit (CFU) (per ml contents)(Reference O'May, Reynolds and Smith25)), the walls of the stomach are colonised with bacteria. In the healthy adult stomach, the predominant organisms isolated include lactobacilli, enterococci, ‘catenabacteria’ and bacilli(Reference Reuter26). Of the bacteria that inhabit the stomach, Helicobacter species have been studied most intensively due to their association with various gastric complaints. Helicobacter pylori is present in the stomach of a subset of the population (10 % of those between 18 and 30 years of age; 50 % of those age 60 and over), where it resides in the mucous layer next to the gastric epithelium(Reference Kerckhoffs, Samson, van Berge Henegouwen, Ouwehand and Vaughan23). Colonisation with Helicobacter pylori can be asymptomatic, but the organism is known to cause symptoms such as acute gastritis (i.e. pain, bloating, nausea and vomiting) and/or chronic gastritis; it has also been associated with peptic ulcers and gastric carcinomas(Reference Kerckhoffs, Samson, van Berge Henegouwen, Ouwehand and Vaughan23).
The small intestine (duodenum, jejunum and ileum)
The environment of the duodenum is acidic (pH 4–5) with lactobacilli and streptococci predominating, and numbers of bacteria are higher than those found in the stomach (102–104 CFU (per ml contents);(Reference O'May, Reynolds and Macfarlane27)).
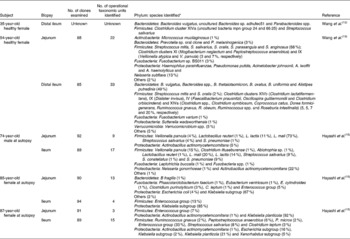
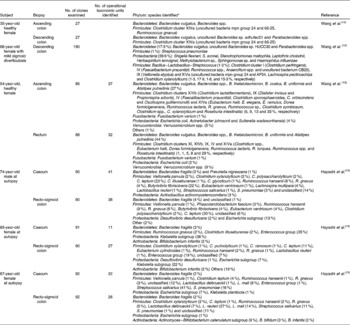
Cultivation studies have shown that lactobacilli, streptococci, veillonellae, staphylococci, actinobacilli and yeasts to be the most prominent in the duodenum and jejunum(Reference Kerckhoffs, Samson, van Berge Henegouwen, Ouwehand and Vaughan23). However, due to limitations in cultivation techniques and the ethical issues surrounding the obtention of biopsy samples from human subjects, our knowledge of the microbiota of the small intestine was poor until recently. Table 4 gives details of the results of recent molecular studies that have provided additional understanding of the microbiota of the small intestine. But these studies are only informative, because only one or a few donors have been used in each study, and their ages have not been representative of the general population. However, the results of the molecular studies appear to confirm those of cultivation-based work.
Table 4 Microbial diversity of the mucosa of the human small intestine as determined by 16S ribosomal ribonucleic acid gene sequence analysis
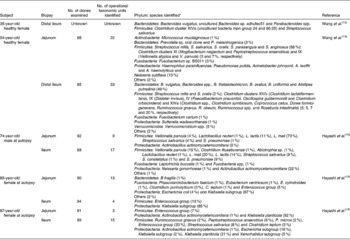
No., number.
* Numbers in parentheses represent proportion of clones ascribed to a particular phylum/genus/cluster where known. Names of nearest phylogenetic relatives are given.
The microbiota changes markedly from the duodenum to the ileum, as the velocity of the intraluminal content decreases, pH increases and oxidation–reduction potentials lower, with bacterial loads increasing to 106–108 CFU (per ml contents)(Reference Kerckhoffs, Samson, van Berge Henegouwen, Ouwehand and Vaughan23). As transit time in the small intestine is rather rapid (2–4 h) and the bacterial density relatively low, its impact in terms of overall fermentation is low compared with the large intestine (see later). The small intestine is also the site of many bacterial infections, such as salmonella and some Escherichia coli. For this reason, the small intestine is also a target for probiotics known to compete with pathogens. Similarly, sialylated acidic oligosaccharides from human milk can block the adhesion of pathogens on the epithelial surface.
The large intestine
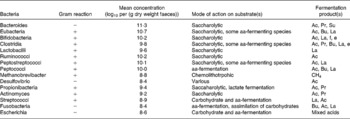
The combination of increased transit time of the large intestine, increased nutrient availability (i.e. undigested food material from the upper GI tract, sloughed-off bacterial cells, microbial cell debris and by-products of microbial metabolism) and a more neutral pH ensures that the large intestine is a highly favourable environment for microbial colonisation. As the environment is strictly anaerobic (>100 mV), in particular obligate anaerobes prevail. Table 5 gives details of some bacteria that have been isolated from the GI microbiota. Table 6 gives details of molecular studies on biopsies from different regions of the large intestine. In addition to characterising the mucosa-associated microbiota, Zoetendal et al. (Reference Zoetendal, von Wright and Vilpponen-Salmela11) demonstrated that the faecal microbiota differs from that inhabiting the GI mucosa.
Table 5 Bacteria, their substrates and products in the human large intestine Taken from Salminen et al. (Reference Salminen, Bouley and Boutron-Ruault377)
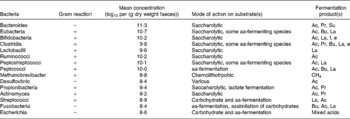
aa, amino acid; Ac, acetate; Pr, propionate; Su, succinate; Bu, butyrate; La, lactate; f, formate; e, ethanol.
Table 6 Microbial diversity of the mucosa of the human large intestine as determined by 16S ribosomal ribonucleic acid gene sequence analysis
No., number.
* Numbers in parentheses represent proportion of clones ascribed to a particular phylum/genus/cluster where known. Names of nearest phylogenetic relatives are given.
Even today, due to the difficulty of obtaining samples from the different regions of the intestine, much of the work done in relation to the ecology and activity of bacteria within the GI tract is carried out using faecal samples. However, the faecal microbiota is not representative of that of the GI tract as a whole(Reference Zoetendal, von Wright and Vilpponen-Salmela11, Reference Eckburg, Bik and Bernstein14), and inferences made from in vitro studies in relation to specific GI diseases, particularly those involving the more-proximal regions of the intestine, should always be made with this in mind. However, a study examining the GI microbiota of sudden-death victims has shown that the faecal microbiota reflects that of the luminal contents of the descending colon in terms of the culturable component(Reference Macfarlane, Macfarlane and Gibson28). Molecular-based methods have been used to examine the faecal microbiota in recent years. Identification of specific strains isolated from faecal samples has become more accurate due to the use of 16S ribosomal ribonucleic acid gene sequence analysis and has improved taxonomic schemes and our understanding of the bacteria involved in specific metabolic processes (e.g. the role of Roseburia spp. in butyrate production(Reference Duncan, Aminov and Scott29), and the identification of the mucin-degrading bacterium Akkermansia muciniphila (Reference Derrien, Vaughan and Plugge30)). This improved characterisation of viable bacteria has also aided in the design of probes for use in fluorescence in situ hybridisation analysis (e.g. Rrec584 for Roseburia spp.(Reference Walker, Duncan and William Leitch31)).
Early cloning studies examined relatively small numbers of clones to generate a phylogenetic inventory of the faecal microbiota of healthy adults. Wilson & Blitchington(Reference Wilson and Blitchington22) generated two clone libraries (one from a 9-cycle PCR (fifty clones, twenty-seven operational taxonomic units) and the other from a 35-cycle PCR (thirty-nine clones, thirteen operational taxonomic units)) from a faecal sample from a healthy 40-year-old male. Of the clones they analysed, 35 % were related to the Bacteroides group, 10 % to the Clostridium coccoides group (Clostridium cluster XIVa) and 50 % to the Clostridium leptum group (Clostridium cluster IV). Less than a quarter of the sequences analysed were derived from a known bacteria. Suau et al. (Reference Suau, Bonnet and Sutren5) found that of the 284 clones they generated from a faecal sample from a 40-year-old male, the majority of the sequences fell into three phylogenetic groups: Bacteroides (31 %), C. coccoides (44 %) and C. leptum (20 %). The remaining clones were derived from Streptococcus salivarius and Streptococcus parasanguinis and bacteria related to Mycoplasma spp., clostridia, the Atopobium group, Verrucomicrobium spinosum and the Phascolarctobacterium faecium subgroup. Seventy-six per cent of the clones analysed were derived from previously unknown bacteria. Blaut et al. (Reference Blaut, Collins and Welling32) used a cloning approach to demonstrate that microbial diversity in faeces increases with age. It was found that the number of operational taxonomic units corresponding to known molecular species was highest in infants and lowest in the elderly subjects, with 92 % of sequences from the elderly subjects corresponding to previously unknown bacteria.
As molecular methods have become more widely available and less time consuming and their relative costs have decreased, more ambitious cloning studies in which thousands of sequences have been examined have been carried out(Reference Eckburg, Bik and Bernstein14, Reference Manichanh, Rigottier-Gois and Bonnaud33). The results of these studies in terms of the groups of bacteria represented by the largest number of clones and the identification of previously unknown bacteria are in accordance with those of Wilson & Blitchington(Reference Wilson and Blitchington22) and Suau et al. (Reference Suau, Bonnet and Sutren5), but are notable for the characterisation of several actinobacterial and proteobacterial sequences from human faecal samples.
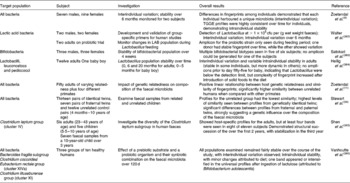
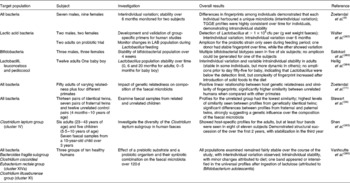
Techniques such as temperature gradient gel electrophoresis and denaturing gradient gel electrophoresis (DGGE) allow higher numbers of samples from more donors to be examined than traditional cloning studies. Temperature gradient gel electrophoresis was used by Zoetendal et al. (Reference Zoetendal, Akkermans and de Vos9) to examine the total bacterial communities of faecal samples from sixteen adults. Host-specific fingerprints were generated, demonstrating interindividual variation in the composition of the faecal microbiota and confirming the results of cultivation studies. Some bands were seen in fingerprints from multiple donors, suggesting that species of the predominant microbiota were common across individuals. In addition, by obtaining samples from two donors over a 6-month period, the authors showed that the profiles of these donors did not differ significantly over time, demonstrating that predominant microbial species were relatively stable without dietary intervention. Excision and sequencing of bands of interest allowed the authors to perform a phylogenetic analysis on their samples, the results of which demonstrated that the majority of bacteria represented in their fingerprints did not correspond to known bacterial species. Of the prominent bands identified in almost all samples, most belonged to different Clostridium clusters, with the remainder identified as Ruminococcus obeum, Eubacterium hallii and Faecalibacterium prausnitzii. Zoetendal et al. (Reference Zoetendal, Akkermans and Akkermans-van Vliet10), using DGGE, demonstrated that host genotype affects the composition of the faecal microbiota. In that study, the authors examined faecal samples from fifty donors of varying relatedness. A higher similarity was seen between fingerprints from monozygotic twins living apart than between those of married couples or pairs of twins. There was a significant difference between the fingerprints of unrelated people grouped by either gender or living arrangements, and no relationship between the fingerprints generated and the age difference of siblings. Temporal temperature gradient gel electrophoresis and DGGE studies examining the faecal microbiota of children and infants have confirmed the impact of host genotype on the composition of the faecal microbiota(Reference Stewart, Chadwick and Murray34). Other studies employing DGGE have used primer sets that allow examination of the composition and dynamics of specific groups of bacteria (Table 7). The detection limit seems to be the main barrier to overcome in these studies, particularly when examining populations such as bifidobacteria and lactobacilli – the commonest prebiotic targets.
Table 7 Details of some TGGE and denaturing gradient gel electrophoresis studies of the faecal microbiota
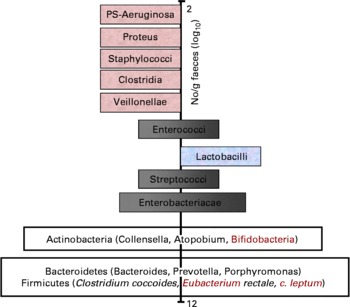
With respect to the prebiotic concept, it is important to understand that apart from knowledge on the complexity of the gut microflora, it is also known that certain bacteria are associated with toxin formation and even pathogenicity when they become dominant. Others are associated with carcinogen generation and the metabolism of other xenobiotics. These potentially harmful bacteria belong to species within groups such as clostridia and bacteroides. Whereas knowledge on overt or latent pathogens has advanced markedly, due to the symptoms they can cause, there is less consensus on what characterises potentially harmful bacteria (without direct pathogenicity) and potentially healthy bacteria. Still potentially healthy bacterial groups are characterised by a beneficial metabolism to the host through their SCFA formation, absence of toxin production, formation of defensins or even vitamin synthesis. They may also inhibit pathogens through a multiplicity of mechanisms. Their cell wall is devoid of lipoplysaccharides or other inflammatory mediators (i.e. mainly Gram positive). Some may also compete with receptor sites on the gut wall and inhibit pathogen persistence and thus reduce the potential risk of infection. They may also compete effectively for nutrients with pathogens. One subject of intensive research is their stimulation of immunological defence systems, as discussed in section ‘Prebiotic effects and immune system’ of the present paper. Acknowledged examples are bifidobacteria and lactobacilli – known as useful probiotics. Intermediate genera like streptococci, enterococci, eubacteria and bacteroides can be classified as potentially beneficial to health or potentially harmful, depending on the species. With regard to some of the most recently identified genera in the major phylla (Firmicutes, Actinobacteria and Bacteroidetes), classification as potentially beneficial to health or potentially harmful still remains to be made. A scheme describing the hypothesis of a balanced microbiota has been proposed by Gibson and Roberfroid(Reference Gibson and Roberfroid3) and recently endorsed by ISAPP (2008) even though it is stillsubject of ongoing discussion. A revised version of that scheme including the most recent knowledge on gut microbiota composition is presented in Fig. 1.
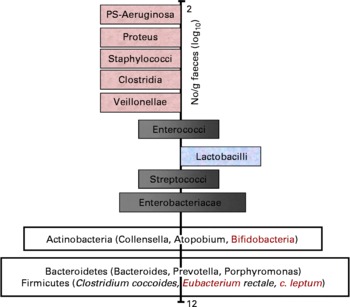
Fig. 1 Schematic representation of an adult gut microbiota. Major phylla and genera are located on a logarithmic scale as no. of CFU/g of faeces. Genera on the left site are likely to be potentially harmful whereas those on the right site are potentially beneficial to health. Those that sit both on the left site and the right site either contain species that are potentially harmful and species that are potentially beneficial to health or contain genera/species that still need to be classified. Indeed many of these have only recently been identified in the gut microbiota and their activity(ies) is/are still largely unknown.
The prebiotic concept is based on the selective stimulation of the host's own beneficial microflora by providing specific substrate for their growth and metabolism. Today, the effect is measured by using bifidobacteria or lactobacilli as markers, but may include others in the future, if their positive nature can be confirmed.
It has been shown by several studies (see section ‘Human studies showing prebiotic effects in healthy persons’ of the present paper) that dietary intervention can selectively modulate the indigenous composition of the gut microbiota. This is the basis of a prebiotic effect and this has been assessed through reliable molecular-based analyses.
Prebiotic effects and fermentation and physiology
Bacterial fermentation in the large gut
It is clear that a complex, resident gut microflora is present in human subjects. While the transit of residual foodstuffs through the stomach and small intestine is probably too rapid for the microbiota to exert a significant impact, this slows markedly in the colon. Colonic micro-organisms have ample opportunity to degrade available substrates(Reference Cherbut35, Reference Flint, Bayer and Rincon36). These may be derived either from the diet or by endogenous secretions(Reference Cummings and Macfarlane37).
Due to the high residence time of colonic contents, as well as a diverse and profuse flora, the colonic microbiota plays a more important role in host health and well-being than is the case in the small intestine. Beneficial effects can be related to their metabolism (i.e. fermentation profiles and end products), capacity for producing vitamins, antioxidants (reduction equivalents), defensins against potentially harmful competitors, exchange of molecular signals between the different genera/species but also with the eukaryotic epithelial cells. Potentially beneficial bacteria are further characterised by the absence of secondary metabolic pathways leading to toxic metabolites of, for example xenobiotics or phytochemicals.
The prebiotic concept emphasises the specific stimulation of such a microbiota leading to a reduction of the metabolic activity of potentially harmful bacterial. This section focusses essentially on primary metabolism, whereas the following ones deal with adverse effects and their prevention.
Substrate utilisation in the large intestine
The colonic microflora derive substrates for growth from the human diet (e.g. non-digestible oligosaccharides, dietary fibre and un-digested proteins reaching the colon) as well as from endogenous sources such as mucins, the main glycoprotein constituents of the mucus which lines the walls of the GI tract(Reference Rowland, Mallett and Wise38). The vast majority of the bacteria in the colon are strict anaerobes and thus derive energy from fermentation. The two main fermentative substrates of dietary origin are non-digestible carbohydrates (resistant starch, NSP, dietary fibres, non-digestible oligosaccharides of plant origin) and proteins which escape digestion in the small intestine(Reference Topping and Clifton39, Reference Lupton40). Of these, carbohydrate fermentation is more energetically favourable, leading to a gradient of substrate utilisation spatially through the colon(Reference Macfarlane, Gibson and Cummings41). The proximal colon is a saccharolytic environment with the majority of carbohydrate entering the colon being fermented in this region. As digesta moves through to the distal colon, carbohydrate availability decreases, proteins and amino acids become increasingly important energy sources for bacteria(Reference Macfarlane, Gibson and Cummings41).
The main substrates for bacterial growth are dietary non-digestible carbohydrates that evade upper intestinal hydrolysis and absorption. Non-digestible carbohydrates comprise resistant starch and resistant dextrins, NSP (e.g. pectins, arabinogalactans, gum Arabic, guar gum and hemicellulose), non-digestible oligosaccharides (e.g. raffinose, stachyose, ITF, galactans and mannans) as well as undigested portions of disaccharides (e.g. lactose) and sugar alcohols (e.g. lactitol and isomalt)(Reference Cummings and Macfarlane37, Reference Bingham, Pett and Day42, Reference Gray43). Resistant starch, NSP, most dietary fibres but also some non-digestible oligosaccharides are fermented by a wide range of the colonic bacterial although the degree of their breaking down might vary(Reference Englyst and Macfarlane44). However, some non-digestible oligosaccharides entering the colon are rapidly and quantitatively but selectively fermented (e.g. raffinose, ITF and galactans) by a small number of bacteria (e.g. bifidobacteria and lactobacilli)(Reference Hudson, Marsh, Gibson and Macfarlane45).
The overall intake of non-digestible carbohydrate in a Western diet is estimated between 20 and 30 g/d(46). Endogenous carbohydrates, chiefly from mucins and chondroitin sulphate, contribute about 2–3 g/d of fermentable substrate(Reference Quigley, Kelly, Gibson and Macfarlane47). The main saccharolytic species in the colonic microflora belong to the genera Bacteroides, Bifidobacterium, Ruminococcus, Eubacterium, Lactobacillus and Clostridium.
The second important group of substances for bacterial growth are proteins, peptides and amino acids: Approximately, 25 g of protein enters the colon daily(Reference Macfarlane, Macfarlane, Gibson and Macfarlane48). Other sources of proteins in the colon include non-digestible food components, bacterial secretions, sloughed off epithelial cells, bacterial lysis products and mucins. The main proteolytic species belong to the genera Bacteroides and Clostridium.
Products of microbial fermentation in the colon and their effects on the host
Carbohydrates in the colon are fermented to SCFA, mainly, acetate, propionate and butyrate(Reference Cummings49–Reference Flint, Logan, Lappin-Scott and Oyston51) and a number of other metabolites such as the electron sink products lactate, pyruvate, ethanol, succinate as well as the gases H2, CO2, CH4 and H2S(Reference Levitt, Gibson, Christl, Gibson and Macfarlane52). As a whole, SCFA acidify the luminal pH which suppresses the growth of pathogens(Reference Blaut53), they also influence intestinal motility(Reference Dass, John and Bassil54). They are rapidly absorbed by the colonic mucosa and contribute towards energy requirements of the host(Reference Cummings49, Reference Engelhardt, Busche, Gros, Cummings, Rombeau and Sakata55, Reference Vogt and Wolever56). Acetate is mainly metabolised in human muscle, kidney, heart and brain propionate that is cleared up by the liver, is a possible gluceogenic substrate and it might contribute to inhibition of cholesterol synthesis. It might also play a role in the regulation of adipose tissue deposition(Reference Reshef, Niv and Shapiro57, Reference Siong, Miyamoto and Shibata58).
Butyrate on the other hand is largely metabolised by the colonic epithelium where it serves as the major energy substrate as well as a regulator of cell growth and differentiation(Reference Cummings, Gibson and Macfarlane50, Reference Williams, Coxhead and Mathers59). It is also acknowledged that it may reduce the risk of colon cancer through stimulating apoptosis. Evidence for the role of butyrate in relation to the administration of ingredient showing a prebiotic effect is described later in the present paper. Rectally administered butyrate was also shown to relieve subjects from inflammatory bowel disease (IBD) symptoms(Reference Scheppach60).
Proteins reaching and/or produced in the colon are fermented to branched chain fatty acids such as isobutyrate, isovalerate and a range of nitrogenous and sulphur-containing compounds. Unlike carbohydrate fermentation products which are recognised as beneficial to health, some of the end products of amino acids metabolism may be toxic to the host, e.g. ammonia, amines and phenolic compounds(Reference Macfarlane, Macfarlane, Gibson and Macfarlane48). Consequently, excessive fermentation of proteins, especially in the distal colon, has been linked with disease states such as colon cancer and IBD, which generally start in this region of the large intestine before affecting more proximal areas. Thus, it is favourable to shift the gut fermentation towards saccharolytic fermentation over a prolonged period of time into the distal parts.
Conclusions
(1) Overall, saccharolytic fermentation leads to the formation of end products (SCFA) that are recognised as being beneficial to the host.
(2) Protein degradation on the other hand is likely to give rise to toxic substances such as ammonia and amines.
(3) Non-digestible carbohydrates with prebiotic effects selectively stimulate the growth of bacterial genera/species characterised exclusively, or preferably, by saccharolytic fermentation. Such a selective effect on gut microflora composition is likely to be more beneficial to host health than the one which would favour the metabolism of both carbohydrates and proteins. This is well established today for prebiotic effects favouring the growth of bifidobacteria and lactobacilli. Emerging genera are Eubacterium, Faecalibacterium and Roseburia – although more evidence is needed on their physiological properties.
In vitro tests for prebiotic effect
In vitro models aim at studying prebiotic effects independently from their passage through the upper parts of the GI tract even if digestion is sometimes partly simulated. These models are thus only indicative of a potential prebiotic effect, however, they do not prove the prebiotic attribute of a particular product as in vivo studies need to be performed to definitively demonstrate that the compound under investigation selectively stimulates the growth and/or activity(ies) of one or a limited number of microbial genus(era)/species in the gut microbiota that confers health benefits to the host. Since, as discussed earlier, the aim of the present paper is not to provide a list of food ingredients/supplements that classify as prebiotics, the following sections will only refer to a few examples to illustrate the potentials and the limits of in vitro tests as well as the advantages and disadvantages of the different experimental models.
Batch culture (pH or non-pH controlled) studies where different substrates are incubated with either pure culture of selected bacteria or faecal slurries subsequently analysed for microbial composition can be used:
(1) to study the selectivity of fermentation (including possible mechanism of selectivity) by, for example, bifidobacteria, lactobacilli of different substrates (e.g. main oligosaccharides contained in soyabeans are raffinose and stachyose which have been found to be good growth promoters of Bifidobacterium infantis but not E. coli, Streptococcus faecalis or Lactobacillus Lactobacillus acidophilus (Reference Tamura61)) or similar substrates differing in molecular weights (e.g. wheat arabinoxylans) showing, e.g. that molecular weight can be an important factor in selectivity(Reference Hughes, Shewry and Li62).
(2) to show changes in faecal microbiota (e.g. increase in bifidobacteria) but also to compare the efficacy of different substrates (e.g. ITF, starch, polydextrose, fructose and pectin, galactans, xylo-oligosaccharides, soyabean oligosaccharides(Reference Wang and Gibson63–Reference Hayakawa, Mizutani and Wada65)).
(3) to measure and to compare the evolution of gas and SCFA production as a result of the fermentation of different substrates(Reference Rycroft, Jones and Gibson64).
Single-stage chemostat studies with ITF were used to compare differing techniques to analyse microbiota composition, demonstrating that discrepancies might exist between classical microbiological techniques and molecular approaches. Agar plate counts showed an increase in the combined populations of bifidobacteria and lactobacilli reaching 98·7 % of the total bacterial flora by steady state. However, 16S ribosomal ribonucleic acid genus-specific probes indicated an initial increase in the bifidobacteria population which decreased after 6 d, while lactobacilli thrived in the low pH fermenter (pH 5·2–5·4) maintaining a high population at steady state. Changes observed in the SCFA profile corresponded well with the population data obtained through probe methods(Reference Sghir, Chow and Mackie66).
Continuous culture systems inoculated with faecal slurries can be used to investigate fermentation profiles showing, for example that in accordance with earlier studies, bifidobacteria, and to a lesser extent lactobacilli preferred ITF to glucose, whereas bacteroides could not grow on these substrates(Reference Gibson and Wang67, Reference Gibson and Wang68). By varying parameters in the chemostat, the conditions for growth of bifidobacteria and inhibition of bacteroides, clostridia and coliforms can be further analysed showing that low pH (pH 5·5), high culture dilution rate (0·3 h− 1) and 1 % (w/v) concentration of carbohydrate (i.e. similar to the physico-chemical environment of the proximal colon) are optimum.
The three-stage gut model reproduces the three segments of the colon (proximal/ascending, transverse and distal/descending). It is used to confirm the effects observed in the previous models. Studies using this model show enhanced proliferation of bifidobacteria and/or lactobacilli by ITF and galactans in conditions resembling the proximal/ascending colon(Reference Gibson and Wang67, Reference McBain and Macfarlane69, Reference McBain and Macfarlane70). Whereas studies using models of vessels two and three (modeling transverse and descending colon respectively) displayed very little change in microbiota when fermenting galactans(Reference McBain and Macfarlane70). In the same model, changes in enzyme activities (β-glycosidase, β-glucuronidase, azoreductase and arylsulphatase) can also be monitored showing their suppression after fermentation of galactans(Reference McBain and Macfarlane70) or soyabean–oligosaccharides(Reference Wada, Watabe and Mizutani71). Investigating the effect of pH and substrate concentration on the fermentation selectivity of galactans alongside other products, Palframan et al. (Reference Palframan, Gibson and Rastall72) reported a strong bifidogenic effect at pH 6 and at 2 % (w/v) and suggested that they may be well fermented in the distal colon. In another study, galactans of rather low molecular weight (1 % w/v) had a strong bifidogenic effect which showed good persistence through the first two vessels, with a weaker response in the third(Reference Tzortzis, Goulas and Gee73).
The simulator of the human intestinal microbial ecosystem model consists of a series of five temperature and pH-controlled vessels that simulate the stomach, small intestine, ascending, transverse and descending colons, respectively. It can be fed with a complex growth medium containing selected substrates (e.g. ITF) to study their fermentation including the monitoring of metabolites and to analyse their effect on enzyme activities and composition of the microbiota by using a multiphase approach consisting of plate counting, quantitative PCR and DGGE(Reference van de Wiele, Boon and Possemiers74). Results have shown a significant increase in lactobacilli in the transverse and descending colon vessels. Low levels of bifidobacteria were recorded in the colon vessels. DGGE analysis revealed that bacteria in the ascending colon vessel grouped together as the bacteria in the other colon vessels did. Bifidobacteria clustered according to the time point rather than the vessel. Quantitative PCR, however, revealed a significant increase in bifidobacteria population in all three-colon vessels. ITF feeding also resulted in an increase in the production of SCFA, particularly propionate and butyrate, indicating a shift towards a more saccharolytic fermentation. The same model system and metabolic analysis can also be used to investigate the effect of different composition of the same substrates (e.g. of ITF with different molecular weight) on fermentation properties(Reference van de Wiele, Boon and Possemiers75).
A more sophisticated in vitro model of fermentation in the proximal large intestine is the TNO-intestinal model-2 model(Reference Minekus, Smeets-Peeters and Bernalier76, Reference Venema, van Nuenen and van den Heuvel77). This consists of a series of linked glass vessels containing flexible walls. This arrangement allows simulation of peristalsis together with temperature regulation by means of pumping water through the space between the glass and flexible walls. The flow is controlled by computer to more accurately simulate peristalitc mixing. The vessels are further equipped with a hollow fibre membrane in the lumen to simulate absorption of water and SCFA. TNO-intestinal model-2 has been used to investigate the population changes on the fermentation of lactulose using culture-based methods coupled with DGGE(Reference Venema, van Nuenen and van den Heuvel77). Increases in lactobacilli and enterococci were seen.
Conclusions
(1) In vitro models allow comparative studies on fermentation by and/or effects of ingredients showing a potential prebiotic effect on isolated or mixture of bacterial strains, including faecal flora, as well as identification and eventually quantification of the resulting fermentation products especially the SCFA. They also allow comparative analysis of the different analytical methods available to identify and quantify the various genera/species.
(2) They further allow the analysis of the potential/absence of toxin formation or change in enzyme activities potentially associated with beneficial or harmful effects.
(3) The multi-stage models that are designed to mimic the different segments of the intestine, especially the proximal/ascending, transverse and distal/descending colon, are useful in localising the site of the selective stimulation of bacterial growth.
(4) The results can be used to select potential candidate showing prebiotic effect(s) for in vivo studies especially in human volunteers, which remain the obligatory steps to definitively prove the prebiotic effect attribute.
Human studies showing prebiotics effect in healthy persons
By reference to the prebiotic concept as defined earlier, criteria for classification as a prebiotic are(Reference Gibson, Probert and Van Loo4)
(1) resistance to gastric acidity, hydrolysis by mammalian digestive enzymes and GI absorption;
(2) fermentation by intestinal microflora;
(3) selective stimulation of the growth and/or activity(ies) of one or a limited number of intestinal bacteria beneficially associated with health and well-being.
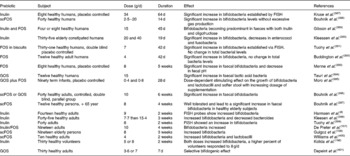
Any dietary component that reaches the colon intact (or partly so) is a potential candidate for prebiotic attribute, however, it is the latter of the three above criteria which is crucial but still the most difficult to fulfil (and which is often ignored when citing ingredients as ‘prebiotics’). Even if in addition to ITF and GOS, several dietary carbohydrates (e.g polydextrose, soyabean oligosaccharides, lactosucrose, isomalto-oligosaccharides, gluco-oligosaccharides, xylylo-oligosaccharides, gentio-oligosaccharides, mannan-oligosaccharides, lactose, hemicellulose, resistant starch, resistant dextrins, oat bran, oligosaccharides from melibiose, β-glucans, N-acetylchito-oligosaccharides, sugar alcohols such as lactitol, sorbitol and maltitol) show some fermentation selectivity when tested in laboratory systems (see section ‘In vitro tests for prebiotic effect’ in the present paper). However, the ultimate test for prebiotic activity (i.e. human volunteer trials) is lacking for the majority of these compounds. As for today, ITF and GOS are the compounds the most extensively tested in human trials that have confirmed their prebiotic effects as evidence by their ability to change the gut flora composition after a short feeding period at reasonably low doses(Reference Gibson and Roberfroid20) (Table 8). ITF, the most extensively tested forms in the literature, occur naturally in several foods such as leek, asparagus, chicory, Jerusalem artichoke, garlic, artichoke, onion, wheat, banana and oats, as well as soyabean. However, these foods contain only trace levels of ITF, so developments have taken the approach of removing the active ingredient from such sources (especially chicory roots) and adding them to more frequently consumed products in order to attain levels whereby a prebiotic effect may occur, e.g. cereals, confectionery, biscuits, infant foods, yoghurts, table spreads, bread, sauces, drinks(Reference Gibson, Probert and Van Loo4). Other food ingredients/additives with potential prebiotic effects are already under investigations and will certainly be further developed in the future from dietary fibres and other non-digestible food ingredients. Very preliminary data already exist for some but many more replicate human studies including the quantitative analysis of a wide variety of bacterial genera in faecal microbiota using the more recent methodologies (as described in section ‘Microbiota of the gastro-intestinal tract – the large intestine’ of the present paper) are needed before this can be the case. Human trials may be carried out on volunteers who are on controlled diets, or are free living. To ensure consistency and exclude incidental findings, more than one human trial is needed and the totality of several human studies for a candidate prebiotic should be considered.
Table 8 Example of human studies (healthy persons) designed to determine the prebiotic effect of short-chain fructo-oligosaccharides (scFOS), fructo-oligosaccharides (FOS), galacto-oligosaccharides (GOS) and inulin
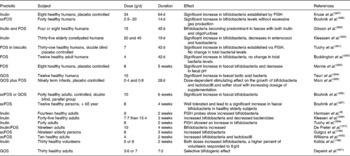
FISH, fluorescent in situ hybridization.
When evaluating a potential prebiotic effect it must be kept in mind that a dose–effect relationship and consequently a minimum effective dose are difficult to establish. Indeed, the major determinant that quantitatively controls the prebiotic effect is the number of targeted bacteria genus/species per gram of faeces the volunteers have before the supplementation with the compound presumed to show a prebiotic effect. This issue has been extensively discussed previously(Reference Roberfroid78).
Conclusions
Apart from protein fermentation, harmful substances may arise from bacterial secondary metabolism.
A prebiotic effect should not lead to stimulate the proteolytic microbiota and thereby reduce overall formation of bacterial metabolism.
Prebiotic effects and immune system
Outline of benefit area
The main authors of this section are Professor Watzl and Dr Wolvers. To provide optimal resistance against a large variety of pathogenic encounters, the immune system has evolved to comprise multiple, functionally differing cell types enabling the development of an immune response that is specifically tailored to clear the pathogen involved. Consequently, a large spectrum of immune parameters involved in various types of responses exist, of which comprehensive descriptions can be found in many textbooks (e.g. Janeway's Immunobiology by Murphy et al. (Reference Murphy, Travers and Walport79)). Some of these may be measurable in human subjects and can be divided into innate v. adaptive, mucosal v. systemic, pro-inflammatory v. anti-inflammatory, etc. Modulating aspects of the immune system may, in theory, serve several clinical purposes. First, boosting or restoring the very purpose of immune function, i.e. the resistance against infections, may serve as a clinical tool to prevent or treat infectious diseases. Secondly, preventing or treating consequences of an aberrant or undesired immune response, such as those occurring with an allergic response or during chronic inflammatory diseases, are other targets with high clinical relevance.
Although there is no single immune marker that accurately reflects or predicts an individual's resistance to infection, parameters can be identified that play a more prominent role in certain types of infections or conditions than others. For instance, if resistance against the common cold, i.e. a viral upper respiratory tract infection, is the topic of interest, it seems appropriate to investigate natural killer (NK) cell and CD8+lymphocyte activity, whereas in case of IBD the balance between pro-inflammatory and immuno-regulatory cytokines will be of interest (see section ‘Prebiotic effects and IBD’ of the present paper). Moreover, in a previous ILSI Europe activity, the suitability of immune markers to measure immuno-modulation by dietary intervention in human subjects was assessed, leading to the identification of four high-suitability markers that are the result of an integrated immune reaction (vaccine-specific serum antibody production, delayed-type hypersensitivity response, vaccine-specific or total secretory IgA in saliva, the response to attenuated pathogens). In addition, a range of medium and low-suitability markers, such as functional activity of cells of the innate immune system (NK cell activity, phagocytosis, T-cell proliferation and various cytokines), were identified(Reference Albers, Antoine and Bourdet-Sicard80). Although the combined measurement of high- and medium-suitability markers may be a way to address aspects of immune status, the ultimate proof of accurate or even improved immune function in practice is a change in the incidence, severity or duration of infectious episodes or conditions with a prominent immune component such as allergies and chronic inflammation.
That modulation of certain aspects of the immune system may result from prebiotic effects and is based on the pivotal interaction between the intestinal microbiota and the host immune system. From several studies in germ-free and gnotobiotic animals, it is clear that the microbiota is essential for an optimal structural and functional development of the immune system(Reference Wagner81–Reference Gaboriau-Routhiau, Rakotobe and Lecuyer84). The interactive co-existence of the immune system and the microbiota is especially apparent in the intestinal tract where the gut-associated lymphoid tissue (GALT) has evolved to provide optimal defense against intestinal pathogens, while at the same time tolerating dietary and self-antigens, as well as large populations of commensal non-pathogenic microbes.
Although specialised cells such as the M-cells and, as discovered more recently, also dendritic cells sample material directly from the intestinal lumen(Reference Rescigno, Urbano and Valzasina85), enterocytes are key intermediates that convey signals from the intestinal lumen to the mucosal immune system(Reference Sanderson86, Reference Artis87) and are thus a target for a prebiotic effect on the immune system.
Prebiotic effects may influence the immune system directly or indirectly as a result of intestinal fermentation and promotion of growth of certain members of the gut microbiota. First, the mere presence of increased numbers of a particular microbial genus or species, or a related decrease of other microbes, may change the collective immuno-interactive profile of the microbiota. Through pattern-recognition receptors, such as the toll-like receptors (TLR), both immune cells and enterocytes interact with the so-called pathogen-associated molecular patterns, such as lipopolysaccharides (LPS, a membrane component of Gram-negative bacteria), lipoteichoic acids and unmethylated C-phosphate-G (CpG) DNA that are in fact present on all the micro-organisms surface regardless of pathogenicity. These interactions, possibly in combination with contextual cues of pathogenicity, result in a variety of downstream events eventually leading to cytokine production steering towards an appropriate immune response for the microbial event(Reference Medzhitov88–Reference Rakoff-Nahoum, Paglino and Eslami-Varzaneh90).
Secondly, microbial products such as SCFA may interact with immune cells and enterocytes and modify their activity. G-protein-coupled receptors (GPR) 41 and GPR 43 are identified as receptors for SCFA and are expressed on leukocytes, especially polymorphonuclear cells(Reference Nilsson, Kotarsky and Owman91, Reference Le Poul, Loison and Struyf92), as well as on enterocytes and enteroendocrine cells in the human colon(Reference Karaki, Tazoe and Hayashi93, Reference Tazoe, Otomo and Karaki94). SCFA modulate chemokine expression in intestinal epithelial cells(Reference Sanderson86), differentially affect pro-inflammatory IL-2 and interferon (IFN)-γ and immuno-regulatory IL-10 production by rat lymphocytes in vitro (Reference Cavaglieri, Nishiyama and Fernandes95), and a recent publication shows the importance of ligation to GPR43 in mice to maintain intestinal homeostasis(Reference Maslowski, Vieira and Ng96).
Thirdly, the potential direct ligation of pattern recognition receptors on immune cells by prebiotic carbohydrate structures may result in immunomodulation, although there is currently very little evidence for the presence of, for example, a fructose-receptor on immune cells.
In summary, there are plausible mechanisms by which prebiotic effects can modulate immune function parameters. The inaccessibility of the human GI immune system complicates the investigation in this area and most human studies rely on the measurement of ex vivo systemic immune markers, of which the predictive value for overall resistance to infections or outcome of immune-related disorders is limited.
Summary of key studies
Several comprehensive reviews have summarised the present knowledge of the immunomodulatory potential of prebiotic effects (especially ITF)(Reference Schley and Field97–Reference Seifert, Watzl, Gibson and Roberfroid101). A limited number of human studies have been performed but most have limitations as they investigated prebiotic effects in combination with the administration of other ingredients or did not include an appropriate control group.
The prebiotic effects on immune markers that represent a more or less integrated immune response, such as response to vaccination, were investigated in only a few studies (Table 9). Bunout et al. (Reference Bunout, Hirsch and Pia102) supplemented healthy elderly with an oligofructose/inulin mix (6 g/d) in combination with a nutrient supplement, while the control group received maltodextrin with the nutrient supplement. No significant differences were observed in antibody titers after vaccination or on secretory IgA levels(Reference Bunout, Hirsch and Pia102). In a second study, the same authors investigated the effect of a supplement with oligofructose on various immune markers including delayed type hypersensitivity and vaccination. Elderly subjects attending a clinic received oligofructose as part of a complex nutritional supplement including Lactobacillus paracasei. Elderly subjects attending another clinic not receiving this supplement served as controls. Delayed type hypersensitivity response and antibody titres after vaccination did not differ between groups(Reference Bunout, Barrera and Hirsch103).
Table 9 The prebiotic effect on immune markers
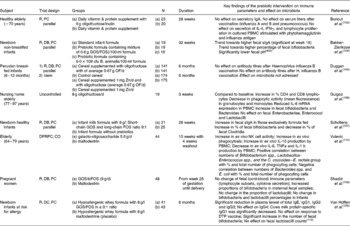
R, PC, randomised, placebo-control; IFNγ, interferon γ PBMC, peripheral blood mononuclear cell; R, DB, PC, randomised, double-blind, placebo-control; GOS, galacto-oligosaccharides; FOS, fructo-oligosaccharides; NK, natural killer; CD, Crohn's disease; CO, crossover; OF, oligofructose; DPRPC, double-parallel, randomised, placebo-control; DTP, diphteria, tetanus, polio.
Human studies are detailed that allow interpretation of the effect of prebiotics alone, not of the combination of prebitocs with other ingredients.
Studies describe the effect on immune markers; studies that focus on clinical endpoints are summarized elsewhere in this paper (pediatrics, inflammatory bowel disease).
In infants aged 6–12 months (87 % breast-fed), the intake of oligofructose as part of an infant cereal had no effect on diarrhoea prevalence (see section ‘Use of prebiotic effects for paediatric disorders – diarrhoeal diseases’ of the present paper) and on vaccination-induced antibody titres to Haemophilus influenza when compared with the infant cereal alone(Reference Duggan, Penny and Hibberd104). Besides, the fact that a rather low dose of oligofructose was supplemented, breast-feeding may already have provided adequate amounts of human milk oligosaccharides in the present study. Also in infants at high risk for allergies, supplementation with a GOS/fructo-oligosaccharides (FOS) mixtures did not change antibody levels after a standard vaccination(Reference van Hoffen, Ruiter and Faber105). In contrast, early-life exposure of non-breast-fed infants to oligosaccharides had an effect on natural Ig production, as a mixture of GOS/FOS was shown to result in significantly higher faecal secreteory Immunoglobulin A (sIgA) concentrations as a consequence of the prebiotic effect(Reference Bakker-Zierikzee, Tol and Kroes106, Reference Scholtens, Alliet and Raes107). Overall, there are currently no data that support beneficial prebiotic effects on the response to vaccination, but data on faecal secretory IgA in infants are promising when supplemented with a specific combination of compounds showing prebiotic effects.
In addition to the effects on integrated immune responses, the prebiotic effect on specific immune markers has been tested in a few studies of varying quality with differential outcomes (Table 9). In healthy elderly people receiving ITF-DPav3-4 (6 g/d), a decrease in phagocytosis and IL-6 mRNA expression in peripheral blood mononuclear cell was found(Reference Guigoz, Rochat and Perruisseau-Carrier108). The present study was a one-arm study using baseline for comparison. Whether the tested ingredient induced the observed immunological changes cannot be answered from the present study. Increased NK cell activity and IL-2 production by peripheral blood mononuclear cell (Lymphokine production by mononuclear cells) were found in a synbiotic study in elderly(Reference Bunout, Barrera and Hirsch103). As this was a synbiotic intervention, a causal conclusion about an immunomodulation of the prebiotic intervention cannot be drawn. No effect was observed on secretion of IL-4, IFNγ and lymphocyte proliferation in cultured peripheral blood mononuclear cell(Reference Bunout, Hirsch and Pia102).
A study investigating the application of ingredients showing a prebiotic effect in pregnant women showed no effect on the composition of lymphocyte subsets or cytokine secretion patterns in circulating lymphocytes of the off-spring as assessed in cord-blood(Reference Shadid, Haarman and Knol109). For safety reasons, the dosage was relatively low in the present study.
A well-designed and controlled human intervention study investigated the effect of a mixture of galactans on the immune system of healthy elderly volunteers. The present study reported that intake of such GOS (galactans) (5·5 g/d) for 10 weeks significantly increased phagocytosis, NK cell activity and the production of the anti-inflammatory cytokine IL-10, while the production of pro-inflammatory cytokines IL-1β, IL-6, TNFα was reduced(Reference Vulevic, Drakoularakou and Yaqoob110). A clear positive correlation between numbers of bifidobacteria in faecal samples and both, NK cell activity and phagocytosis, was observed. The present study suggests that a mixture of galactans beneficially affects the immune system and that the achieved effects may be indirect and mediated via a prebiotic effect, i.e. a change in microbiota composition. A few of the trials described earlier also show changes in immune markers alongside changes in the faecal microbiota, mainly increase in bifidobacteria. These studies thus provide data for the suggested link between a change in the flora and immunomodulation, but more studies showing correlative findings are required for convincing evidence.
Only a few studies that investigated the prebiotic effect on immune-related clinical end points such as resistance to infections, allergies and IBD have also included measurements on immune markers. Combining clinical end points with such functional markers may provide a possible mechanistic explanation for the observed effects. In a small number of patients with moderately active Crohn's disease, consumption of 15 g ITF/d reported positive clinical outcomes (see section ‘Prebiotic effects in Crohn's disease’ of the present paper), while IL-10 production by mucosal dendritic cells isolated from biopsies was increased as did expression of TLR-2 and TLR-4(Reference Lindsay, Whelan and Stagg111). Although some of the findings correlate with those found in animals studies(Reference Hoentjen, Welling and Harmsen112), the open label character of the study needs to be considered.
In infants at high risk of allergies, a mixture of GOS/FOS supplemented for 6 months reduced plasma level of total IgE, IgG1, IgG2 and IgG3, whereas no effect on IgG4 was observed. In addition, cow's milk protein-specific IgG1 was significantly decreased(Reference van Hoffen, Ruiter and Faber105). This may be beneficial change in infants at risk of allergies, and although no direct correlations were reported, the same study found a significant reduction in the incidence of atopic dermatitis in a subpopulation of the GOS/FOS group(Reference Moro, Arslanoglu and Stahl113).
Experimental data from animal studies indicate that, besides the systemic immune system, the GALT may be the primary target of immunomodulatory prebiotic effects. Biomarkers to assess functional changes in the GALT include sIgA, cytokine production and lymphocyte numbers. Prebiotic effects have been shown to increase sIgA concentration in the intestinal lumen, to increase B cell numbers in Peyer's patches, and, in intestinal tissues, to enhance IL-10 protein secretion and to decrease mRNA expression and protein concentrations of pro-inflammatory cytokines(Reference Watzl, Girrbach and Roller98–Reference Seifert, Watzl, Gibson and Roberfroid101). Genes related to intestinal immune responses seem to be a primary target of the prebiotic effects(Reference Fukasawa, Murashima and Matsumoto114). Further, functional activities of NK cells and phagocytes isolated from various immune tissues were significantly increased but depending on the source of immune cells (Peyer's patches, mesenteric lymph nodes, intraepithelial lymphocytes) the prebiotic effects may differ(Reference Roller, Pietro and Caderni115–Reference Girrbach, Schroeder and Breves117). This illustrates the need to differentially study the prebiotic effects of on various immune compartments. The lack of sufficient tools to investigate prebiotic effects in the human GALT hampers insights into the possible differential impact on the mucosal v. the systemic immune system.
Key points
(1) Plausible hypotheses exist that ingredients showing a prebiotic effect may potentially affect the immune system as a direct or indirect result of the change in the composition and/or fermentation profile of the microbiota.
(2) There is currently limited, yet promising evidence that such ingredients modulate immune markers in human subjects. Well-designed human intervention studies are few.
(3) Data that show increased faecal sIgA levels in infants are promising and need to be confirmed.
(4) While several studies report changes in the faecal microbial composition alongside with changes in immune markers, only one study so far has correlated these findings. More studies addressing such correlation are needed to establish a firm link between changes in the microbiota and immune markers.
(5) Despite the wealth of evidence that compounds with prebiotic effects affect the intestinal microbiota, and modulate immune parameters, it is of importance to know whether these immunomodulatory effects result in a clinically relevant outcome, i.e. improved resistance against infections, or impairment of allergies and inflammation. Preliminary yet promising clinical end point studies exist which integrate the measurement of immune markers as possible explanation of prebiotic efficacy.
(6) Animal studies indicate that immunological effects may vary depending upon the anatomical site of origin of the immune cell (e.g. Peyer's patches v. intraepithelial lymphocytes). However, as the human GALT as primary target of the prebiotic effects cannot be easily addressed in human intervention studies, insights are difficult to obtain and thus still limited.
Recommendations
Data from well-designed, controlled human intervention studies with healthy subjects do not allow a final conclusion about the effects of ingredients showing a prebiotic effect on the immune system. Data so far are available for ITF and GOS, but few studies have been published so far. Therefore, further studies with adequate methodology, investigating immune parameters such as laid out by the ILSI Task Force on Nutrition and Immunity in Man(Reference Albers, Antoine and Bourdet-Sicard80), are warranted to obtain further insights on how prebiotic effects may modify immune function markers. Furthermore, tools should be developed to measure the impact of prebiotic effects on the GALT in human subjects, so an understanding of the tissue-specific effects can be achieved. Findings of such immunomodulation should lead to hypotheses on the potential use of compounds with prebiotic effects in relevant health-related conditions, which could then be tested in well-designed clinical end point studies. In addition, effects of different prebiotic chemical structures of prebiotics, dosing and timing of supplementation have to be studied.
Prebiotic effects in paediatrics
Oligosaccharides and prebiotic effects in infant formulae
The main authors for this section are Professor Szajewska and Dr Stahl. The use of non-digestible carbohydrates in infant formulae and follow-on formulae has been commented on by the Committee on Nutrition of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition(Reference Agostoni, Axelsson and Goulet118). Based on the evidence obtained in a search up to January 2004, the Committee concluded that only a limited number of studies have evaluated the effects of the addition of substances with prebiotic effects to dietetic products for infants. Only one type of oligosaccharide mixture of galactans and ITF consisting of GOS and a high molecular weight fraction of inulin in a ratio of 9:1 (GOS/FOS) was evaluated. The Committee stated that although the administration of oligosaccharides with prebiotic effects has the potential to increase the total number of bifidobacteria in faeces and may also soften stools, there is no published evidence of any clinical benefits after addition of oligosaccharides with prebiotic effects to dietetic products for infants. No general recommendation on the use of oligosaccharide supplementation in infancy for preventive or therapeutic purposes can be made. The available data on the oligosaccharide mixtures in infant formulae do not demonstrate adverse effects. Validated clinical outcome measures of prebiotic effects in infants should be characterised in further well designed and carefully conducted randomised controlled trials (RCT), with relevant inclusion/exclusion criteria and adequate sample sizes. Such trials should also define the optimal quantities, types and intake durations.
A number of studies have been published thereafter on the addition of ingredients showing a prebiotic effect to dietetic products for infants and recently reviewed(Reference Boehm and Moro119). These ingredients have been used either as one compound(Reference Yap, Mohamed and Husni120–Reference Fanaro, Marten and Bagna123) or as a mixture of different neutral and acidic oligosaccharides(Reference Magne, Hachelaf and Suau124). Collectively, these studies confirm that the administration of oligosaccharides with prebiotic effects in dietetic products have the potential to increase dose dependently the total number of bifidobacteria in faeces, although at present, it is not possible to define the number of bifidobacteria that would constitute normal/optimal microbiota and to soften stools. Furthermore, prebiotic effects modulate stool pH, SCFA pattern similar to those of breast-fed infants. Whether any of these effects per se is of benefit is currently not well established. Clinical outcomes related to the use of dietetic products for infants supplemented with prebiotic effects are discussed in the forthcoming sections (e.g. effect on allergic diseases, infections).
Currently, the Directive 2006/141/EC on infant formulae and follow-on formulae specifically allows the addition of GOS–FOS in a ratio of 9/1 and in a quantity of 0·8 g/100 ml prepared product(125). The GOS and FOS were specified as ‘a combination of 90 % oligogalactosyl-lactose and 10 % high molecular weight oligofructosyl-saccharose’. This Directive also states that other combinations and maximum levels of FOS and GOS may be used if they satisfy the nutritional requirements of infants in good health as established by generally accepted scientific data.
Use of prebiotic effects in complementary foods for children
One controlled trial (RCT)(Reference Moore, Chao and Yang126) conducted in fifty-six healthy, term infants aged 4–12 months evaluated the tolerance and GI effects of an infant cereal supplemented with either ITF or placebo for 28 d. Compared with the control group, stool consistency was less often described as ‘hard’ and more likely to be described as ‘soft’ or ‘loose’ in the ITF-supplemented group. There was no difference between the groups in crying, spitting-up or colic. No difference in stool pH between the groups was found. There was also no significant difference in growth between the two groups. Clinical outcomes were not reported. The limitations of the present study include the use of non-validated tool for parental assessment of stool consistency, a small sample size and a short follow-up period.
Another double-blind RCT(Reference Scholtens, Alles and Bindels127) involving thirty-five infants aged 4–6 months studied the effect of adding GOS/FOS to solid foods results in an increase in the faecal proportion of bifidobacteria in the intestinal microbiota. Intention-to-treat analysis revealed no significant difference between the two study groups. Only per-protocol analysis involving twenty children who complied with the protocol showed that the faecal percentage of bifidobacteria increased from 43 to 57 % (P = 0·03) from weeks 0 to 6 but did non-significantly change in the control group (36 and 32 %, respectively, P = 0·4). There were no statistically significant differences in stool frequency and consistency.
More recently an indication for a prebiotic effect with ITF-fortified milk in children aged 7–8 years has also been reported(Reference Lien do, Nhung and Khan128).
Use of prebiotic effects for paediatric disorders
The effect of prebiotics in paediatric diseases has to be seen under the different aspect either of treatment or of prophylaxis. Theoretically, – and also clearly demonstrated in this part of the manuscript – prebiotics are more effective in prophylaxis more than in treatment. That seems logically because the prebiotic effect can only be seen after a certain period of time which is needed for the development of the microbiota (and which is significantly longer than the duration of an acute diarrhoea). In consequence, prebiotics are ideal candidates for prophylaxis but not for treatment.
Diarrhoeal diseases
It can be hypothesised that the continuous use of products with prebiotic effects might, by providing an immunologic stimulus (see section ‘Prebiotic effects and immune system’ of the present paper), be useful in preventing infectious diseases commonly encountered by young children.
In a large well-designed RCT performed in infants aged 6–12 months (n 282), Duggan et al. (Reference Duggan, Penny and Hibberd104) compared an infant cereal supplemented with oligofructose with a non-supplemented cereal. There was no difference in the number of diarrhoeal episodes, episodes of severe diarrhoea or episodes of dysentery. No significant difference was found in the mean duration of diarrhoea. During a second part of the same trial involving 349 subjects, Zn was added to both oligofructose-supplemented and control cereals(Reference Duggan, Penny and Hibberd104). Again, no significant difference was found in any of the outcomes studied between the groups. In the both trials, post-immunisation titres of the antibody to Haemophilus influenzae type B were similar in all groups, as were gains in height (no data on weight), number of visits to the clinic, hospitalisations and use of antibiotics. The prebiotic dose was with 0·25 g/kg d lower that the prebiotic level mentioned in the EC directive with 0·8 g/100 ml – assuming an intake of 150–200 ml/kg and day – thus resulting in 1·2–1·6 g prebiotics/kg and day(125).
More recently, Bruzzese et al. (Reference Bruzzese, Volpicelli and Squeglia129) evaluated the effect of an infant formula containing the prebiotic mixture GOS/FOS compared with a standard infant formula in an open placebo-controlled involving 342 healthy infants with 12 months follow-up. Compared with the controls, the use of prebiotic-supplemented formula was associated with a significant reduction in the incidence of gastroenteritis (0·12 ± 0·04 v. 0·29 ± 0·05 episodes/child per 12 months; P = 0·015), and in the rate of children with ≥ 1 episode of acute diarrhoea (10/96 v. 26/109, Relative Risk (RR) 0·44 (95 % CI 0·22, 0·86)). The findings regarding the prevention of GI infections are promising for efficacy. However, there are some methodological limitations to the study, including no allocation concealment, no blind control and no intention-to-treat analysis (this analysis aims to test for effectiveness under field conditions); this may result in selection, performance and/or attrition biases. The impact on respiratory tract infections is discussed under section ‘Respiratory tract infections’.
One RCT(Reference Arslanoglu, Moro and Boehm130) found similar number of episodes of diarrhoea in the group of infants fed extensively hydrolysed whey formula supplemented either with 0·8 g GOS/FOS or with maltodextrin as placebo.
Acute infectious gastroenteritis
The efficacy and safety of administering a mixture of non-digestible carbohydrates, including soya polysaccharide 25 %, α-cellulose 9 %, gum Arabic 19 %, oligofructose 18·5 %, inulin 21·5 % and resistant starch 7 %, as an adjunct to oral rehydration therapy in the treatment of acute infectious diarrhoea was assessed in one RCT involving 144 boys with mild-to-moderate dehydration. It was hypothesised that with the incorporation of non-digestible carbohydrates, some of them (e.g. galactans and ITF) with prebiotic effects might promote fermentation in the colon, and thus, decrease faecal volume and the duration of the diarrhoeal illness. Intention-to-treat analysis (relevant for effectiveness) did not show a significant difference in the mean 48-h stool volume, the duration of the diarrhoea after randomisation, the duration of hospital stay and unscheduled intravenous rehydration. No significant adverse effects were noted(Reference Hoekstra, Szajewska and Zikri131). An explanation that no effects could be detected could originate from the type and the amount of non-digestible carbohydrates added to the oral rehydration solution. An average dose of 10–15 g per episode in relatively mild diarrhoea may be simply insufficient to achieve a shorter duration of diarrhoea. Furthermore, it is possible that the timing of the intervention was inappropriate, making the addition of non-digestible carbohydrates to exclusive oral rehydration therapy an insufficient measure.
Antibiotic-associated diarrhoea
The rationale for the use of ingredients showing a prebiotic effect for the prevention of antibiotic-associated diarrhoea is based on the assumption that the use of antibiotics leads to intestinal dysbiosis and that this is a key factor in the pathogenesis of antibiotic-associated diarrhoea(Reference Surawicz132). In contrast to probiotics(Reference D'Souza, Rajkumar and Cooke133–Reference Szajewska, Ruszczynski and Radzikowski137), there is a paucity of data on the prebiotic effects in preventing antibiotic-associated diarrhoea. One paediatric double-blind RCT(Reference Brunser, Gotteland and Cruchet138) involved 140 children (1–2 years of age) who were treated with amoxicillin for acute bronchitis. The present study revealed no significant difference in the incidence of diarrhoea in children receiving ITF administered in a milk formula (4·5 g/l) for 21 d after completion of antibiotic treatment compared with placebo (10 % v. 6 %, RR 0·6, 95 % CI 0·2–1·8). However, ingredients showing a prebiotic effect in a milk formula increased faecal bifidobacteria early after amoxicillin treatment.
Respiratory tract infections
In the most recent RCT by Bruzzese et al. (Reference Bruzzese, Volpicelli and Squeglia129) described earlier, it was found that compared with controls, the use of an infant formula with GOS/FOS was associated with a similar number of episodes of upper respiratory tract infections (P = 0·4), similar number of children with greater than three episodes upper respiratory tract infections (17/60 v. 29/65; P = 0·06), although the number of children with multiple antibiotic courses per year was lower in children receiving ingredients showing a prebiotic effect (24/60 v. 43/65; P = 0·004).
One RCT(Reference Arslanoglu, Moro and Boehm130) found that infants fed extensively hydrolysed whey formula supplemented with 0·8 g GOS/FOS compared with the placebo group had fewer episodes of physician-diagnosed overall and upper respiratory tract infections (P < 0·01), fever episodes (P < 0·00 001) and fewer antibiotic prescriptions (P < 0·05).
Prebiotic effects and atopy
Atopic eczema is an itchy inflammatory skin condition with associated epidermal barrier dysfunction. Therapeutic options (emollients and topical steroids for mild-to-moderate eczema; topical or systemic calcineurin inhibitors, UV phototherapy, or systemic azathioprine for moderate-to-severe eczema) are relatively limited and often unsatisfactory, prompting interest in alternative treatment methods.
The rationale for using prebiotic effects in preventing atopic disorders is based on the concept that prebiotic effects modify the intestinal flora of formula-fed infants towards that of breast-fed infants. The intestinal flora of atopic children has been found to differ from that of controls with atopic subjects having more clostridia and tending to have fewer bifidobacteria than non-atopic subjects(Reference Kalliomaki, Kirjavainen and Eerola139). Thus, there is indirect evidence that differences in the neonatal gut microbiota may precede or coincide with the early development of atopy. This further suggests a crucial role for a balanced commensal gut microbiota in the maturation of the early immune system.
The Cochrane Review published in 2007(Reference Osborn and Sinn140) aimed at determining the effect of different ingredients showing a prebiotic effect (GOS/FOS, only FOS, GOS together with polydextrose and lactulose) on the prevention of allergic disease or food hypersensitivity in infants. Only two RCT of reasonable methodological quality according to the reviewers and involving 432 infants reported outcomes related to allergic disease. The reviewers concluded that there is insufficient evidence to determine the role of prebiotic supplementation of infant formula for the prevention of allergic disease and food hypersensitivity.
One of the included RCT(Reference Osborn and Sinn140) investigated the effect of the prebiotic mixture (GOS/FOS; dosage: 8 g/l) on the intestinal flora and the cumulative incidence of atopic dermatitis during the first 6 months of life in infants at risk for allergy (with at least one parent with documented allergic disease confirmed by physician). Of the 259 infants, 206 (79·5 %) infants who were randomly assigned to receive extensively hydrolysed whey formula supplemented either with 0·8 g GOS/FOS (experimental group, n 102) or with maltodextrin as placebo (control group, n 104) were included in the per-protocol analysis. The frequency of atopic eczema in the experimental group was significantly reduced compared with the placebo group (9·8 % v. 23·1 %, RR 0·42 (95 % CI 0·2, 0·8)), number needed to treat eight (95 % CI 5, 31). In a subgroup of ninety-eight infants, the parents provided fresh stool samples for microbiological analysis using plating techniques; the faecal counts of bifidobacteria were significantly higher in the group fed the GOS/FOS formula compared to the placebo group. No significant difference was found for the lactobacilli count between groups. Follow-up of the present study showed that at 2 years the cumulative incidences of atopic dermatitis, recurrent wheezing and allergic urticaria were higher in the placebo group (27·9, 20·6 and 10·3 %, respectively) than in the intervention group (13·6, 7·6 and 1·5 %) (P < 0·05). This is the first observation that prebiotic effects are able to reduce the incidence of atopic diseases and that this effect persists beyond the intervention period. This assessment is based on a per protocol evaluation which aims at testing efficacy; due to the high drop-out rate (20 % at 6 months and 48 % at 2 years of age) and lacking intention-to-treat analysis, effectiveness for field practice needs to be confirmed(Reference Arslanoglu, Moro and Schmitt141) (see section ‘Prebiotic effects and mineral absorption’ of the present paper).
Conclusions
(1) Only two dietary non-digestible oligosaccharides fulfil the criteria for prebiotic classification. These are galactans and ITF. Only a limited number of RCT evaluating the efficacy and safety of in paediatric population are available. Some of these studies had methodological limitations.
(2) Typically, the studies could show efficacy, i.e. statistical effects based on per protocol analysis. However, they may need to be confirmed by effectiveness using intention-to-treat analysis.
(3) Supplementation with such ingredients has the potential to increase the total number of bifidobacteria in faeces and reduce some pathogens. It also can reduce stool pH, increase the concentrations of faecal SCFA like observed in breast-fed infants. The clinical meaning of these findings is still under debate.
(4) There is evidence from controlled trials that effects are able to reduce the incidence of atopic diseases and that this effect persists beyond the intervention period. Confirmation of these data for effectiveness is needed.
(5) A reduction in the risk of some infectious diseases is likely, but needs to be confirmed for effectiveness.
(6) The available data on prebiotic effects do not demonstrate adverse effects.
Prebiotic effects and gastro-intestinal disorders
Prebiotic effects and gastro-intestinal infections
The main authors for this section are Professor Guarner and Dr Respondek (IBS), Dr Whelan (IBD) and Professor Rowland (colon cancer and bacterial activities). In adults, the use of ingredients showing a prebiotic effect in the fight against infections has hardly been studied. A few studies, dealing with different infectious problems, have been reported.
One study dealing with traveller's diarrhoea reports that consumption of 10 g ITF/d for a 2-week pre-travel period continued during a 2-week travel period to high and medium risk destinations had no effect on the prevention of traveller's diarrhoea, although the sense of ‘well-being’ was improved(Reference Cummings, Christie and Cole142). Furthermore, a study of patients consuming 12 g ITF/d while taking broad-spectrum antibiotics for 7 d, followed by another 7 d of the same treatment reported no difference from the placebo group regarding diarrhoea incidence, Clostridium difficile infection and hospital stay, while the number of faecal bifidobacteria increased significantly(Reference Lewis, Burmeister and Cohen143). In contrast, continued consumption of 12 g ITF/d for 30 d after the cessation of C. difficile-associated diarrhoea reduced the relapse rate, while increasing bifidobacteria levels(Reference Lewis, Burmeister and Brazier144).
Overall, the number of studies on the efficacy of ingredients showing a prebiotic effect in the prevention of infectious diseases is limited. Some positive outcomes exist alongside studies reporting no-effects. Clearly, a rationale is present for the use of such ingredients. However, any direct effect of the studied ingredients on the immune system cannot be excluded and the measurement of the putative associated effect on the microbiota is not always included in these studies, hindering the formation of any conclusions on possible underlying mechanisms.
Prebiotic effects and irritable bowel syndrome
The irritable bowel syndrome (IBS) is a functional bowel disorder manifested by chronic, recurring abdominal pain or discomfort associated with disturbed bowel habit, in the absence of structural abnormalities likely to account for these symptoms(Reference Spiller, Aziz and Creed145). The symptomatic array may include abdominal pain, discomfort, distension, cramping, distress, bloating, excess flatulence and variable changes in frequency and form of stools. Such symptomatic episodes may be experienced by almost every individual, and in order to separate IBS from transient gut symptoms, experts have emphasised the chronic and relapsing nature of IBS and have proposed diagnostic criteria based on the recurrence rate of such symptoms(Reference Longstreth, Thompson and Chey146). IBS is one of the most common intestinal disorders both in industrialised and in developing countries, and it is known to generate significant health care costs(Reference Spiller, Aziz and Creed145).
A precise aetiology for IBS is not recognised. However, epidemiological studies have identified a series of pathogenetic factors, including genetic and early environmental conditioning, cognitive/emotional adaptation, altered response to stress and inflammatory post-infectious processes of the gut mucosa, etc.(Reference Spiller, Aziz and Creed145). It has been shown that IBS patients have abnormal reflexes and perception in response to gut stimuli(Reference Serra, Salvioli and Azpiroz147). In subsets of patients, the underlying defects appear to be altered GI motility, visceral hypersensitivity, small bowel bacterial overgrowth, excess gas production, abnormalities in the composition of the gut microbiota (Table 10) or combinations of them(Reference Spiller148).
Table 10 Comparison of faecal microbiota between irritable bowel syndrome (IBS) and healthy control subjects

Among the modifications of the gut microbiota, a decrease of bifidobacteria and more specifically Bifidobacterium catenulatum has been observed in IBS patients in comparison to healthy subjects(Reference Balsari, Ceccarelli and Dubini149–Reference Maukonen, Satokari and Mattö155).
Hypothetically, some of these disturbances may be corrected or counteracted by prebiotic effects. Indeed, compounds showing such effects are known to modulate the digestive microbiota and particularly to stimulate the growth of bifidobacteria especially when the initial level is low(Reference Rycroft, Jones and Gibson64). Furthermore, human studies with ITF or lactulose have shown that such prebiotics modulate gut transit(Reference Spiller148, Reference Nyman156), decrease putrefactive activity within the gut lumen(Reference de Preter, Vanhoutte and Huys157), prevent GI infections(Reference Cummings, Christie and Cole142, Reference Lewis, Burmeister and Brazier144) and mitigate inflammatory responses(Reference Lindsay, Whelan and Stagg111, Reference Furrie, Macfarlane and Kennedy158, Reference Casellas, Borruel and Torrejon159).
Indirect evidence for beneficial effects of ingredients showing a prebiotic effect on abdominal well-being was initially obtained in human trials addressing other primary end points. For instance, Cummings et al. (Reference Cummings, Christie and Cole142) tested the effectiveness of ITF in preventing diarrhoea in 244 healthy subjects, travelling to high and medium risk destinations for travellers' diarrhoea (see section ‘Prebiotic effects and gastro-intestinal infections’ of the present paper for discussion of the effects on risk of intestinal infections). This randomised, double-blind, placebo-controlled study showed that consumption of 10 g ITF daily gave a significantly better sense of ‘well-being’ during the holiday, as recorded in post-study questionnaires. Likewise, Casellas et al. (Reference Casellas, Borruel and Torrejon159) performed a prospective, randomised, double-blind, placebo-controlled trial to test the effect of ITF (12 g/d) in patients with active ulcerative colitis (UC). Interestingly, the study observed a significant decrease in abdominal symptoms with treatment but not with placebo, as assessed with the validated questionnaire of dyspepsia-related health scale(Reference Cook, Rabeneck and Campbell160).
Few studies have investigated the effect of ingredients showing a prebiotic effect in patients with IBS. The study by Olesen & Gudmand-Hoyer(Reference Olesen and Gudmand-Hoyer161) tested a high dose of finally 20 g ITF during 12 weeks. The authors hypothesised that IBS symptoms may be provoked by large quantities of fermentable carbohydrates in the colon. After 4–6 weeks on treatment, IBS symptoms worsened, as expected, in patients on 20 g ITF/d and improved in patients on placebo. However, continuous treatment for 12 weeks resulted in adaptation and there were no differences between groups: symptoms improved in 58 % of the ITF group and in 65 % of the placebo group, and symptoms worsened in 8 % of the ITF group and in 13 % of the placebo group. Large doses of any fermentable carbohydrates should not be recommended to IBS patients.
Hunter et al. (Reference Hunter, Tuffnell and Lee162) found no effect of 2 g ITF (three times daily) against placebo in a reduced group of IBS patients studied in a double-blind crossover trial. The Rome team of experts on functional bowel disorders do not recommend the use of a crossover design for IBS treatment trials as they have the potential disadvantages of carryover effects and unmasking the study product by differences in taste and palatability(Reference Irvine, Whitehead and Chey163). Dughera et al. (Reference Dughera, Elia and Navino164) reported a positive effect of a synbiotic (including short-chain ITF at 2·5 g/d) on clinical manifestations and intestinal function in patients with IBS. However, this was an open-label and uncontrolled study, and IBS studies with subjective outcomes are prone to study bias(Reference Spiller148).
To date, there are two published studies of adequate study design reporting the effects of an ingredient showing a prebiotic effect in IBS. The first study screened 2235 subjects and recruited and randomised 105 patients with IBS fulfilling Rome II criteria with minor intensity of symptoms as assessed by an initial questionnaire. Treatment with short-chain ITF at 5 g/d for 6 weeks reduced incidence and intensity of symptoms as compared to the placebo product. Prebiotic treatment also improved functional digestive disorders related quality of life(Reference Paineau, Payen and Panserieu165).
The second study randomised forty-four subjects according to Rome II criteria into three groups either receiving 7 g/d placebo, 3·5 g/d of ingredient showing a prebiotic effect and 3·5 g/placebo and 7 g/d of the tested ingredient for 6 weeks. The prebiotic treatment significantly improved flatulence, bloating and composite score of symptoms as well-subjective global assessment. It also increased the proportion of bifidobacteria in faecal samples(Reference Silk, Davis and Vulevic166).
In summary, the two available studies with up to date standard provided positive outcomes for both the ITF and GOS tested up to 7 g. Results with less positive outcomes used either higher or lower doses.
Recommendations
Ingredients showing a prebiotic effect are likely to play a role in the symptomatic control of IBS. Evidence accumulated so far in well-designed clinical studies is limited, but suggests possible benefits at moderate doses. Further studies with adequate methodology are warranted.
Key points
(1) The IBS is a functional bowel disorder manifested by chronic, recurring abdominal pain or discomfort in the absence of structural abnormalities.
(2) The symptomatic array includes abdominal distension, cramping, distress, bloating, excess flatulence and variable changes in frequency and form of stools. Such symptomatic episodes may be experienced by almost every individual.
(3) The underlying defects appear to be altered GI motility, visceral hypersensitivity, small bowel bacterial overgrowth, excess gas production and abnormalities in the composition of the gut microbiota or combinations of these.
(4) Ingredient showing a prebiotic effect may counteract these disturbances as they were shown to modulate gut transit, decrease putrefactive activity within the gut lumen, prevent GI infections and mitigate inflammatory responses.
(5) To date, there are only two published studies of adequate study design testing such ingredient in IBS. Both studies improved the subjects' symptoms.
Prebiotic effects and inflammatory bowel disease
IBD is a chronic relapsing and remitting disorder characterised by inflammation, ulceration and stricturing of the GI tract. UC and Crohn's disease (CD) are the two main types of IBD. In Europe, the incidence ranges from 1·5 to 20·3 cases per 100,000 person-years for UC and from 0·7 to 9·8 cases per 100,000 person-years for CD, meaning that up to 2·2 million people in Europe currently live with IBD(Reference Loftus167).
UC causes continuous mucosal inflammation that is restricted to the colon, whereas CD causes discontinuous transmural inflammation anywhere throughout the GI tract, although it most frequently affects the terminal ileum(Reference Travis, Stange and Lemann168). Symptoms common to both UC and CD include diarrhoea, faecal urgency and incontinence. Severe abdominal pain and rectal bleeding are common and complications such as fissuring and abscesses may occur. These symptoms can have a profound impact on patients, with evidence of impaired nutritional status(Reference Lucendo and De Rezende169) and quality of life(Reference Irvine170).
The primary treatment approach in IBD is usually drug therapy. Patients can be treated with a variety of drugs, including 5-ASAs (e.g. mesalazine), steroids (e.g. prednisolone) and immunosuppressants (e.g. azathioprine). In addition, patients with CD may also receive new biological drugs such as monoclonal antibodies (e.g. the anti-TNF-α antibody infliximab) when standard drug treatment fails(Reference Schwartz and Cohen171). Despite their general efficacy, such drugs can carry a significant burden. They are not only expensive but also side effects are common, with an incidence of 28 % for immunosuppressants, rising to 50 % for steroids(Reference Carter, Lobo and Travis172). In addition, approximately 30 % of patients with UC and 50 % of patients with CD will require surgery at some point in their life(Reference Carter, Lobo and Travis172). In the case of UC, a colectomy and formation of an ileo-anal pouch may be curative. However, following this procedure, a minority of patients will experience relapsing, remitting pouch inflammation, described as pouchitis.
Nutritional approaches to treating IBD have been investigated. In clinical trials, enteral nutrition has been shown to induce remission in 60–85 % of patients with CD, however, it remains less effective than steroids(Reference Zachos, Tondeur and Griffiths173) and patients report problems with palatability and abstinence from food(Reference Teahon, Pearson and Levi174). In view of these findings, safe and effective interventions that induce and maintain remission in IBD with a low incidence of side effects are urgently needed.
In order to identify potential therapeutic targets for IBD, examination of its pathogenesis is required. Although the precise mechanisms are not yet known, it appears that IBD results from a heightened mucosal immune response to the GI microbiota in genetically susceptible individuals.
The immunological processes underlying IBD involve alterations in the balance of proinflammatory and immuno-regulatory cytokines within the mucosal immune system. Much of the inflammation is mediated via cytokines released by activated Th1/Th17 lymphocytes. In addition, TNF (TNF)-α has been shown to play a key role, exerting its effects via stimulation of other proinflammatory cytokines such as IL (IL)-1, IL-6 and IFN-γ. Each of these proinflammatory cytokines have been shown to be elevated during active IBD(Reference Neuman175), and biological therapies such as anti-TNF-α-antibodies directly target this immunological cascade. Other proinflammatory cytokines include IL-12 and IL-18, both of which are involved in IFNγ production. In contrast, the immuno-regulatory response is mediated by cytokines such as IL-10, which down-regulates IFNγ production(Reference Lindsay and Hodgson176). Furthermore, some animal studies have indicated immuno-regulatory roles for IL-4 and transforming growth factor (TGF)-β in IBD(Reference Brown and Mayer177).
There is convincing evidence that the inflammation observed in IBD is driven by the GI microbiota. For example, it has been shown that animal models of IBD do not develop inflammation when reared in germ-free conditions, whereas they subsequently develop inflammation once transferred to non-sterile conditions or are artificially colonised with bacteria(Reference Sellon, Tonkonogy and Schultz178). Similar observations have been described in human subjects with IBD. In patients with colonic CD, formation of an ileostomy, which diverts the faecal stream away from the site of inflammation, results in disease remission in 65 % of patients, while reversal of this procedure results in disease relapse in 60 %, implying that the content of the faecal stream is in part responsible for driving inflammation(Reference Fasoli, Kettlewell and Mortensen179). Patients with active IBD also have elevated GI permeability, thereby increasing the exposure of the mucosal immune system to the resident microbiota(Reference Chichlowski and Hale180). An underlying pathogenic mechanism linking CD and the GI microbiota was realised when it was found that mutations in the caspase-activating recruitment domain 15 (CARD15) gene, involved in bacterial recognition, were found to result in a 38-fold increase in risk for CD(Reference Hugot, Chamaillard and Zouali181). Interestingly, this mutation does not result in a higher risk of UC and further genome wide association studies have identified numerous other mutations associated with increased risk of either UC or CD but that are unrelated to bacterial recognition or sensing(Reference Zhang, Massey and Tremelling182). Therefore, there are clearly genetic and environmental triggers related to the onset of IBD other than those involving the GI microbiota.
Despite the evidence that the GI microbiota is necessary to drive the inflammation in IBD, some bacteria may indeed protect the mucosa from such inflammation. Studies in both animals models and patients with IBD have shown that some bacteria decrease abnormal GI permeability(Reference Miyauchi, Morita and Tanabe183, Reference Garcia, De Lourdes De Abreu Ferrari and Oswaldo Da Gama184), thereby reducing exposure of the mucosal immune system to the GI microbiota. Meanwhile, some probiotics, in particular bifidobacteria, up-regulate immuno-regulatory IL-10 production by dendritic cells(Reference Hart, Lammers and Brigidi185, Reference Ng, Plamondon and Hart186), the production of which is therapeutic in animal models of IBD(Reference Lindsay and Hodgson176). In view of this, studies have shown some success of both antibiotics and probiotics in the management of IBD and these have been extensively reviewed elsewhere(Reference Sartor187, Reference Hedin, Whelan and Lindsay188).
Components of the GI microbiota therefore drive proinflammatory and/or immuno-regulatory cytokine production during IBD. Interestingly, numerous studies demonstrate alterations in the GI microbiota of patients. Such studies are varied, utilising a wide variety of microbiological techniques (e.g. traditional culture and molecular microbiology) in different samples (i.e. faeces, inflamed mucosa, non-inflamed mucosa). Comparisons have been made between UC and/or CD and/or healthy controls, and these vary as to whether patients were in relapse or remission. Consequently, studies of the GI microbiota in IBD are too varied to review in detail here. However, some conclusions can be drawn regarding the alterations in GI microbiota in IBD that suggest that ingredients showing a prebiotic effect may be of potential benefit in its treatment or maintenance.
In general, studies adopt two different approaches to investigating the microbiota in IBD. Some investigate differences in concentration, proportion or diversity of microbial communities (i.e. dysbiosis theory), whereas others investigate the presence or absence of selected species (i.e. single strain theory). For example, patients with inactive CD have been shown to have lower proportions of faecal bifidobacteria(Reference Seksik, Rigottier-Gois and Gramet189, Reference Sokol, Seksik and Furet190), whereas both patients with active UC or active CD have lower faecal bifidobacteria, C. coccoides and C. leptum compared with healthy controls(Reference Sokol, Seksik and Furet190). Lower concentrations of bifidobacteria(Reference Macfarlane, Furrie and Cummings191, Reference Mylonaki, Rayment and Rampton192) and higher concentrations of bacteroides(Reference Swidsinski, Ladhoff and Pernthaler193) have also been found in the mucosa of both patients with UC or CD. Meanwhile, another study has shown that some patients with CD or UC have lower numbers of mucosal Firmicutes and Bacteroidetes(Reference Frank, St Amand and Feldman194). Increased presence of E. coli has been demonstrated in patients with UC or CD(Reference Sokol, Lepage and Seksik195, Reference Martinez-Medina, Aldeguer and Gonzalez-Huix196) and more recently, lower concentrations of F. prausnitzii were found in the faeces of patients with CD or UC compared with controls(Reference Sokol, Seksik and Furet190). This is important as F. prausnitzii is immuno-regulatory and higher mucosal concentrations are associated with longer maintenance following surgically induced remission of CD(Reference Sokol, Pigneur and Watterlot197).
In view of the role of the certain components of the GI microbiota in driving intestinal inflammation, combined with the apparent dysbiosis in IBD, the use of ingredients showing a prebiotic effect as an approach to modifying the microbiota in order to induce or maintain remission in IBD has been investigated.
The prebiotic concept is defined as the selective stimulation of growth and/or activity of one or a limited number of microbial genera, species or strains in the gut microbiota that confers health benefits to the host. Ingredients showing a prebiotic effect have been shown to increase faecal and mucosal bifidobacteria in healthy subjects(Reference Kolida and Gibson198, Reference Langlands, Hopkins and Coleman199). This is relevant because bifidobacteria are present in lower concentrations in the faeces and mucosa of patients with IBD(Reference Sokol, Seksik and Furet190, Reference Mylonaki, Rayment and Rampton192), while in vitro experiments have shown that some species of bifidobacteria stimulate IL-10 production, potentially via interaction with TLR on lamina propria dendritic cells(Reference Hart, Lammers and Brigidi185). In addition, prebiotic ITF have recently been shown to increase concentrations of F. prausnitzii in healthy subjects(Reference Ramirez-Farias, Slezak and Fuller200), although this has not yet been confirmed in patients with IBD. Furthermore, SCFA, produced through the fermentation of such ingredients, modulate inflammation, with cell-culture studies showing that butyrate inhibits pro-inflammatory IL-2 and IFNγ production and acetate and propionate increases immuno-regulatory IL-10 production(Reference Cavaglieri, Nishiyama and Fernandes95). In addition, mucin production is stimulated by both propionate and butyrate(Reference Burger-van, Vincent and Puiman201). Mucins (such as MUC2) are required for the maintenance of the mucous layer that enables epithelial protection and which may be lower in patients with IBD(Reference Pullan, Thomas and Rhodes202).
Numerous experiments have been conducted to investigate the impact of these ingredients on chronic intestinal inflammation in animal models of IBD, and these have been reviewed elsewhere(Reference Leenen and Dieleman203). However, at the current time, their use among patients with IBD remains relatively low(Reference Hedin, Graczer and Sanderson204). However, over the last decade, there has been an increase in the number of clinical trials investigating their use in inducing or maintaining remission in IBD (Table 11).
Table 11 Clinical trials on the prebiotic effect in inflammatory bowel disease
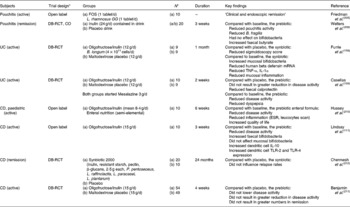
FOS, fructo-oligosaccharides; DB-RCT, double-blind randomised controlled trial; CO, crossover; UC, ulcerative colitis; CD, Crohn's disease; ESR, erythrocyte sedimentation rate; TLR-2, toll-like receptor 2.
* Numbers recruited to each group.
Prebiotic effects in pouchitis
Two studies have been identified that investigate the use of ingredients showing a prebiotic effect in patients with pouchitis. The first, published in abstract form only, involved ten patients with active pouchitis who were treated with a synbiotic combination of Lactobacillus rhamnosus GG and ITF in an open label study in whom ‘all patients experienced complete clinical and endoscopic remission’(Reference Friedman and George205). Unfortunately, further details of the outcomes are limited and the cause of any benefit, be it a placebo effect, the probiotic, a prebiotic effect or a combination, is unclear. In a larger, controlled study, twenty patients with inactive pouchitis were randomised to consume 24 g/d ITF or placebo for 3 weeks in a crossover study(Reference Welters, Heineman and Thunnissen206). There was a significant reduction in pouchitis disease activity index during the ITF intervention, despite nobody having active disease. In addition, there was a reduction in faecal Bacteroides fragilis and an increase in butyrate. Interestingly, bifidobacteria remained unchanged, perhaps due to the absence of a colon preventing the complete fermentation and prebiotic effects of the ITF to be realised. Clearly, larger parallel controlled trials in both active and inactive pouchitis are warranted.
Prebiotic effects in ulcerative colitis
Two trials have used ingredients showing a prebiotic effect to investigate their efficacy in the management of UC. The first was a pilot study of eighteen patients with active UC, who were randomised to receive either a synbiotic (6 g/d of ITF and Bifidobacterium longum) or a placebo. Only fourteen completed the study (eight intervention and six control) and there was no difference in clinical scores between the intervention and control groups, but there was a lower degree of inflammation(Reference Furrie, Macfarlane and Kennedy158). In addition, there was an increase in mucosal bifidobacteria and decrease in TNF-α, IL-1α and antimicrobial human β-defensin peptides in the synbiotic group. Although this data suggest promising effects, the use of a synbiotic combination makes it difficult to ascertain the specific effects of the prebiotic on clinical outcome.
In another pilot study in active UC, nineteen patients were randomised to receive either an ingredient showing a prebiotic effect (12 g/d of ITF) or a placebo, in conjunction with 3 g/d mesalazine for 2 weeks(Reference Casellas, Borruel and Torrejon159). Only fifteen patients completed the study (seven intervention and eight control) and although there was a reduction in disease activity, this occurred in both groups, potentially due to them both starting concomitant drug therapy. However, compared with the placebo, the intervention group had significantly lower concentrations of the inflammatory marker faecal calprotectin. This trial provides the first indicator that a prebiotic alone may be of benefit in treating active UC. Its major limitations include low numbers in each group, that increase the chance of type 2 errors, and a short treatment duration that may be insufficient to allow a prebiotic effect to translate into a clinical effect(Reference Casellas, Borruel and Torrejon159).
In addition to these, a number of studies in UC have investigated the use of compounds that although described as prebiotic are not generally considered to be so. Trials of these fibre compounds have therefore not been included in Table 11. For example, a series of studies have shown that germinated barley foodstuff increases remission rates when used to treat active UC(Reference Kanauchi, Mitsuyama and Homma207) and results in longer remission when used in maintenance of UC(Reference Hanai, Kanauchi and Mitsuyama208). More recently a trial of psyllium or the probiotic B. longum did not result in a significant improvement in quality of life or reduction in serum C-reactive protein, whereas when used together they did(Reference Fujimori, Gudis and Mitsui209).
There remains little data on the clinical, microbiological and immunological effects of prebiotics specifically in maintaining remission in UC.
Prebiotic effects in Crohn's disease
In a small, open-label study a semi-elemental enteral formula containing ingredients showing a prebiotic effect (4 g/l of ITF) was fed via nasogastric tube as a sole source of nutrition for 6 weeks to ten children with active CD(Reference Hussey, Issenman and Persad210). There was a reduction in disease activity alongside improvements in markers of inflammation including reduced erythrocyte sedimentation rate and improved white cell scans. In light of the evidence for the efficacy of enteral nutrition in inducing remission in active CD(Reference Zachos, Tondeur and Griffiths173), the present study design does not allow the clinical consequences of the prebiotic effect to be separated from those of the enteral nutrition.
A small open label study of ingredients ITF (15 g/d) in patients with active CD demonstrated a significant reduction in disease activity after 3 weeks, with four out of ten patients entering disease remission(Reference Lindsay, Whelan and Stagg111). In addition, faecal, but not mucosal, bifidobacteria increased and there was an increase in dendritic cell IL-10 production together with TLR-2 and TLR-4 expression. Clearly caution is required in interpreting and applying the results of this small uncontrolled trial.
The same group have recently presented the clinical data from a large double-blind, randomised, placebo-controlled trial of ITF (15 g/d) in 103 patients with active CD(Reference Benjamin, Hedin and Koutsoumpas211). Analysed on an intention-to-treat basis, there were no significant differences in disease activity or the numbers entering disease remission between groups. However, as the data have only been presented as a conference abstract, there is currently limited clinical data and no microbiological and immunological data published.
Finally, one study has investigated the effect of ingredients showing a prebiotic effect on preventing relapse in thirty patients following surgically induced remission of CD. The present study supplemented a synbiotic (Pediacoccus pentoseceus, Lactobacillus raffinolactis, L. paracasei susp paracasei 19, Lactobacillus plantarum, 2·5 g β-glucans, 2·5 g ITF, 2·5 g pectin, 2·5 g resistant starch) or a placebo for 24 months(Reference Chermesh, Tamir and Reshef212). In view of the long follow-up period, only nine patients completed the study (nine intervention and two control) and there were no differences in relapse rates between groups. It is noteworthy that the amount of the used ingredient contained within the synbiotic was relatively low.
Limitations of existing studies on prebiotic effects in inflammatory bowel disease
Of the identified clinical trials of ingredients showing a prebiotic effect in IBD, numerous limitations in their reporting and trial design have been highlighted. First, a number have only been published as conference abstracts(Reference Friedman and George205, Reference Hussey, Issenman and Persad210, Reference Benjamin, Hedin and Koutsoumpas211), therefore impeding detailed data extraction. Many of the studies used different compounds, some with unconfirmed prebiotic properties, and in different doses. In addition, many of the studies use a synbiotic combination, making it unclear whether the probiotic, the prebiotic or the combination is effective. The majority of the studies have poor study design, with numerous small pilot studies, some of which do not have control groups. Where control groups are used they do not always receive a placebo, making subjective outcomes such as patient reports of disease activity or quality of life difficult to interpret. This is important in view of the high placebo rates reported in clinical trials of IBD(Reference Su, Lewis and Goldberg213, Reference Su, Lichtenstein and Krok214). Furthermore, of the trials in CD none have analysed the influence of disease location, which may be important as ingredients showing a prebiotic effect may have different efficacy in colonic and ileal disease, due to the site of fermentation and augmentation of bacterial growth.
Key points
IBD results from a heightened mucosal immune response to the GI microbiota in genetically susceptible individuals.
Patients with IBD have a GI dysbiosis characterised by, among other things, lower concentrations of luminal and mucosal bifidobacteria, suggesting potential for prebiotic intervention. Prebiotic effects have potential benefits in IBD by increasing luminal and mucosal bifidobacteria and SCFA concentrations and stimulating immuno-regulatory cytokine production.
Numerous small pilot studies have been conducted in pouchitis, UC and CD indicating potential benefit in treating active disease.
Although some larger trials have been conducted, they are generally limited in study design, interpretation and analysis, therefore definitive conclusions regarding the clinical efficacy of the prebiotic effect in IBD are not yet possible. One large RCT has demonstrated no clinical benefit of treating active CD with ingredients showing a prebiotic effect.
So far, results are substance and study specific, but do not warrant a conclusion for prebiotic effects in general.
None of the trials conducted thus far have reported concerns regarding the safety of ingredients showing a prebiotic effect in patients with IBD, and so their use at the doses used would appear safe.
Recommendations
Further large, multi-centre randomised, double-blind, placebo-controlled trials of ingredients showing a prebiotic effect in IBD are required. There is a particular lack of research on maintenance of remission of IBD and for the treatment colonic IBD (either UC or colonic CD).
Inter-disciplinary research is required that addresses clinical, as well as mechanistic, outcomes that are validated and relevant to this patient population.
In vivo and in vitro research is also required to further understand the mechanisms by which ingredients showing a prebiotic effect may achieve their potential benefit.
Health care professionals should keep informed of the latest evidence relating prebiotic effect in IBD. Not only is this an emerging area of research, with clinical trials currently underway, but it is also an area of interest to patients.
Prebiotic effects and colon cancer
Colon carcinogenesis – the role of diet and gut microbiota
Evidence suggests that diet plays an important role in the aetiology of colorectal cancer, However, identifying conclusively which constituents (e.g. vegetables, meat, fibre, fat and micronutrients) exert an effect on risk has been more problematic due to inconsistent data. The 2007 World Cancer Research Fund report(215) concluded that the epidemiological evidence was convincing or probable for associations between overweight and obesity (in particular waist circumference), processed meat, alcohol and increased risk of colorectal cancer. Fibre, garlic, milk and Ca are associated with decreased risk. There are no published epidemiological studies on ingredients showing a prebiotic effect and cancer risk.
Evidence from a wide range of sources supports the view that the colonic microbiota is involved in the aetiology of cancer(Reference Rowland216) and that bacterial metabolism of unabsorbed dietary residues and endogenous secretions is the origin of many of the genotoxic and tumour-promoting agents found in faeces(Reference Hughes, Rowland, Fuller and Perdigon217).
Prebiotic effects and colorectal cancer
It follows from the above that modification of the gut microbiota may interfere with the process of carcinogenesis, and this opens up the possibility for dietary modification of colon cancer risk. Prebiotic modulation of the microbiota by increasing numbers of lactobacilli and/or bifidobacteria in the colon has been a particular focus of attention in this regard. Evidence that such an effect can influence carcinogenesis is derived from a variety of sources:
(1) Effects on bacterial enzyme activities.
(2) Antigenotoxic effects in vivo.
(3) Effects on pre-cancerous lesions in laboratory animals.
(4) Effects on tumour incidence in laboratory animals.
(5) Epidemiological and experimental studies in human subjects.
Prebiotic protective effects and bacterial activities
Prebiotic effects and secondary bacterial enzyme activities
The ability of the colonic microbiota to generate a wide variety of mutagens, carcinogens and tumour promoters including N-nitrosocompounds, secondary bile acids, ammonia, phenols and cresols from dietary and endogenously produced precursors is well documented(Reference Rowland216, Reference Rowland, Macfarlane and Gibson218). In addition, the bacterial enzyme β-glucuronidase is involved in the release in the colon from their conjugated form of a number of dietary carcinogens, including polycyclic aromatic hydrocarbons.
Ingredients showing a prebiotic effect should not stimulate bacteria capable for such metabolism. During in vivo experiments, this should result in an overall decrease in toxic substances.
In general, species of Bifidobacterium and Lactobacillus have low activities of enzymes involved in carcinogen formation and metabolism by comparison to other major anaerobes in the gut such as bacteroides, eubacteria and clostridia(Reference Saito, Takano and Rowland219). This suggests that increasing the proportion of these two lactic acid bacteria in the gut could modify, beneficially, the levels of xenobiotic-metabolising enzymes. It may lead to decrease in certain bacterial enzymes purported to be involved in the synthesis or activation of carcinogens, genotoxins and tumour promoters. Such manipulations have been suggested to be responsible for decreased levels or preneoplastic lesions or tumours in animal models(Reference Reddy and Rivenson220, Reference Rowland, Rumney and Coutts221) and suggest a reduction in the damaging load.
In general, studies in laboratory animals have shown that ITF and galactans decrease caecal enzyme activities(Reference Hidaka, Eida and Takizawa222, Reference Rowland and Tanaka223). However, human studies have yielded inconsistent or negative results on such enzyme activities or on production of toxic bacterial metabolites such as ammonia and phenols(Reference Hayakawa, Mizutani and Wada65, Reference Tanaka, Takayama and Morotomi224, Reference Gostner, Blaut and Schaffer225).
Prebiotic and synbiotic effects on pre-cancerous lesions in laboratory animals
Aberrant crypts (AC) are putative pre-neoplastic lesions seen in the colon of carcinogen-treated rodents. In many cases, a focus of two or more crypts is seen and is termed an aberrant crypt focus (ACF). AC are induced in colonic mucosa of rats and mice by treatment with various colon carcinogens such as azoxymethane (AOM) and dimethylhydrazine (DMH)(Reference Pretlow, O'Riordan and Somich226).
Ingredients showing a prebiotic effect alone appear to give inconsistent results on carcinogen-induced ACF which may be partly a consequence of differences in carcinogen and treatment regimes used. For example, Rao et al. (Reference Rao, Chou and Simi227) reported that ITF (10 % in diet) had no significant effect on total ACF in colon, or their multiplicity, in F344 rats, although curiously a significant decrease in ACF/cm2 of colon was reported. A study by Gallaher et al. (Reference Gallaher, Stallings and Blessing228) on Bifidobacterium spp and FOS (2 % in diet) gave inconsistent results with only one out of three experiments showing a decrease in DMH-induced ACF. In contrast, Verghese et al. (Reference Verghese, Rao and Chawan229) reported a dose-dependent decrease in the incidence of ACF and total crypts (P < 0·01) after ITF supplementation (0, 2·5, 5 and 10 g/100 g diets) in AOM-challenged rats.
The effects of prebiotics on ACF may be dependent on the chain length of the ITF, since a number of studies report more potent inhibition by longer than by shorter chains(Reference Reddy, Hamid and Rao230–Reference Poulsen, Molck and Jacobsen232). For example, Buddington et al. (Reference Buddington, Donahoo and Buddington231) reported that inulin (10 % in diet), but not oligofructose fed mice, had significantly lower ACF numbers than the controls.
Some studies have found that ITF have differential effects on ACF and tumours. For example, Jacobsen et al. (Reference Jacobsen, Poulsen and Dragsted233) reported that oligofructose or long-chain inulin (15 % in diet) increased the number of ACF but significantly reduced the tumour incidence. A study by Caderni et al. (Reference Caderni, Femia and Giannini234) showed similar results when rats were fed the synbiotic-containing ITF alongside Lactobacillus GG, L. delbrueckii subsp. Rhamnosus and Bifidobacterium lactis Bb12. Supplementation caused increased ACF multiplicity after 16 weeks, however, significantly reduced tumour incidence following 32 weeks in AOM-challenged rats.
There are limited studies on ingredients showing a prebiotic effect other than ITF in this area. Challa et al. (Reference Challa, Rao and Chawan235) demonstrated a small reduction (22 %) in total ACF in AOM-treated F344 rats when the synthetic, non-digestible disaccharide lactulose was incorporated in the diet at 2 %. Hsu et al. (Reference Hsu, Liao and Chung236) compared the influence ITF (60 g/kg) and xylo-oligosaccharides supplementation on DMH-induced AC in ratsreporting a decrease in the mean number of multicrypt clusters of AC by 56 and 81 %, respectively (P < 0·05). Wijnands et al. (Reference Wijnands, Schoterman and Bruijntjes237) compared AOM-induced ACF in F344 rats fed diets containing low or high GOS (5 v. 20 % w/w of a GOS syrup comprising 38 % GOS). There were no significant differences between the dietary groups in total ACF after 7 or 13 weeks of treatment although there was a significant decrease in ACF multiplicity in the high GOS fed group (4·4 v. 3·07; P < 0·5).
Both Challa et al. (Reference Challa, Rao and Chawan235) and Rowland et al. (Reference Rowland, Rumney and Coutts221) studied the effect of combined treatment of probiotic and prebiotic on ACF numbers. The combination of B. longum and lactulose resulted in a 48 % inhibition of colonic ACF, which was significantly greater than that achieved by either B. longum or lactulose alone(Reference Challa, Rao and Chawan235). Similarly Rowland et al. (Reference Rowland, Rumney and Coutts221) reported a decrease in total ACF of 74 % in rats given B. longum+ITF (by comparison to 29 and 21 % reduction achieved by B. longum or ITF alone). Importantly, the combined administration of probiotic and prebiotic reduced large ACF by 59 %, whereas the individual treatments had no effect. Nakanishi et al. (Reference Nakanishi, Kataoka and Kuwahara238) showed that supplementation with C. butyricum (CB) in AOM-challenged rats had no significant effect on ACF occurrence. However, CB supplemented alongside high amylose maize starch (a poorly digestible carbohydrate) decreased the number of ACF significantly (P < 0·05) indicating a degree of synbiotic activity.
Prebiotic effects and colon tumour incidence in laboratory animals
There are fewer reports on prebiotic and synbiotics than on probiotics in terms of tumour incidence but overall the studies indicate protective effects. Jacobsen et al. (Reference Jacobsen, Poulsen and Dragsted233) compared the incidence of tumours in AOM-challenged rats following consumption of ITF (15 % diet w/w). Significantly less rats developed colon tumours in the treated group (P < 0·05) compared to the control diet. The total number of tumours developed per rat was significantly reduced following both oligofructose (P < 0·01) and inulin (P < 0·05) supplementation. However, supplementation had no effect on the malignancy of the tumours. Wijnands et al. (Reference Wijnands, Appel and Hollanders239) compared the effect of cellulose and GOS syrup on induction of DMH-induced colorectal tumours in Wistar rats consuming basal diets containing low-, medium- or high-fat content. The cellulose diets contained 4·5–5·2 % w/w (low cellulose) or 22·6–24·5 % (high cellulose) and the GOS syrup diets 8·3–9·5 % (low GOS) or 26·3–28·7 % (high GOS). The GOS syrup used comprises 38 % GOS with additional lactose, glucose and galactose, thus the high GOS diets contained about 10·5 % dry weight GOS. The cellulose content of the diet had no effect on total tumours, but high cellulose increased adenomas and significantly decreased carcinomas. There were no significant effects of high GOS diets on tumour incidence. Multiplicity of tumours (i.e. number per tumour-bearing animal) both adenoma and carcinoma, was significantly decreased in the high-GOS-fed group.
Femia et al. (Reference Femia, Luceri and Dolara240) investigated the protective effects of prebiotic (ITF), probiotic (B. lactis Bb12 and L. rhamnosus GG, (5 × 108 CFU/g diet) or synbiotic combination of the two against AOM-induced colon tumours in rats. Prebiotic-fed groups (prebiotic and synbiotic groups) resulted in lower adenoma (P < 0·001) and adenocarcinoma (P < 0·05) incidence than in the rats not given prebiotic (probiotic and control). Interestingly, in the groups treated with probiotics (probiotic and synbiotic groups) the proportion of cancers relative to the total number of tumours was significantly lower (P = 0·04) (nine cancers out of eighty-four tumours (11 %)) than in the control and prebiotic groups (nineteen cancers out of eighty-three tumours (23 %)), suggesting a protective effect of probiotics, but not ingredients showing a prebiotic effect, on development of malignant tumours.
In the transgenic Min mice model, the mice develop spontaneous adenomas throughout the small intestine and colon within a few weeks. Results from studies on ITF in this model have been conflicting, with both inhibitory and stimulatory effects on tumours reported. In one study, Min mice were fed various diets containing wheat bran, resistant starch or oligofructose (5·8 % in diet) for 6 weeks. Tumour numbers remained unchanged from the control (low (2 %) fibre diet) in the mice fed either wheat bran or resistant starch, but a significant reduction in colon tumours was observed in rats receiving the diet supplemented with oligofructose. Furthermore, four out of the ten oligofructose fed animals were totally free of colon tumours(Reference Pierre, Perrin and Champ241). These results contrast with those of Mutanen et al. (Reference Mutanen, Pajari and Oikarinen242) using the same model. In the first of their studies, Min mice fed a purified high-fat diet (40 % energy) with 2·5 % ITF showed NS increases in adenomas in the small and large intestines compared with the control animals fed the high-fat, fibre-free diet alone. A subsequent study(Reference Pajari, Rajakangas and Paivarinta243) using a higher ITF dose (10 %) confirmed these results with increases, again NS, being seen in the number of adenomas in the small intestine and colon and significant increases in tumours in the distal small intestine after 9 weeks of treatment. Interestingly, although the adenoma size in the small intestine was significantly increased in the inulin-fed mice, in the colon the size was reduced from 3·72 to 2·54 mm (non significant). In some articles, it has been suggested that the reasons for the discrepancies in the Min mouse studies are due to major differences in the basal diet fed: high-fat, high glucose diet in the Mutanen studies and high-starch diet in the studies of Pierre et al. (Reference Roberfroid78, Reference Pool-Zobel244).
Taper & Roberfroid(Reference Taper and Roberfroid245) investigated the effects in mice of ITF or pectin (15 % in the diet) on the growth of intramuscularly transplanted mouse tumours, belonging to two tumour lines – TLT (a mammary tumour) and EMT6 (a liver tumour). The growth of both tumour lines was significantly inhibited by supplementing the diet with non-digestible carbohydrates. In subsequent studies, the same authors demonstrated that ITF (15 % in diet) reduced the incidence of mammary tumours induced in Sprague–Dawley rats by methylnitrosourea and decreased the incidence of lung metastases of a malignant tumour implanted intramuscularly in mice(Reference Taper and Roberfroid246).
Prebiotic effects in human intervention studies
For human intervention trials, cancer is an impractical end point in terms of numbers of subjects, cost, study duration and ethical considerations. An alternative strategy employed in recent studies is to use early or intermediate biomarkers of cancer such as DNA damage and cell proliferation in colonic mucosa and genotoxic activity of faecal extracts (‘faecal water’)(Reference Gill and Rowland247).
In a larger scale, randomised, double-blind, placebocontrolled trial, patients with resected polyps (n 37) or colon cancer (n 43) were given a synbiotic food supplement composed of ITF and the probiotics L. rhamnosus GG and B. lactis Bb12 for 12 weeks(Reference Rafter, Bennett and Caderni248). The effect of synbiotic consumption on a battery of intermediate biomarkers for colon cancer was examined. The intervention significantly reduced colorectal proliferation as assessed by in vitro (3H) thymidine incorporation and autoradiography in colorectal biopsy samples. Given the correlation between colorectal proliferativeactivity and colon cancer risk, these results suggest that synbiotics might be beneficial for patients with an increased risk of colon cancer. In addition, in the polyp patients, the synbiotic intervention was associated with a significant improvement in barrier function as assessed by trans-epithelial resistance of Caco-2 cell monolayers after exposure to faecal water samples. This anti-promotion effect may reflect changes in the balance of SCFA and secondary bile acids (deoxycholic acid and lithocholic acid) in the samples because these gut microbial metabolites have trans-epithelial resistance, beneficially and adversely respectively, in this system. Genotoxicity assays of colonic biopsies and faecal water indicated a decreased exposure to genotoxins in the polyp patients at the end of the intervention period.
Thus, several colorectal cancer biomarkers were altered favourably by the intervention and the results show consistency with animal studies conducted in parallel(Reference Femia, Luceri and Dolara240).
Also of interest was the observation that the polyp patients and cancer patients appeared to respond differently to the synbiotic, as evidenced by the different effects observed on each biomarker. This may have been due to the fact that the intestinal microbiota was more refractory to changes induced by the synbiotic in the cancer patients than in the polyp patients.
Mechanisms of anticarcinogenicity and antigenotoxicity
Prebiotic effects and in vivo prevention of genotoxicity
More direct evidence for protective properties of probiotics and ingredients showing a prebiotic effect has been obtained by assessing the ability to prevent DNA damage and mutations (which are considered to be early events in the process of carcinogenesis) in cell cultures or in animals.
Using the technique of single-cell microgel electrophoresis (Comet assay), the prebiotic effect of lactulose on DNA damage in the colonic mucosa has been evaluated. Rats that were fed a diet containing 3 % lactulose and given DMH, exhibited less DNA damage in colon cells than similarly treated animals fed a sucrose diet. In the latter animals, the percentage of cells with severe DNA damage comprises 33 % of the total compared with only 12·6 % in the lactulose-fed rats(Reference Rowland, Bearne and Fischer249).
Klinder et al. (Reference Klinder, Forster and Caderni250) also showed that the prebiotic effect of ITF and probiotic supplementation (8 months) caused a reduction in the genotoxicity of faecal and caecal samples obtained from AOM-treated rats.
Rafter et al. (Reference Rafter, Bennett and Caderni248) investigated the influence of 12 weeks synbiotic supplementation (L. rhamnosus GG (LGG)+B. lactis Bb12+ITFmix) on selected cancer biomarkers in patients with resected colonic polyps or cancer. Synbiotic supplementation resulted in significant reductions in DNA damage in the colonic mucosa of polyp patients. The results provide evidence that both supplementation of lactic acid bacteria and prebiotic effects may be protective against the early stages of colon cancer.
Another important aspect to be considered in relation to the anti-toxic potential associated with a prebiotic effect is the formation of reducing equivalents, such as glutathione. Food-borne carcinogens such as heterocyclic amines and polycyclic aromatic hydrocarbons are often conjugated with glutathione and thus inactivated. The enzyme involved, glutathione transferase, is found in the liver and in other tissues including the gut. Challa et al. (Reference Challa, Rao and Chawan235) showed in a study of the effect of a synbiotic (B. longum and lactulose) on AOM-induced AC foci (ACF) in the rat colon that glutathione transferase in the colonic mucosa was inversely related to the ACF numbers and higher with the synbiotic intervention Such an effect would be effective against a wide range of oxidative damage.
Effects on bacterial enzymes and metabolite production
As described in section ‘Microbiota of the gastro-intestinal tract’ of the present paper, the increase in concentration of lactic acid bacteria in the gut as a consequence of consumption of ingredients showing a prebiotic effect leads to decreases in certain bacterial enzymes purported to be involved in synthesis or activation of carcinogens, genotoxins and tumour promoters. This would appear to be due to the low-specific activity of these enzymes in lactic acid bacteria(Reference Saito, Takano and Rowland219). Such changes in enzyme activity or metabolite concentration have been suggested to be responsible for the decreased level of preneoplastic lesions or tumours seen in carcinogen-treated rats given probiotics and prebiotics(Reference Reddy and Rivenson220, Reference Rowland, Rumney and Coutts221). Although a causal link has not been demonstrated, this remains a plausible hypothesis.
Production of anti-cancer metabolites
Luminal SCFA, in particular butyrate, are potential anti-carcinogenic agents within the gut. Butyrate is the preferred energy source of colonocytes and has been implicated in the control of the machinery regulating apoptosis and cellular differentiation. Perrin et al. (Reference Perrin, Pierre and Patry251) studied the effect of different forms of dietary fibre, a starch-free wheat bran, a type three-resistant starch and ITF on the prevention of ACF in rats. Their hypothesis was that only fibres capable of releasing butyrate in vitro would be capable of preventing colon cancer. The resistant starch diet and the ITF diet both produced large quantities of butyrate and inhibited ACF formation, in contrast to the wheat bran diet that neither generated large amounts of butyrate nor protected against ACF formation.
Stimulation of protective enzymes
Many of the food-borne carcinogens such as heterocyclic amines and polycyclic aromatic hydrocarbons are known to be conjugated to glutathione, which appears to result in inactivation. The enzyme involved, glutathione transferase, is found in the liver and in other tissues including the gut. Challa et al. (Reference Challa, Rao and Chawan235) investigated the effect of B. longum and lactulose on AOM-induced ACF in the colon and showed that the activity of glutathione transferase in the colonic mucosa was inversely related to the ACF numbers. Such a mechanism of protection would be effective against a wide range of dietary carcinogens.
Apoptotic effects
The control of gene expression, cell growth, proliferation and cell death in multi-cellular organisms is dependent upon the complex array of signals received and transmitted by individual cells. Apoptosis or programmed cell death is one of the primary mechanisms by which multi-cellular organisms control normal development and prevent aberrant cell growth. Up-regulation of apoptosis has received some attention recently as a potential mechanism of action of probiotics and ingredients showing a prebiotic effect.
Hughes & Rowland(Reference Hughes and Rowland252) fed three groups of rats with one of the three diets: basal, basal with oligofructose (5 % w/w) or basal with long-chain inulin (5 % w/w), for 3 weeks. All the animals were then dosed with 1,2-DMH and sacrified 24 h later. The mean number of apoptotic cells per crypt was significantly higher in the colon of rats fed oligofructose (P = 0·049) and long-chain inulin (P = 0·017) as compared with those fed the basal diet alone. This suggests that such ingredients exert protective effects at an early stage in the onset of cancer, as the supplements were effective soon after the carcinogen insult. Comparison of the apoptotic indices between the two oligosaccharide diets showed no significant difference even though the mean apoptotic index was higher in animals fed long-chain inulin.
Effects on tight junctions
Other studies have looked at cellular and physiological events associated with tumour promotion in the colon. For example, one feature of colonic tumour promotion is a decrease the in epithelial barrier integrity.
Commane et al. (Reference Commane, Shortt and Silvi253) showed using an in vitro model of tight junction integrity (trans-epithelial resistance) that metabolic products (probably SCFA) derived from probiotics and ingredients showing a prebiotic effect fermentations were capable of improving tight junction integrity, suggesting that synbiotics may have anti-tumour-promoting activity.
Summary and conclusion
(1) Data from animal models as well as preliminary evidence in human study suggest reduction in the risk of colon cancer development associated with the prebiotic effects.
(2) Data from animal models, with endpoints such as DNA damage, AC foci and tumours in the colon, suggest that reduction in the risk of colon cancer development is associated with prebiotic effects.
(3) Limited animal studies also indicate that combinations of prebiotics and probiotics may be more effective than either agent alone.
(4) A prebiotics and probiotics study in human subjects using putative biomarkers of cancer risk showed improvements in some, including a reduction in DNA damage and cell proliferation in colon biopsies. Further studies are needed.
(5) A number of potential mechanisms for reduction in cancer risk by prebiotic effect, including changes in gut bacterial enzyme activities, up-regulation of apoptosis and induction of protective enzymes have been explored in animal models, but currently evidence for such effects in human subjects is lacking.
Prebiotic effects and mineral absorption
The main authors of this section are Dr Coxam, Dr Davicco, Dr Léotoing and Dr Wittrant.
Accumulating knowledge prompted the scientific community to consider compounds showing prebiotic effects as a source for putative innovative dietary health intervention for the improvement of mineral retention. This particular effect of ingredients showing a prebiotic effect is indeed especially challenging because, among the bone builders, Ca is critical in achieving optimal peak bone mass and modulating the rate of bone loss associated with ageing, and is the most likely to be inadequate in terms of dietary intakes. Consequently, this specific property of prebiotics has been investigated extensively because if the mineral is inadequate during growth, the full genetic programme for skeletal mass acquisition cannot be achieved. Then, if Ca intake is not enough to offset obligatory losses, acquired skeletal mass cannot be maintained, leading to osteoporosis, a major public health problem.
Moreover, biological properties of ingredients showing a prebiotic effect could extend far beyond, with potential improvement of other minerals bioavailability, including Mg, Fe or Zn.
Rationale behind the prebiotic effects on mineral absorption
Calcium
The most compelling data have demonstrated that ingredients showing a prebiotic effect lead to increased Ca absorption. As such ingredients are resistant to hydrolysis by small intestinal digestive enzymes, they reach the colon virtually intact, where they are selectively fermented by the microbiota(Reference Roberfroid254, Reference Roberfroid255). This colonic fermentation produces SCFA and other organic acids that contribute to lower luminal pH in the large intestine which, in turn, elicits a modification of Ca speciation and hence solubility in the luminal phase so that its passive diffusion is improved(Reference Remesy, Levrat and Gamet256–Reference Lopez, Coudray and Levrat-Verny258). SCFA are also likely to contribute directly to the enhancement of Ca absorption via a cation exchange mechanism (increased exchange of cellular H+ for luminal Ca2+)(Reference Lutz and Scharrer259).
Further, these ingredients may also modulate transcellular active Ca transport by increasing calbindin D9K expression in the cecum and colorectum (the intracellular carrier protein involved in the translocation of Ca to the basolateral membrane of mucosal epithelial cells)(Reference Ohta, Motohashi and Sakai260, Reference Takasaki, Inaba and Ohta261).
Another way to contribute to the enhanced mineral absorption is the trophic effect of prebiotics on the gut (cell growth and functional enhancement of the absorptive area)(Reference Raschka and Daniel262). It has been suggested that this is mediated by an increased production of butyrate and/or certain polyamines(Reference Roberfroid254). Remesy et al. (Reference Remesy, Levrat and Gamet256) have shown that inulin is able to stimulate ornithine decarboxylase, the rate-limiting enzyme for polyamine synthesis. Nevertheless, Scholz-Ahrens & Schrezenmeier(Reference Scholz-Ahrens and Schrezenmeir263) failed to show that polyamines mediate this effect.
In summary, ingredients showing a prebiotic effect help to increase Ca bioavailability by extending the site of mineral absorption (through the tight junctions between mucosal cells in the small intestine) towards the large intestine.
Other minerals
With regard to Mg, most of the potential of ingredients showing a prebiotic effect on its absorption are similar to those described for Ca. They include increased Mg solubility and absorption due to reduced colonic pH(Reference Heijnen, Brink and Lemmens264). Nevertheless, significant effects on Mg retention have been demonstrated in dogs, despite the lack of any change in faecal pH(Reference Beynen, Baas and Hoekemeijer265). It is also possible that SCFA affect Mg absorption(Reference Rayssiguier and Remesy266), butyrate being more efficient than propionate or acetate(Reference Leonhard-Marek, Gabel and Martens267), probably via a cation exchange mechanism. Indeed, butyric acid is able to enhance the intestinal uptake by activation of an apical Mg2+/2H+ antiport through the provision of protons within the epithelial cell.
Fe and Zn balance can be improved by consumption of these ingredients however, animal studies have failed to show any significant effect on Cu bioavailability(Reference Delzenne, Aertssens and Verplaetse268).
Summary of key studies
Table 12 provides the list of review papers dealing with the effect of prebiotics on mineral metabolism.
Table 12 Published reviews on the prebiotic effect on mineral metabolism
FOS, fructo-oligosaccharides; GOS, galacto-oligosaccharides; SOS, soy-oligosaccharides; TOS, transgalacto-oligosaccharides; ITF, inulin-type fructans.
Animal study
Animal studies targeting the effect of prebiotics on Ca absorption are listed in the Tables 13 and 14. The points arising from these studies are the following:
(1) Different types of molecules have been studied, including ITF-Dpav 3-4, ITF-Dpav12, ITF-Dpav25, ITF-MIX, GOS, lactulose or resistant starch.
(2) Dietary supplementation with ITF enhances the uptake of Ca, improves bone mineral content (BMC) in growing rats and alleviates the reduction in bone mineral content and bone mineral density (BMD) which follows ovariectomy or gastrectomy in rats.
Table 13 The prebiotic effects on bone metabolism in the rat

GOS, galacto-oligosaccharides; FOS, fructo-oligosaccharides; AAS, atomic absorption spectrophotometry; ICPMS, inductively coupled plasma MS; DP, degree of polymerisation; BMC, bone mineral content; BMD, bone mineral density; OF, oligofructose; DEXA, dual-energy X-ray absorptiometry; ITF, inulin-type fructans; DPD, deoxypyridinoline; OVX, ovariectomized. Femoral mechanical testing (three-point bending test).
Table 14 The prebiotic effects on mineral absorption in the rat

FOS, fructo-oligosaccharides; GOS, galacto-oligosaccharides; TOS, transgalactosylated oligosaccharides; OF, oligofructose; AAS, atomic absorption spectrometry; ICPOES, inductively coupled plasma optical emission spectroscopy; DP, degree of polymerisation; ITF, inulin-type fructans; ICPMS, inductively coupled plasma MS; OVX, ovariectomized.
Apparent absorption: Ca intake (I) − Ca fecal excretion (F).
Net retention: Ca intake (I) − (Ca fecal excretion (F)+Ca urinary excretion (U)).
True intestinal Ca absorption: (Ca45 Ca44) = (I − F)+f (endogenous net Ca excretion).
Fractional Ca absorption: Ca47, Sc49 ratio (I − F).
Ca balance: 4–7 d balance period (I, F, U using metabolic cages) % Ca45 absorption: % Ca45 oral dose/% Ca45 IP dose × 100.
Clinical trials
In infants
The only available study targeting the prebiotic effect on mineral metabolism in infants was conducted in 6–12 months healthy formula-fed babies (Tables 15 and 16). Even though, ITF did not elicit any modulation of faecal SCFA concentration, a beneficial effect on both Fe and Mg absorption and retention was reported. No significant difference was observed for Ca, Cu or Zn(Reference Yap, Mohamed and Yazid269).
Table 15 The prebiotic effects on mineral absorption in the human

Sc, short chain; R, randomized; DB, double-blind; AAS, atomic absorption spectrometry; CO, crossover; OF, oligofructose; ICPMS, inductively coupled plasma MS; ScFOS, short-chain fructo-oligosaccharides; ITF, inulin-type fructans; TIMMS, thermal ionisation magnetic sector MS; PC, placebo control; CPP, casein phosphopeptide.
Fractional Ca: (Ca44, Ca43) ratio; (Ca46, Ca42) ratio.
Table 16 The prebiotic effects on human bone health
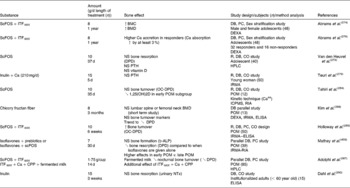
ScFOS, short-chain fructo-oligosaccharides; ITF, inulin-type fructans; BMC, bone mineral content; BMD, bone mineral density; DB, double-blind; PC, placebo control; DEXA, dual-energy X-ray absorptiometry; DPD, deoxypyridinoline; CO, crossover; PTH, parathyroid hormone; IRMA, immunoradiometric assay; POM, postmenopausal women; ICPMS, inductively coupled plasma MS; OC-DPD, osteocalcin deoxypyridinoline; PP, caseinophosphopetide; b-ALP, bone specific alkaline phosphatase; ELISA, enzyme-linked immunosorbent assay; CPP, casein phosphopeptide; NTx, N-Telopeptide.
In adolescents
As far as adolescents are concerned, in 1999, van den Heuvel et al. (Reference van den Heuvel, Muys and van Dokkum270) demonstrated that a daily consumption of 15 g of ITF for 9 d stimulated fractional Ca absorption by 10 % in young boys (14–16 years). Later on, Griffin et al. (Reference Griffin, Davila and Abrams271) provided the evidence that modest intake of ITFMIX, corresponding to 8 g/d, stimulated Ca absorption in sixty girls at or near menarche. The increase reached about 30 % after 3 weeks of consumption, when compared with oligofructose only or placebo intakes.
This effect was mostly observed in girls with lower Ca absorption status(Reference Griffin, Hicks and Heaney272). Moreover, when given for 36 d to adolescent girls (12–14 years), 10 g of ITF-Dpav 3-4 were able to stimulate Mg absorption (18 %), without affecting Ca absorption, vitamin D or parathyroid hormone (PTH) serum concentration or urine concentration which are used as markers of bone resorption(Reference van den Heuvel, Muijs and Brouns273).
The longest and most compelling study is a 1-year intervention trial on pre-pubertal girls and boys (n 100) that found significantly increased Ca absorption in the group receiving ITF-MIX (8 g/d) after 8 weeks. The effect lasted throughout the intervention period resulting, after 1 year, in improved whole body bone mineral content and significantly increased BMD, compared with the controls(Reference Abrams, Griffin and Hawthorne274). This demonstrates a beneficial effect on long-term use of this particular mixture on Ca absorption and bone mineralisation in young adolescents(Reference Cashman275). A further study by Abrams et al. (Reference Abrams, Griffin and Hawthorne276) showed that responders to the ‘treatment’ had greater Ca absorption and increased accretion of Ca to the skeleton, and thus concluded on the importance of such a strategy to enhance peak bone mass, as the extra absorbed Ca is deposited in bones.
In adults
It has been previously shown, using the metabolic balance methodology, that addition of up to 40 g/d of ITF and sugarbeet fibres to a normal mixed diet for 28 d improved Ca balance, without adverse effects on the retention of other mineral(Reference Coudray, Bellanger and Castiglia-Delavaud277). However, a study carried out by van den Heuvel et al. (Reference van den Heuvel, Schaafsma and Muys278) in healthy young adults found no significant differences in mineral absorption, irrespective of the treatment (which consisted of a constant basal diet supplemented for 21 d with 15 g/d ITF, or GOS, or not supplemented) followed by a 24 h urine collection. It was hypothesised that a 24 h period of urine collection, used in the study, was too short to include the colonic component of Ca absorption and thus to make up a complete balance necessary to detect the effect of ITF. In a similar way, Teuri et al. (Reference Teuri, Karkkainen and Lamberg-Allardt279) investigated a combination of 15 g of ITF and 210 mg of Ca added to 100 g of cheese given at breakfast to fifteen adult healthy women with an average age of 23 years old. The study failed to show any significant influence of the diet on blood ionised Ca or parathyroid concentration over the 8 h assessment period. Nevertheless, measuring serum parathyroid and ionised Ca do not provide direct information about Ca absorption, as do isotope techniques, and it has been suggested that the length of the trial was probably too short. Moreover, the addition of 1·1 g ITF-Dpav3-4 or caseinophosphopeptides to Ca-enriched milks, a valuable source of well-absorbed Ca, did non-significantly increase Ca absorption in adults (25–36 years), independently of sex(Reference Lopez-Huertas, Teucher and Boza280). Finally, Abrams et al. (Reference Abrams, Hawthorne and Aliu281) gave a supplementation containing 8 g of ITF-MIX for 8 weeks to thirteen young adults (average age of 23 years). Eight of the thirteen volunteers were classified as responders, based on their level of Ca absorption.
In postmenopausal women
Ducros et al. (Reference Ducros, Arnaud and Tahiri282) carried out a clinical trial in postmenopausal women (age between 50 and 70 years with at least 2 years of menopause). The volunteers were provided with 10 g/d ITF-Dpav3-4 or a placebo for 5 weeks using a crossover design. They demonstrated that consumption of ingredients showing a prebiotic effect was associated with increased Cu absorption, while no significant effect could be demonstrated on Zn or Se bioavailability.
In a similarly designed double-blind randomised, crossover design, post-menopausal women without hormone replacement therapy were given 10 g of ITF-Dpav3-4 daily for 5 weeks. Mg absorption and status was determined using mass spectrometer analysis in faeces, urine and blood. Results showed that the ITF-Dpav3-4-enriched diet increased Mg absorption by 12·3 %, compared to the placebo sucrose control group(Reference Tahiri, Tressol and Arnaud283). In the same experiment, Tahiri et al. (Reference Tahiri, Tressol and Arnaud284) showed that over 5 weeks of a moderate daily dose (10 g) of ITF-Dpav3-4 failed to modify intestinal Ca absorption in the early postmenopausal phase, while in the subgroup of late phase (women who had been going through menopause for more than 6 years), an increase in Ca absorption was observed.
Twelve older postmenopausal women (of at least 5 years past the onset of menopause) drank 100 ml of water containing 5 or 10 g of lactulose or a reference substance at breakfast for 9 d. True fractional Ca absorption was calculated using Ca isotope ratios, and consumption of lactulose was found to increase Ca absorption in a dose–response way(Reference van den Heuvel, Muijs and van Dokkum285).
In a crossover trial, twelve postmenopausal women were given a 200 ml yogurt to drink twice a day (at breakfast and lunch) containing either GOS (20 g) or sucrose for 9 d; a greater true Ca absorption (16 %) was observed after consumption of a product rich in GOS. In addition, no increased urinary Ca excretion was observed, suggesting that GOS could also indirectly increase the uptake of Ca by bones and/or inhibit bone resorption(Reference van den Heuvel, Schoterman and Muijs286).
Adolphi et al. (Reference Adolphi, Scholz-Ahrens and de Vrese287) tested the hypothesis that, in postmenopausal women (between 48 and 67 years and who had been postmenopausal for 10·5 (sem 0·7) years consumption of fermented milk (supplemented with Ca) at bedtime could prevent the nocturnal peak of bone resorption by decelerating its turnover and that this effect could be improved by adding Ca absorption enhancers. Actually, they showed that indeed such a practice can reduce the nocturnal bone resorption and that supplementation with Ca had no additional effect unless absorption enhancers such as ITF and caseinphosphopeptides were added.
Kim et al. (Reference Kim, Jang and Lee288) who investigated the effects of ITF supplementation (8 g/d for 3 months) in postmenopausal women (mean age: 60 year) showed that apparent Ca absorption was significantly increased by 42 % in the ITF group, while a 29 % decrease was observed in the placebo group. This was associated with lower alkaline phosphate plasma levels (a parameter which is actually not specific of bone formation) and a trend towards a slight reduction in urinary deoxypyridinolin (a biomarker for bone resorption). As expected, due to the very short length of exposure, BMD was not modified by the treatment.
Finally, fifteen women (who were a minimum of 10 year past the onset of menopause and had taken no hormone replacement therapy for the past years) were treated with 10 g/d of a specific mixture of ITF for 6 weeks, according to a double-blind, placebo-controlled crossover design. True fractional Ca absorption, measured by dual isotopes before and after treatment, was significantly increased (+7 %) in women with lower initial BMD(Reference Holloway, Moynihan and Abrams289).
In institutionalised patients
Bone resorption, used as indicator of Ca retention, remained unchanged in institutionalised adults after 3 weeks of treatment with 13 g/day of ITF-fortified beverages(Reference Dahl, Whiting and Isaac290).
Outline of general rules
Involvement of the colon
The main points arising from the available studies are that the Ca sparing effect elicited by a prebiotic effect involves colonic absorption. Indeed, using in vitro Ussing chambers, Raschka & Daniel(Reference Raschka and Daniel262) provided the evidence of the effect of ITF-MIX on transepithelial Ca fluxes in large intestine of rats.
Levrat et al. (Reference Levrat, Remesy and Demigne291) showed that dietary ITF given in the range of 0–20 % in the diet-stimulated intestinal Ca absorption in a dose-dependent manner, coinciding with a progressive decrease in caecal or ileal pH, hypertrophy of caecal walls and a rise in caecal pool of SCFA.
Moreover, Ohta et al. (Reference Ohta, Ohtsuki and Baba257) demonstrated that in rats fed a ITF-containing diet, but not in those given a control diet, the ratio of Ca or Mg to Cr (Cr being used as an unabsorbable marker to calculate apparent absorption of Ca and Mg) were correlated with the fractional length of transit along the colon and rectum, indicating linear disappearance of Ca and Mg during the colorectal passage. Consequently, in cecectomised rats, ITF failed to increase Ca absorption(Reference Ohta, Ohtuki and Takizawa292).
Similarly, in patients with conventional ileostomy, data analysis of ITF effects on mineral absorption and excretion (Mg, Zn, Ca, Fe) showed no significant influence(Reference Ellegard, Andersson and Bosaeus293).
This offers an explanation as to why Van den Heuvel et al. (Reference van den Heuvel, Schaafsma and Muys278) found no significant differences in mineral absorption in healthy young adults, irrespective of the treatment they received (consisting of a constant basal diet supplemented for 21 d with 15 g/d ITF, or GOS, or not supplemented), as the 24 h period of urine collection used in the present study was too short to include the colonic component of Ca absorption and thus to make up a complete balance necessary to detect the effect of fructans.
Indeed, Abrams et al. (Reference Abrams, Hawthorne and Aliu281) gave 8 g of ITF-MIX to young adults (average age of 23 years) for 8 weeks and confirmed that Ca absorption after treatment occurred principally in the colon (69·6 ± 18·6 %).
Nevertheless, it is still unclear whether the Ca-sparing effect results from induction of specific bacterial strains or from their ‘colonic food’ activity(Reference Scholz-Ahrens, Schaafsma and van den Heuvel294).
Dose effect
Various doses of ITF have been investigated ranging from 1·1 to 17 g/d (and even 40 g/d in one case). A minimum level of 8 g/d seems to be required to elicit an improvement on both Ca absorption and bone mineralisation. Indeed, Lopez-Huertas et al. (Reference Lopez-Huertas, Teucher and Boza280) explained the lack of effect of the addition of 1·1 g ITF or caseinophosphopeptides to Ca-enriched milks in adults by the very low dose provided in the diet.
However, with regard to animal studies, ITF appears to exhibit a dose-dependent effect on Ca absorption, as well. Levrat et al. (Reference Levrat, Remesy and Demigne291) showed that dietary ITF given in the range of 0–20 % in the diet-stimulated intestinal Ca absorption in a dose-dependent manner. Similarly, in the study carried out by Brommage et al. (Reference Brommage, Binacua and Antille295), a near linear increase in Ca absorption was demonstrated in rats fed a 5 and 10 % lactulosecontaining diet. Nevertheless, it appears that when a minimum is reached, Ca absorption enhancement occurs whatever maybe the dose, as a diet supplemented with either 10 % of ITF(Reference Delzenne, Aertssens and Verplaetse268) or 5 % of oligofructose or other non-digestible carbohydrates(Reference Brommage, Binacua and Antille295) leads to a similar increase (about 60–65 %) of the apparent absorption of Ca, even though, raising the content of oligofructose in the diet from 2·5 to 10 % in ovariectomised rats, a bone-sparing effect has been shown, independent of the dose by Scholz-Ahrens et al. (Reference Scholz-Ahrens, Acil and Schrezenmeir296).
Test substances
Various substances such as the different types of ITF, GOS, soya-oligosaccharides, lactulose, or resistant starch have provided evidence of a positive effect on Ca absorption, at least in the rat. However, the biological effect is likely to be related to the rate of fermentation which is mainly dependent on the degree of polymerisation (DP), as well as the solubility and the structural arrangement of the carbohydrates. In rats fed ITF with different degrees of polymerisation (ITF-Dpav3-4, ITF-Dpav25, ITF-MIX), Kruger et al. (Reference Kruger, Brown and Collett297) showed that the various ITF do not have the same effect on Ca retention, femoral bone density, bone Ca content and excretion of collagen degradation products in the urine.
From the available data, it can be concluded that the higher biological effects were elicited by a combination of ingredients showing a prebiotic effect with different chain length. Indeed, ITF-MIX outperformed the traditional molecules given alone with regard to Ca absorption. Indeed, in adolescent girls, such a combination increased the true Ca absorption by almost 20 %, while oligofructose alone did not show any significant effect(Reference Griffin, Davila and Abrams271). This conceptual rule is even more apparent in animal experiments. Coudray et al. (Reference Coudray, Tressol and Gueux298) compared different types of fructans which differed in both sugar chain length and chain branching, and found a synergistic effect of a combination of ITF with different chain lengths in adult male rats.
A potential mechanism for the improved efficiency of such a mixture could be the larger distribution of fermentation along the colon, depending on the chain length, which is critical to obtain maximum efficacy at low daily doses. Actually, the short-chain components such as oligofructose are most active in the proximal part of the colon, while the long-chain molecules have their effect in the distal part. The combination of both molecules offers a synergistic effect on Ca absorption, the fermentation process taking place over the full length of the colon, thus maximising the mucosal surface through which the extra solubilised Ca can migrate(Reference Coxam299).
Influence of physiological status
It appears that some subjects are more likely to benefit from consumption of inulin, according to their physiological status.
Initial status in calcium
First, of all, Griffin et al. (Reference Griffin, Hicks and Heaney272) demonstrated that the most consistent identifiable determinant of a beneficial effect on Ca absorption was the fractional Ca absorption at baseline with those individuals with lower absorption during placebo period showing the greatest benefit. These data were corroborated by data published by Holloway et al. (Reference Holloway, Moynihan and Abrams289) who showed that, in fifteen postmenopausal women (who were a minimum of 10 year past the onset of menopause) treated with 10 g/d of ITF-MIX for 6 weeks, true fractional Ca absorption, measured by dual isotopes before and after treatment, was significantly increased only in those with lower initial BMD.
Oestrogen permeation
From human data, we can conclude that an improvement in Ca absorption is possible in adolescents or young adults. Similarly, a positive effect has been reported in older women. However, ITF failed to modulate Ca absorption during the first 5 years after the onset of menopause, a period, actually, predominantly characterised by hormonal disturbances. In fact, menopausal status is the overriding factor in determining bone loss in women in their early fifties. Thus, given the tremendous impact of gonadal hormones on bone health, a high- Ca intake will not offset osteopenia that occurs immediately following menopause.
However, ITF could still remain a source for putative innovative dietary health intervention to prevent post-menopausal osteoporosis by modulating phytoestrogens bioavalability. Setchell et al. (Reference Setchell, Brown and Lydeking-Olsen300) have found that intestinal metabolism of isoflavones (the major class of phytoestrogens) would be the more important clue to the clinical efficacy of soya foods in preventing osteopenia. Thus, because a greater efficacy of phytoestrogens can be expected if converted into equol by the intestinal microbiota, there is a good rationale for considering non-digestible carbohydrates with prebiotic effects, targeting an increase of isoflavones bioavailability. Nevertheless, available data are still conflicting. In animal studies, it has been shown that dietary oligofructose may increase β-glucosidase activity in the large intestine, leading to an enhancement of the large intestinal absorption of these compounds(Reference Uehara, Ohta and Sakai301). Furthermore, in ovariectomised mice(Reference Ohta, Uehara and Sakai302) or rats(Reference Mathey, Puel and Kati-Coulibaly303), two experimental models for postmenopausal osteoporosis, oligofructose consumption has been shown to augment the bone sparing effect of isoflavones by improving equol production. Again, Devareddy et al. (Reference Devareddy, Khalil and Korlagunta304) demonstrated that although the combination of ITF and soya had no additive effect on BMD, it had a greater effect in reversing the loss of certain microarchitectural parameters such as tibial trabecular number, separation and thickness. By contrast, from a rat experiment, Zafar et al. (Reference Zafar, Weaver and Jones305) concluded that isoflavones could enhance Ca absorption, without synergy from ITF, and that actually ITF decreased equol production.
In postmenopausal women, Piazza et al. (Reference Piazza, Privitera and Melilli306) showed that the presence of ITF in the diet (3·6 g twice a day) facilitated the absorption of isoflavones. As far as bone metabolism is concerned, Mathey et al. (Reference Mathey, Puel and Kati-Coulibaly303) demonstrated that ITF consumption was able to improve the protective effect of isoflavones on bone resorption.
From mineral absorption to health benefits
The key question of whether the extra absorption of minerals may exhibit substantial benefits needs to be addressed.
Minerals
Ohta et al. (Reference Ohta, Baba and Takizawa307) showed that, in rats fed ITF-Dpav3-4 (1 or 5 %in the diet), apparent Mg absorption was increased, as compared with controls. The highest dose (and sufficient Mg in the diet, i.e. 0·5 mg/g) resulted in a reduction of auricular and facial peripheral hyperaemia and hemorrhage and improved inflammation in Mg-deficient rats. Similarly, in Fe-deficient animals, ITF-Dpav3-4 feeding not only increased Fe, Ca and Mg absorption but also improved recovery from anaemia, as well(Reference Ohta, Ohtsuki and Baba308). Kobayashi et al. (Reference Kobayashi, Nagatani and Magishi309) also found that soya polysaccharides could enhance Fe absorption and improve anaemia.
Consequently, these studies provide the evidence that ITF are able to elicit health improvement by enhancing mineral and Ca absorption. Further studies are necessary to assess this possibility.
Calcium and bone health
The adequate consumption of Ca in conjunction with optimisation of its absorption is likely to optimise bone mass. It is thus necessary to prove that the benefits of ingredients showing a prebiotic effect on Ca absorption persist and can be translated into benefits to bone health, in other words, whether the extra absorbed Ca is deposited in bones, as such a substantial bone benefit may have important implications for future preventative strategies for osteoporosis.
Even though animal data provide promising results on the role of ingredients showing a prebiotic effect on bone health, they need to be confirmed by human intervention trials. Most of the scientific evidence of the bone sparing is based on the animal studies, in which they not only improve Ca absorption but also prevent bone loss in conditions of oestrogen deprivation. Actually, the major available data come from the Abrams's team(Reference Abrams, Griffin and Hawthorne274) and the study with ITF-MIX is the only published data dealing with long-term effect. Thus, because when targeting bone mineralisation process, Ca is the most likely to be inadequate in terms of dietary intake, the enhancement of Ca accretion in bones, and hence BMD, in adolescents given ITF-MIX for 1 year, is very interesting. Indeed, adequate Ca intake in childhood is critical for the formation and retention of a healthy skeleton. However, if those molecules may help to optimise peak bone mass, their effect in older people, when bone turnover is increased, needs to be ascertained.
Moreover, because bone strength is the ultimate hallmark of bone quality, the issue of persistence of the beneficial effect on the skeleton is another issue important to consider, in order to assess their potential in the prevention of the risk of fracture.
Key points
(1) Ingredients showing a prebiotic effect are able to improve mineral absorption (and especially Ca) in the animals.
(2) Most data are available for ITF, in particular ITF-Dpav3-4 as well asITF-MIX.
(3) ITF have been found to increase Mg absorption in human subjects, nevertheless available data are very limited.
(4) These ingredients are able to enhance Ca absorption in human, depending on their physiological status (no effect in early postmenopausal women).
(5) The benefits on Ca absorption can be translated into benefits to bone health in animals.
(6) More interestingly, ITF-MIX given for 1 year to adolescents was able to elicit not only an enhancement of Ca accretion in bones but also BMD. In this light, such or similar may have important implications for future preventative startegies for osteoporosis.
(7) A combination of molecules with different degrees of polymerisation appears to be more efficient as shown with the research on ITF-MIX in comparison with the small and high MW fractions given alone.
Recommendations (future targets for research)
(1) Further studies are required to investigate the underlying mechanisms of the prebiotic effects on absorption of minerals, with special attention to the role of the specific changes in gut microbiota. Indeed the question still remains open of whether these effects are due to the changes in colonic microbiota composition (prebiotic effect) or any other mechanisms. In this regard, high-throughput methodologies such as metabolomics, for example, are warranted.
(2) Results from ITF, in particular ITF-MIX, need to be confirmed in other ingredients showing a prebiotic effect for a generalisation.
(3) Further long-term well-designed clinical trials need to be implemented to prove that the benefits of these ingredients persist in the longer term (because bone strength is the ultimate hallmark of bone quality, the issue of persistence of the effect of ITF-DPav3-4 on the skeleton is important to consider) to assess their potential in the prevention of the risk of fracture.
(4) With regards to the bone target, it is interesting to focus on relevant populations, i.e. during childhood and during ageing.
(5) It is still challenging to investigate the potential synergy between the prebiotic effect and other nutrients (such as phytoestrogens) endowed with bone-sparing effect.
Prebiotic effects in weight management and obesity-related disorders
The main authors of this section are Professor Delzenne, Dr Cani and Dr Neyrinck.
Several reviews report the interest of non-digestible carbohydrates – which are prone to be fermented by the gut microbiota in the control of obesity and related metabolic disorders (Table 17). Carbohydrates showing a prebiotic effect have received special attention in this context, since they have been shown – mostly in experimental animal studies – to regulate food intake and weight gain, as well as metabolic disorders associated with obesity, such as liver steatosis, dyslipidemia, diabetes and/or even hypertension(Reference Cani and Delzenne310). Most of the data published to date have been obtained through the supplementation with ITF as prebiotics. The relevance of changes in gut microbiota in the modulation of obesity and related disorders is discussed, taking into account both animal and human studies published so far.
Table 17 Experimental data supporting the prebiotic effects on body weight and fat mass development

FOS, fructo-oligosaccharides; GOS, galacto-oligosaccharides; BW, body weight; HF, high fat; HC, high carbohydrate; EAT, epididymal adipose tissue; IAT, inguinal adipose tissue; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; wk, weeks.
Description of the prebiotic effects on obesity and related metabolic disorders
Prebiotic effects and regulation of food intake, fat mass and body weight
Animal studies
Numerous data have described the effect of prebiotics (5–10 % in feed) feeding on the evolution of body weight and fat mass in experimental animal models (Table 17). The observed decrease in fat mass had sometimes occurred without significant effect on body weight and has been observed in all the types of white adipose tissue (epididymal, visceral and or subcutaneous). In numerous studies of rodent models (lean, genetic or nutritional induced obese mice or rats), this decrease in fat mass following feeding with ingredients showing a prebiotic effect was associated with a reduction of food/energy intake. The decrease in food/energy intake is not observed when ITF prebiotics are substituted by non-fermentable dietary fibre (microcrystalline cellulose), suggesting that at least the colonic fermentation plays a role in the modulation of food intake(Reference Daubioul, Rousseau and Demeure311, Reference Cani, Possemiers and van de312).
Potential mechanism
The decrease in food intake associated with prebiotics feeding in animals might be linked to the modulation of GI peptides involved in the regulation of food intake. Endocrine cells present in the intestinal mucosa secrete peptides involved in the regulation of energy homeostasis. Among those peptides, glucagon-like peptide (GLP)-1, peptide Y Y (PYY), ghrelin and oxyntomodulin have recently been proposed as important modulators of food intake and energy expenditure(Reference Chaudhri, Salem and Murphy313–Reference Knauf, Cani and Perrin316).
Several data obtained in rats and mice show that ITF-DPav3-4 reduce food intake, body weight gain and fat mass development, these features being associated with a significant increase in the portal plasma levels of anorexigenic peptides GLP-1 and PYY; some data also report a decrease in the serum level of orexigenic ghrelin upon prebiotics feeding(Reference Cani, Dewever and Delzenne317–Reference Reimer and Russell321). Dietary intervention with ingredients showing a prebiotic effect in postnatal diets causes a rapid increase in GLP-1 in rats, and this influences fat mass and glycaemia in adulthood(Reference Maurer, Chen and McPherson322).
Prebiotics feeding promotes GLP-1 synthesis (mRNA and peptide content) in the proximal colon namely by a mechanism linked to the differentiation of precursor cells into enteroendocrine cells(Reference Cani, Hoste and Guiot323). The overproduction of GLP-1 of mice supplemented with short-chain ITF could constitute a key event explaining several systemic effects of prebiotics, since the decrease in food intake and in fat mass after fructans treatment is abolished in GLP-1. Receptor knock-k out mice or in mice treated chronically with a GLP-1 receptor antagonist – Exendin 9–39(Reference Cani, Knauf and Iglesias320).
Human data
In healthy human subjects, feeding 16 g/d of ITF-DPav3-4 (short-chain ITF) promotes satiety following breakfast and diner, and reduces hunger and prospective food consumption after the dinner. This is accompanied by a significant 10 % lower total energy intake(Reference Cani, Joly and Horsmans324). Similarly, Archer et al. (Reference Archer, Johnson and Devereux325) have demonstrated that the gut microbiota fermentation of ITF, added to food as fat-replacer, is able to lower energy intake during a test day. ITF feeding (20 g/d) increased plasma GLP-1 in one interventional study performed in patients presenting gastric reflux. The present study was not aimed at demonstrating an effect on food intake and/or satiety(Reference Piche, des Varannes and Sacher-Huvelin326). The authors suggested that the ‘kinetics’ of fermentation – assessed by H breath test – is important to take into account when assessing the influence of fermented nutrients on circulating gut peptides. The increase in H expired (marker of fermentation) correlates with the modulation of plasma GLP-1 level, which could explain the link between intestinal fermentation and gut peptide secretion.
According to this observation, we have recently demonstrated that the prebiotics-induced gut microbiota fermentation was associated with increased postprandial GLP-1 and PYY and subsequent changes in appetite sensations(Reference Cani, Lecourt and Dewulf327).
A recent study demonstrated that supplementation with ITF-MIX not only benefited bone mineralisation, but also had a significant benefit on the maintenance of an appropriate BMI, and fat mass in primarily non-obese young adolescents(Reference Abrams, Griffin and Hawthorne328). Daily intake of yacon syrup, allowing to bring 0·14 g FOS/kg per day, over 120 d, resulted in an increase in satiety sensation and a decrease in body weight, waist circumference and BMI in obese pre-menopausal women(Reference Genta, Cabrera and Habib329). Interestingly, the relevance of gut hormone modulation in the management of obesity and metabolic syndrome in human subjects is supported by some data. A recent clinical trial supports the evidence that ITF-DPav3-4 (short-chain ITF) decrease food intake, body weight gain and fat mass development in obese subjects. The authors found a higher plasma PYY levels as well as a drop in ghrelin following meal, however, they failed to observe an increase GLP-1 plasma concentrations over a 6-h meal tolerance test(Reference Parnell and Reimer330). The effect of acute treatment with 8 g ITF with or without 0·3 g β-glucans over 2 d did not have any effect on appetite, satiety or food intake, suggesting that an adaptative process (linked to the modulation of gut microbiota?) may be necessary to observe the satietogenic effect of prebiotics(Reference Peters, Boers and Haddeman331).
Prebiotic effects and glucose homeostasis
Animals
An improvement of glucose homaeostasis by ingredients showing a prebiotic effect has been observed in rats or mice in several nutritional, genetic or toxic conditions leading to glucose intolerance and/or diabetes: high-fructose(Reference Busserolles, Gueux and Rock332) or high-fat diet-fed animals(Reference Cani, Knauf and Iglesias320, Reference Kok, Taper and Delzenne333–Reference Delmee, Cani and Gual335), genetically obese or diabetic mice(Reference Cani, Possemiers and van de312), streptozotocin-induced diabetic rats(Reference Cani, Daubioul and Reusens336). The improvement of glycaemic response can be explained on either increasing insulin secretion or insulin sensitivity, depending on the model.
In streptozotocin-treated rats, characterised by a diabetes linked to the destruction of β-cells, prebiotics feeding improves glucose tolerance and increases plasma insulin. In this model, the treatment with ITF allows a partial restoration of pancreatic insulin and β-cells mass. Endogenous GLP-1 production is increased in diabetic rats received ITF as compared to other groups(Reference Cani, Daubioul and Reusens336). This GLP-1 overproduction might be part of the protective effect of dietary ITF because:
(1) It has been shown that in diabetes prone-BB rats that are characterised by a default of production of gut peptides, no effect of ITF was shown(Reference Perrin, archesini and Rochat337);
(2) GLP-1 has been shown to increase β-cells differentiation; and
(3) that beneficial effect of ITF is not due to the satietogenic effect alone, since the improvement of glucose tolerance and pancreatic β-cell mass observed in streptozotocin-ITF fed rats is not reproduced through the sole pair-feeding restriction.
It is likely that a more direct effect of GLP-1 could be due to its effect on pancreatic β-cells differentiation.
ITF improve hepatic insulin sensitivity and increase plasma insulin in diet-induced diabetes and obesity (high-fat fed mice)(Reference Cani, Knauf and Iglesias320). As shown by an increase in food intake and body mass, genetic and pharmacological disruption of the GLP-1 receptor action abolished the beneficial effect of the treatment on both glucose tolerance and insulin sensitivity, suggesting a key role for this gut peptide(Reference Cani, Knauf and Iglesias320). In diet-induced obese dogs, 1 % short-chain fructans given in the diet for 6 weeks resulted in a decrease in insulin resistance assessed by euglycaemic/hyperinsulinaemic clamp, and these effects occurred in parallel with changes in the expression of genes involved in glucose and lipid metabolism in the adipose tissue(Reference Respondek, Swanson and Belsito338).
Altogether, these data support the relevance of the prebiotic modulation of gut microbiota by using dietary in the control of glucose homeostasis in different models of diabetes. The implication of gut peptides may be involved in this effect, however, other metabolic mechanisms, such as a decrease in inflammatory tone, could also contribute to the improvement of glucose homeostasis upon treatment with ingredients showing a prebiotic effect (see later).
Human studies
Several papers have been published, which have focused on the influence of ingredients showing a prebiotic effect on glucose homeostasis in human subjects. Luo et al. (Reference Luo, Rizkalla and Alamowitch339) have shown that 20 g short-chain fructans given for 4 weeks to healthy subjects decreased basal hepatic glucose production, but had no detectable effect on insulin-stimulated glucose metabolism. They tested the same approach in type 2 diabetic patients but no significant modification of glucose homeostasis (plasma glucose level, hepatic glucose production) occurred in the prebiotics-treated patients(Reference Luo, Van Yperselle and Rizkalla340). In a similar study conducted in hypercholesterolaemic patients, prebiotics (short-chain fructans) treatment reduced the postprandial insulin response, but the clinical relevance of this effect remains unclear(Reference Giacco, Clemente and Luongo341). In a recent study, a 2-week supplementation with 16 g/d ITF, compared with the same amount of maltodextrin used as placebo, increased GLP-1 production and lessen the postprandial glucose response after a standardised breakfast(Reference Cani, Lecourt and Dewulf327).
Prebiotic effects and lipid homeostasis, including steatosis and hepatic alterations
Animal studies
Ingredients showing a prebiotic effect are able to modulate hepatic lipid metabolism in rats or hamsters, resulting in changes in either TAG accumulation in the liver (steatosis) or serum lipids(Reference Delzenne and Cani342). In non-obese rats and/or hamsters fed a high carbohydrate diet, a decrease in hepatic and serum TAG was observed, when ITF were added to the diet at concentrations ranging from 2·5 to 10 % for several weeks (from 2 to 12 weeks)(Reference Delzenne and Williams343). In animals, reduced triglyceridaemia or steatosis is often linked to a decrease in de novo lipogenesis in the liver(Reference Delzenne and Williams343). In rats fed a lipid-rich diet containing fructans, a decrease in triglyceridaemia also occurs without any protective effect on hepatic TAG accumulation and lipogenesis, suggesting a possible peripheral mode of action(Reference Kok, Taper and Delzenne333). By contrast, in obese Zucker rats, dietary supplementation with ITF lessens hepatic steatosis, with no effect on postprandial triglyceridaemia when added to the standard diet(Reference Daubioul, Taper and De Wispelaere344). This effect is likely to be mainly of a lower availability of NEFA coming from adipose tissue, since fat mass and body weight are decreased by the treatment. In obese dogs, a 6 weeks treatment with short-chain fructans was able to increase uncoupling protein 2 and carnitine palmitoyltransferase 1 expression in the adipose tissue, thereby suggesting a higher substrate oxidation in adipocyte that occurred without any significant change in triglyceridemia(Reference Respondek, Swanson and Belsito338).
The decrease in TAG synthesis and accumulation of dietary prebiotics compounds could be linked to several events. First, a decrease in glycaemia could be part of the process, since glucose (together with insulin) is a driver of lipogenesis. Secondly, the SCFA produced through the fermentation process could play a role in the regulation of lipid metabolism. The high proportion of propionate produced in the caecum, which reaches the liver through the portal vein, is, at least in animals, a key event in explaining a lower hepatic TAG synthesis(Reference Morand, Remesy and Demigne345, Reference Delzenne, Daubioul and Neyrinck346). Interestingly, acetate, when supplied in the diet of diabetic mice at a dose of 0·5 % for 8 weeks, activates AMPkinase in the liver, a phenomenon that is related to the inhibition of de novo lipogenesis(Reference Sakakibara, Yamauchi and Oshima347). The incubation of rat hepatocytes with acetate (0·2 mm) activates AMPkinase and decreases sterol response element binding protein-1c expression, two factors clearly implicated in the regulation of lipogenesis. Therefore, the classical deleterious role attributed to acetate as a precursor of lipogenesis might be modulated taking into account its regulatory effect on key molecular factors involved in fatty acid synthesis in the liver.
Several studies have also reported a decrease in total serum cholesterol after dietary supplementation with inulin (10 %) in mice or rats(Reference Delzenne and Williams343, Reference Levrat, Favier and Moundras348–Reference Fava, Lovegrove and Gitau351). Experiments in apoE-deficient mice support the fact that dietary inulin (mainly long-chain inulin) significantly lowers total cholesterol levels by about one third. This is accompanied by a significant decrease in the hepatic cholesterol content. The authors suggest that the decrease in serum cholesterol could reflect a decrease in TAG-rich lipoproteins which are also rich in cholesterol in apo-E deficient animals(Reference Rault-Nania, Gueux and Demougeot350).
With regard to the hypocholesterolaemic effect of prebiotics, several mechanisms have been proposed. The modulation of the intestinal metabolism of bile acids, (e.g. steroid-binding properties) may be involved, which are independent of the fermentation of the ingredient showing a prebiotic effect in the lower intestinal tract(Reference Delzenne and Williams343, Reference Trautwein, Forgbert and Rieckhoff352, Reference Adam, Levrat-Verny and Lopez353). A recent study, performed in rats supplemented with GOS/FOS, did not support the involvement of changes in the bile salt pool size and kinetics in the modulation of lipid and energy metabolism(Reference van Meer, Boehm and Stellaard354).
Human data
Reported effects of prebiotics on circulating blood lipids in both normo- and moderately hyperlipidaemic human subjects are variable(Reference Brighenti355). Both positive and negative outcomes have been obtained from a small number of well-designed human studies, devoted to analyse the effect of dietary supplementation with fructans (doses ranging from 8 to 20 g/d) exhibiting prebiotic properties. The effect of ITF supplementation on lipogenesis has been shown in human volunteers: the hepatic capacity of TAG synthesis is lowered by this ingredients showing a prebiotic effect as previously shown in rats(Reference Diraison, Moulin and Beylot356). In patients with non-alcoholic steatohepatitis, short-chain ITF supplementation leads to a decrease in serum activity of amino-transferases, suggesting an improvement of hepatic alterations in those patients(Reference Daubioul, Horsmans and Lambert357), thereby suggesting that a prebiotic approach could be useful in the management of hepatic disease associated with obesity.
Prebiotic effects and obesity-associated inflammation
Obesity and insulin resistance are associated with a low-grade inflammation (for review, see Cani & Delzenne(Reference Cani and Delzenne310, Reference Cani and Delzenne358). The gut microbiota takes part of this component of the metabolic disorder associated with obesity. In fact, LPS has been considered to be the triggering factor for the early development of inflammation and metabolic diseases(Reference Cani, Amar and Iglesias359). The excessive intake in dietary fat facilitates the absorption of highly pro-inflammatory bacterial LPS from the gut, thereby increasing plasma LPS level leading to ‘metabolic endotoxemia’(Reference Cani, Amar and Iglesias359). Interestingly, several reports have shown that obesity induced following dietary manipulations (high-fat feeding)(Reference Cani, Amar and Iglesias359–Reference Cani, Bibiloni and Knauf362) or genetic deletion (leptin-deficient models)(Reference Waldram, Holmes and Wang363) is characterised by changes in gut microbiota towards a decreased number of bifidobacteria. Importantly, this group of bacteria has been shown to reduce intestinal LPS levels in mice and to improve the mucosal barrier function(Reference Wang, Xiao and Yao364–Reference Ruan, Shi and Xia367). Feeding mice with ITF-\av3-4 restores the number of intestinal bifidobacteria and reduces the impact of high-fat diet induced-metabolic endotoxaemia and inflammatory disorders(Reference Cani, Knauf and Iglesias320, Reference Cani, Neyrinck and Fava361). With regard to the possible mechanism of action of these ingredients, data obtained in obese ob/ob mice showed that they increase the production of a gut peptide secreted by endocrine cells of the colon, namely GLP-2, which plays a role on the intestinal tissue itself, by restoring tight junction protein expression and repartition, and thereby decreasing gut permeability, endotoxemia, and associated metabolic disorders(Reference Cani, Possemiers and van de312).
The relevance of endotoxemia on metabolic disorders due to fat excess, and diabetes in human is supported by several recent studies. However, the impact of the prebiotic approach on endotoxemia and inflammation in obese and diabetic patients has not yet been demonstrated. This area of research may be very interesting and important, since inflammation is considered as an important event that drives a lot series of metabolic alterations linked to obesity (CVD, non-alcoholic steato hepatitis, insulin resistance, etc.).
Relation between prebiotic effects and improvement of obesity and associated disorders
Relative specificity of prebiotics effects v. other ‘dietary fibres’ on physiological targets regulating appetite and metabolic disorders
It has been proposed before that the secretion of gut peptides might be part of the effects of fermentable carbohydrates with prebiotics properties. Some of those effect can also been driven by dietary compounds for which a prebiotic effect has not yet been shown. Resistant starch has also been shown to increase GLP-1 and PYY in several rodent studies, with consequences on fat mass development(Reference Keenan, Zhou and McCutcheon368, Reference Zhou, Martin and Tulley369).
An increase in the postprandial response of GLP-1 was observed after ingestion of β-glucan-rich rye bread by healthy subjects(Reference Juntunen, Niskanen and Liukkonen370). The administration of guar gum (together with galactose) promoted the increase in GLP-1 in women, and this was related to a significant increase in satiety(Reference Adam and Westerterp-Plantenga371). An increase in the level of non-ndigestible carbohydrates (barley-kernel bread) in the evening meal resulted in an increase in satiety and in a decrease glucose response following breakfast, an event that can be linked to an increase in GLP-1, to the extent of fermentation (assessed through the H breath test) and which is related to a lower proinflammatory cytokine level (IL6)(Reference Nilsson, Ostman and Holst372).
These data suggest that some effect described for ‘well-established’ prebiotics can also be the attribute of other non-digestible/fermentable carbohydrates. The relevance of the gut microbiota composition and activity in this process remains poorly explored. In that view, recent data suggest that butyrate is able to improve insulin sensitivity and energy expenditure in rodents(Reference Gao, Yin and Zhang373) thereby supporting the hypothesis that besides the changes in the composition of the microbiota, the gut microbiota and the pattern of fermentation could also be important to take into account.
What is the contribution of changes in gut microbiota composition in the improvement of metabolic alterations by prebiotics?
A recent study has shown, for the first time in human subjects, that differences in specific ‘healthy’ bacteria in gut microbiota may precede the development of becoming overweight(Reference Kalliomaki, Collado and Salminen374). The authors found that Bifidobacterium spp. during the first year of life was higher in number in children who exhibited a normal weight at 7 years than in children becoming overweight. More importantly, and according to the results obtained in experimental models, they found that the faecal numbers of Staphylococcus aureus were lower in children remaining normal weight than in children becoming overweight. These results unequivocally imply that the gut microbiota profile in favour of a higher number bifidobacteria and a lower number of S. aureus in infancy may provide protection against overweight and obesity development. The authors proposed that S. aureus may act as a trigger of low-grade inflammation(Reference Lundell, Adlerberth and Lindberg375), contributing to the development of obesity. Experimental data in mice suggest that the promotion of bifidobacteria by the intake of ingredients showing a prebiotic effect – may be helpful per se. On the one hand, intervention studies relating concomitantly the changes in gut microbiota composition (and activity), and, on the other hand, behavioural (appetite) or physiological changes are therefore necessary to proof the relevance of the gut microbial changes in the effects.
Methodological aspects
Key questions remain open concerning the adequacy of the experimental protocol to estimate the relevance of ingredients showing a prebiotic effect in the management of obesity and associated disorders. The choice of a placebo is rather difficult, and the type of placebo compounds is different when experiments are conducted in animals or in human subjects. There may also be differences when considering end points such as fat mass development or satiety, or glucose/lipid homeostasis.
In animal studies, the authors often add ingredients showing a prebiotic effect at a relatively high dose (1–10 % wt/wt in the diet) to compare the data obtained in animals receiving the basal diet alone. The interpretation of results would then require the difference in energy/nutrients intake and/or an experimental group with the same intake of energy upon the treatment (pair-fed animals) to be taken into account. Other authors propose to replace the amount of ingredients showing a prebiotic effect by a non-digestible–non fermentable carbohydrate such as microcrystalline cellulose as placebo. This allows a comparison based on differential fermentation properties.
For human studies, the dose of ingredients showing a prebiotic effect is much lower (from 1 to 30 g/d). The organoleptic and physico-chemical properties of the placebo are very important to take into account. Several placebos are proposed in the literature, e.g. a digestible carbohydrate, such as maltodextrin – i.e. alone(Reference Cani, Joly and Horsmans324, Reference Cani, Lecourt and Dewulf327), or in combination with aspartame(Reference Giacco, Clemente and Luongo341) – or saccharose(Reference Luo, Rizkalla and Alamowitch339, Reference Luo, Van Yperselle and Rizkalla340), dietary fibres such as oat fibre(Reference Peters, Boers and Haddeman331).
The choice of the adequate placebo is really difficult and will depend on the end point and duration of the treatment. When estimating the influence on glucose/lipid metabolism, one must consider a placebo that does not change postprandial glucose level or has a minor impact as lipogenic substrate, for example.
For studies aiming at controlling appetite and energy, one has to choose an adequate placebo which does not exert an effect per se. When estimating a long-term effect on body weight composition, the consequence of placebo treatment on global energy intake must be taken into account.
There are, therefore, several possibilities and the interpretation and discussion of the results might also take into account the differences that could be due to the placebo effect in a specific context.
Conclusions and future trends
Collectively, these studies provide support for the beneficial effect of prebiotics on energy homeostasis and body weight gain. Only a few human studies are available to date, but some of them support a role of gut peptide modulation by ingredients showing a prebiotic effect as a potential mechanism occurring in the gut, and appetite regulation. The question of the relevance of gut microbiota modulation in these effects remains unexplored in most of the studies performed in human subjects. In mice, an inverse relationship has been established between the level of faecal bifidobacteria and some features of the metabolic alterations linked to obesity (endotoxemia, fat mass, glucose intolerance). Some other non-digestible carbohydrates or dietary fibres (i.e. resistant starch, insoluble fibre form barley) – for which prebiotic effect has not yet been established – would be able to modulate gut peptides production with consequences on appetite, inflammation and other components of the metabolic syndrome. The analysis of the gut microbiota changes will be crucial in further research and clinical approach, in order to clearly relate those changes with the improvement of metabolic alterations of the host. This will be the way to propose a ‘targeted approach in the modulation of gut microbiota by ingredients showing a prebiotic effect’ as relevant in the context of obesity.
Conclusion and perspectives
Which data to support the hypothesis of a causal relationship between a prebiotic effect and health effects/benefits?
The author of this section is Professor Marcel B. Roberfroid. A prebiotic effect exists and is now a well-established scientific fact. A large number of human intervention studies have demonstrated that dietary consumption of food products/ingredients/supplements results in statistically significant changes in the composition of the faecal (and in some cases, the mucosal) gut microbiota. Most of the available data concern the selective stimulation of bifidobacteria (but also lactobacilli). Other purportedly beneficial genera such as Roseburia and Eubacterium may be more fully investigated in the future – although further evidence of their beneficial effects is required. Some, but not all, studies have reported a reduction in the concentration of pathogenic bacteria such as clostridia and salmonella. The more data are accumulating, the more it will be recognised that such changes in the composition of the faecal microbiota, especially increase in bifidobacteria can be regarded as a marker of intestinal health. This is already supported by scientific publications(Reference Gronlund, Gueimonde and Laitinen376–Reference Salminen, Collado and Isolauri380).
Research on the impact of the prebiotic effect on the activity (metabolic, regulatory and signalling) of the microbiota is ongoing and appropriate relevant methodologies are being developed, validated and applied.
(1) Results from experimental models but also in a few human studies, food products/ingredients/supplements with a demonstrated prebiotic effect have been shown to modulate certain immunological biomarkers and affect activity(ies) of the immune system. Whether changes in immune function markers or immune-health benefits are related to a prebiotic-induced change in the composition of the gut microbiota is an area for future investigation. While several studies report changes in the faecal microbial composition alongside changes in immune markers, only one study sofar has correlated these findings. Although these observations make the link between immuno-modulation and microbiota changes likely, convincing evidence needs to be established by further studies showing clear correlations between parameters of immune function and changes in the microbiota. Although a causal relationship is virtually impossible to establish in human subjects, current plausible hypotheses and future correlative findings will help to establish the correlation between prebiotic modulation of the intestinal microbiota and changes in immune function.
(2) The effect of breast-feeding on infant gut microbiota composition is well established and mother's milk is known to contain a complex mixture oligosaccharides with prebiotic (especially bifidogenic) effects, therefore, infant formulae/foods have been supplemented with prebiotics. Confirming the studies in adults, it has been demonstrated that such supplementation increases the faecal concentration of bifidobacteria. This concomitantly improves stool quality (soft and loose stools), reduces the risk of gastro-enteritis, improves general well-being and reduces the frequency of atopic eczema. It is plausible that these effects were microbiota-induced changes.
(3) Changes in the gut microbiota composition are classically considered as one of the many factors involved in the pathogenesis of either IBD or IBS. The use of particular food products/ingredients/supplements with prebiotic effects has thus been tested in clinical trials with the objective to improve the well-being of patients with such disease states. Promising beneficial effects have been demonstrated in some but still preliminary studies with changes in gut microbiota composition (especially increase in bifidobacteria concentration) being associated. Again, it is feasible to conclude that the mechanism of these effects is linked to the prebiotic effect.
(4) Colon cancer is another pathology for which a possible role of gut microbiota composition has been hypothesised. Numerous experimental studies in mice and rats have reported reduction in incidence of tumours and cancers after feeding specific food products/ingredients/supplements with prebiotic effects. Some of these studies (including one human trial) have also reported that, in such conditions, gut microbiota composition was modified (especially due to increased concentration of bifidobacteria), however, role of such changes in the eventual anti-cancer effect of these specific food products/ingredients/supplements remains to be definitively proven.
(5) Dietary intake of particular food products/ingredients/supplements with a prebiotic effect has been shown, especially in adolescents, but also tentatively in postmenopausal women to increase Ca absorption as well as bone Ca accretion and BMD. No correlation has been reported between such a beneficial effect and changes in gut microbiota composition – although this is plausible but not exclusive. However, other food products/ingredients/supplements that do not show prebiotic effect (e.g. lactose, miscellaneous dietary fibres) have also been reported to exert similar effects. Moreover, a study in adolescents revealed the existence of a genetic component in response (with one-third of non-responders) to increased Ca absorption. It is thus likely that improved Ca absorption is not uniquely caused by changes in gut microbiota composition and might be a consequence of a combination of different effects. Preliminary data have reported, mainly in experimental models, that specific food products/ingredients/supplements with prebiotic effects could also increase the absorption of other minerals (e.g. Mg, Fe). More research is needed to confirm these data and, eventually, to demonstrate if their mechanism involves changes in gut microbiota composition.
(6) Recent data, both from experimental models and from human studies, support the beneficial effects of particular food products/ingredients/supplements with prebiotic properties on energy homeostasis, satiety regulation and body weight gain. Together with data that correlate obesity with differences in gut microbiota composition, these studies have led to hypothesise that gut microbiota composition (especially the number of bifidobacteria) may contribute to modulate metabolic processes associated with syndrome X, especially obesity and diabetes type 2. In a study on the mechanism of action of a prebiotic food ingredient in reducing obesity, an inverse correlation between bifidobacteria faecal concentration, and gut permeability and metabolic endotoxemia (plasmatic LPS), has been reported. However, non-prebiotic dietary fibres have also shown some similar effects, the question of the specific benefits that can specifically be attributed to prebiotic effects remains open.
(7) By reference to the present knowledge (mostly based on the data obtained with the various ITFs and the GOS) on the prebiotic effect and its possible multiple physiological consequences, it appears likely that different compounds (food ingredients or food supplements) including chemically identical compounds with e.g. different chain lengths (like in the ITF group) will have:
(a) different prebiotic effects will influence differently the composition of the microflora in the different segments of the intestine, especially in the large bowel;
(b) different physiological effects and thus will not affect similarly the same functions (as this is clearly the case for Ca absorption, a function that is more influenced by ITF-MIX than by the different ITFs given separately.
Any effect of one particular compound with a prebiotic effect can never be generalised to another compound, unless this has been scientifically substantiated for each particular food ingredient/supplement(Reference Roberfroid78).
The majority of successful human trials on the prebiotic effects show significantly increased intestinal levels of bifidobacteria. Often, these are associated with improvement in well-characterised and accepted markers of health as shown by the extensive and growing body of evidence, outlined in this report. This strongly associates prebiotic-induced increases in numbers of bifidobacteria in the gut with a range of GI and systemic health benefits. Although it could be argued that these studies alone do not necessarily indicate causality, when considered with the results of trials in human subjects and animals supplemented with live bifidobacteria they do indeed provide compelling evidence that the relationship between intestinal bifidobacteria and health might well be causal(Reference Gronlund, Gueimonde and Laitinen376–Reference Salminen, Collado and Isolauri380).
Even so, key questions still remain such as
(1) Which effect(s) (see Table 2) is/are causally linked to selective change(s) in gut microbiota composition?
(2) Which of the physiological and/or pathophysiological well-being and health benefits are directly linked with a particular composition of the gut microbiota or (a) selective change(s) therein?
(3) Which, among the physiological and/or pathophysiological well-being and health benefits, is (are) not linked to a particular composition of the gut microbiota or (a) selective change(s) therein but is (are) the consequence(s) of other mechanism(s) of the product claimed to have a prebiotic effect?
(4) Which protocol(s) is (are) now validated to demonstrate change(s) in microbiota composition?
(5) Which protocol(s) and methodology(ies) is (are) now available and validated to demonstrate links between a particular composition of the gut microbiota or a selective change therein and a particular physiological and/or pathophysiological well-being and health benefit?
Over the last two decades, data have and continue to accumulate improving our knowledge of the gut microbiota composition but also, through the metabonomic approaches, gut microbiota activities. It has convincingly demonstrated that particular food products/ingredients/supplements can, upon feeding, selectively modulate that composition and possibly these activities. Dietary consumption of some of these specific food products/ingredients/supplements has also been reported to exert a series of beneficial health effects that may justify improved function and/or reduction of disease risk claims(Reference Cummings, Antoine and Azpiroz21, Reference Bellisle, Diplock and Hornstra381). A causal relationship between the induced change(s) in gut microbiota composition and/or activity(ies) and these health effects is more than plausible – given our knowledge that prebiotics are known to be specifically metabolised by the gut microbiota. The more we understand the complexity of the gut microbiota, its interactions with the gut epithelium, its roles in modulating epithelial cell differentiation and epithelial cell functions and, beyond, in the whole body, the more we will be in a position to recommend these food ingredients for their health-promoting values. It is becoming more and more clear that gut microbiota plays key roles in modulating human/animal physiology even far beyond the GI tract. Specific food products/ingredients/supplements with prebiotic properties are unique tools to study such effects but also offer unique opportunity to develop new functional foods/food ingredients/food supplements to improve host health. One major contribution of this review article summarising the state of the art in the research on the metabolic and health effects of these compounds is to recommend where research efforts should be concentrated to improve understanding of the activities and the physiological roles of the gut microbiota and in particular the importance of its qualitative composition and the consequences of that modulation. Through this, it should be possible to better address the continuing burden of gastro-intestinally mediated disorders. Importantly, tools exist to underpin this with mechanistic explanations of effect leading to effective hypothesis-driven research.
Acknowledgements
The authors thank Dr Patrice Lebecque (Unité de Nutrition Humaine, UMR1019, INRA Clermont-Ferrand/Theix) for his help in writing the section on mineral absorption and Dr Sarah Schenker who kindly reviewed the English consistency of the present paper. The authors also thank Professor Salminen and its co-authors for enabling us reproducing the table from the following article: S. Salminen, C. Bouley, M. -C. Boutron, J. H. Cummings, A. Franck, G. R. G., E. Isolauri, M.-C. Moreau, M. R. and I. R., ‘Functional food science and gastrointestinal physiology and function’, Br J Nutr 80(Suppl. 1), S147–S171, (1998). This work was commissioned by the Prebiotics Task Force of the European branch of the International Life Sciences Institute (ILSI Europe). Industry members of this task force are Cargill, Cereals Partner Worldwide, Clasado, Colloïdes Naturels International, Cosucra Groupe Warcoing, Danone, Danisco, Friesland Campina, Kellogg Europe, Kraft Foods, Mead Johnson Nutrition, Puratos, Roquette Frères, Sensus, Südzucker/BENEO Group, Syral, Tate & Lyle, Ulker Bisküvi and Unilever. M. R., G. R. G., L. H., A. L. M. C., R. R., I. R., B. W., H. S., F. G., F. R., K. W., N. M. D., P. D. C. and A. M. N. have no conflict of interest to declare. DW is an employee of Unilever, BS is an employee of Danone, FR is an employee of Syral, AM is an employee of ILSI Europe. The team of VC, MJD, YW and LL is doing collaborative research with Cosucra Groupe Warcoing. Prof. Dr. med. habil. Günther Boehm is also an employee of Danone Research, Center for Specialised Nutrition, Friedrichsdorf, Germany. For further information about ILSI Europe, please call +32 2 771 00 14 or email: [email protected]. The opinions expressed herein are those of the authors and do not necessarily represent the views of ILSI Europe.