Experimental studies provide evidence of the neurotrophic and protective properties of lithiumReference Dell'Osso, Del Grande, Gesi, Carmassi and Musetti1 and its potential use for the treatment and prevention of neurodegenerative disorders; yet, the translation of such knowledge into a clinical perspective is still limited by the small number of controlled intervention trials, in addition to a few case series and uncontrolled studies.Reference Forlenza, Aprahamian, de Paula and Hajek2 Epidemiological and imaging studies in bipolar disorder show that long-term lithium treatment is associated with lower rates of dementiaReference Nunes, Forlenza and Gattaz3,Reference Kessing, Søndergård, Forman and Andersen4 and potentially beneficial brain responses, such as increased grey matter densityReference Moore, Bebchuk, Wilds, Chen and Manji5,Reference Zung, Souza-Duran, Soeiro-de-Souza, Uchida, Bottino and Busatto6 and better metabolic integrity of the cerebral tissue.Reference Hajek, Bauer, Pfennig, Cullis, Ploch and O'Donovan7 A recent, nationwide, population-based study conducted in Denmark indicated a negative association between trace lithium in ground water and the incidence of dementia across different geographical regions.Reference Kessing, Gerds, Knudsen, Jørgensen, Kristiansen and Voutchkova8 Similarly, lithium concentrations in drinking water in several counties in Texas, USA were negatively associated with mortality rates as a result of Alzheimer's disease.Reference Fajardo, Fajardo, LeBlanc and MacPherson9
This body of evidence underpins the potential use of lithium as a disease-modifying therapy for Alzheimer's disease.Reference Morris and Berk10 Four clinical trials have so far tested the effects of the administration of lithium salts for patients with, or at risk of, dementia.Reference Macdonald, Briggs, Poppe, Higgins, Velayudhan and Lovestone11–Reference Nunes, Viel and Buck14 These trials are methodologically heterogeneous regarding patient sample, study design, dose regimen, duration of treatment and primary end-points, and have therefore produced discordant results. Nonetheless, a meta-analysis indicated through pooled analysis of data from three of these studies that lithium treatment may indeed be associated with a significant decrease in cognitive decline.Reference Matsunaga, Kishi, Annas, Basun, Hampel and Iwata15 The objective of the present study is to determine the potential clinical and biological benefits of low-dose, long-term lithium treatment for patients with amnestic mild cognitive impairment (MCI), a clinical condition that comprises the transitional state between normal cognitive ageing and incipient dementia, and is associated with a high risk of Alzheimer's disease-related dementia.
Method
The present study was conducted in an academically oriented psychogeriatric service located at a tertiary public hospital in Sao Paulo, Brazil. The study protocol was approved by the local Ethics Committee (CAPPesq-HCFMUSP) and was conducted within the tenets of the Helsinki Declaration and Good Clinical Practice recommendations. Participants were community-dwelling out-patients living in the hospital's catchment area, recruited from a larger cohort of older adults with varying degrees of cognitive impairment, ranging from normal cognition to mild dementia. A total of 106 patients was invited to participate in the study, and 76 were considered eligible according to inclusion criteria: age ≥60 years; diagnosis of amnestic MCI according to Mayo Clinic criteria;Reference Petersen, Smith, Waring, Ivnik, Tangalos and Kokmen16 no clinical history of major psychiatric disorders; and no evidence of relevant/uncontrolled medical diseases. Fifteen eligible patients declined participation and 61 were enrolled to the study after providing written informed consent.
The study was designed as a single-centre, intervention trial starting with a 2-year long, double-blinded, randomised controlled trial followed by a single-blinded extension phase for an additional 24 months (trial registration at clinicaltrials.gov: NCT01055392). An interim analysis of this trial, addressing the modification of clinical and biological outcome variables after 12 months in a smaller sample has been published previously;Reference Forlenza, Diniz, Radanovic, Santos, Talib and Gattaz13 the present set of data corresponds to the analysis of primary and secondary outcome variables in the complete sample undertaking the full period of follow-up. A single physician (O.V.F.) who did not take part in the assessment of baseline or outcome variables performed the recruitment, diagnostic screening and allocation of eligible participants into study groups, in addition to prescribing medications throughout the trial.
Upon enrolment, all participants underwent systematic examination guided by a comprehensive clinical and neuropsychological protocol. For group allocation, we used the permuted blocks and stratified randomisation method, accounting for age and education levels. After randomisation into experimental (lithium) or comparison (placebo) groups (1:1 ratio) participants were longitudinally reassessed at 3-month intervals by raters who were unaware of group allocation. An independent geriatrician systematically performed physical examination and administered the 56-item UKU Side Effect Scale.Reference Lingjaerde, Ahlfors, Bech, Dencker and Elgen17 Peripheral blood samples were obtained prior to each visit for safety monitoring. A panel of laboratory tests (blood cell count, electrolytes, renal and thyroid function tests, fasting glucose and insulin, hepatic enzymes, lipid profile, urinalysis, electrocardiogram) were routinely performed; other diagnostic tests could be required on demand according to clinician's judgement.
An experienced neurologist (M.R.) performed all lumbar punctures at baseline and after 12 and 36 months for the collection of cerebrospinal fluid (CSF) samples. The third lumbar puncture was postponed for 12 months, instead of being performed at the end of the double-blind phase, for the sake of tolerability and adherence, warranting a longer interval between subsequent procedures, and to better account for long-term changes. Alzheimer's disease-related CSF biomarkers were determined by commercially available kits (INNo-BiaAlzBio3 assay, Innogenetics, Ghent, Belgium) using a multiplexed method (xMAP-Luminex).
Target lithium levels were defined at a subtherapeutic window (0.25–0.5 mEq/L). Identical tablets holding 150 mg, 300 mg, 450 mg or 600 mg of lithium carbonate or placebo, produced by the Central Pharmacy HCFMUSP and packaged into identical coded blisters, were used for prescription. A dedicated pharmacist was responsible for dispensation of lithium or placebo tablets, according to coded instructions provided by the prescribing physician. After each visit, participants received from the pharmacist two batches containing either lithium or placebo tablets. Blisters were identified for use in the mornings and/or evenings, and patients were instructed to take one tablet orally once or twice daily (as specified), preferably with meals. Treatment was started with a single daily tablet of lithium carbonate 150 mg or placebo taken orally in the evenings for a week. Participants in the comparison group were instructed to take a second placebo tablet in the morning from the second week onwards. For participants in the lithium group, daily doses were titrated by adding a second lithium (150 mg) or placebo tablet in the morning, and then adjusted to target serum levels at weekly visits, using distinct combinations of lithium/placebo tablets (one in the morning and one at night) to fulfil total doses of 150 mg, 300 mg, 450 mg or 600 mg per day. Therefore, the prescribing physician was able to titrate or taper the daily doses of lithium or to maintain the placebo regimen by regulating the combinations of tablets, without needing to change the number of daily tablets.
Serum lithium levels were determined weekly in the titration phase and at 3-month intervals throughout the study. In the morning prior to each new medical appointment, blood samples were collected for determination of serum lithium concentrations at an in-house lab facility (ion selective electrode method) allowing 12 h after the previous evening intake. In cases of occurrence of relevant side-effects, the lithium dose was tapered down to the highest previously tolerated dose within the target window. Once stable lithium levels were achieved, the prescription was maintained for the following 3 months, until the next scheduled visit when the same monitoring procedures were performed. In the meantime, patients were instructed to report any symptoms suggestive of adverse events or modifications made to other ongoing prescriptions; if necessary, the maintenance of lithium treatment was to be re-evaluated.
Primary outcomes were based on changes in cognitive and functional parameters during the double-blind phase of the study (end-point 2 years), namely: global cognitive state, as indicated by the cognitive subscale of the Alzheimer's Disease Assessment Scale (ADAS-Cog);Reference Rosen, Mohs and Davis18 and functional performance, according to the Clinical Dementia Rating (CDR) Sum of Boxes (SoB).Reference Morris19 Secondary outcomes were changes in neuropsychological test scores with emphasis on memory, attention and executive functionsReference Army Individual Test Battery20,Reference Wechsler21 ; changes in CSF concentrations of the amyloid-beta peptide (Aβ1−42), total tau and phosphorylated tau at threonine 181; conversion from MCI to dementia; safety/tolerability data; and changes in peripheral biomarkers. The latter two sets of data are not shown in this manuscript: safety and tolerability data pertaining to this study have been published elsewhere,Reference Aprahamian, Santos, Santos, Talib, Diniz and Radanovic22 and the analysis of peripheral biomarkers will be presented in a future publication. Details on study design and procedures can be found in supplementary Table 1 available at https://doi.org/10.1192/bjp.2019.76 (CONSORT checklist).
Statistical analysis
Differences in the distribution of demographic, clinical and biological variables between lithium and placebo groups at baseline were statistically examined using chi-squared (χ2), Fisher's and independent sample Student's t-tests. Interim analyses of primary outcome variables were performed with data collected annually. The analysis of cognitive and functional outcome variables was limited to the double-blind phase, and therefore included data collected at baseline and at 12 months and 24 months of follow-up. The analysis of Alzheimer's disease-related biomarkers was based on CSF samples collected at baseline and after 12 and 36 months. For these sets of data, we used a linear mixed effects model to determine the longitudinal changes in cognitive, functional and biological outcome variables, taking into account group allocation (treatment), duration of the intervention (time) and treatment/time interactions at distinct time points. Paired sample t-tests were additionally used to address differences in continuous variables between baseline and end-point within treatment groups. Non-parametric tests (Mann-Whitney U-test and Wilcoxon rank test) were further carried out for analysis of biological data in subanalyses with limiting sample sizes. Normative data generated in our laboratory were further used to classify subgroups of patients according to baseline amyloid burden (‘low-’ or ‘high-’ CSF Aβ1−42) using 416 pg/mL as a threshold.Reference Forlenza, Radanovic, Talib, Aprahamian, Diniz and Zetterberg23 The analysis of conversion from MCI to dementia took into account the full period of the trial (48 months); in this case, the single-blinded extension phase was included in the censoring to maximise the duration of follow-up, taking into account the possibility that this outcome might take longer to occur. Kaplan–Meier curves were therefore built to compare the conversion rates in lithium versus placebo groups, censoring the changes in functional state according to modifications in CDR scores (from CDR <1 to CDR ≥1). Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) version 18.
Results
In total, 61 participants were randomised to receive lithium (50.8%, n = 31) or placebo (49.2%, n = 30). Both groups at baseline had similar distributions of sociodemographic, clinical and biological variables (supplementary Table 2). The mean age of the total sample was 72.6 years (s.d. = 4.8), although patients in the lithium group were slightly younger compared with those in the placebo group. No statistically significant differences were observed between the two groups in other sociodemographic, clinical and biological variables at baseline, nor in the frequency of common comorbidities such as systemic hypertension, osteoarthritis, dyslipidaemia, hypothyroidism, diabetes mellitus and minor depression (data not shown).
In total, 52 patients (85.2%, n = 52) completed the double-blind phase of the study, with similar rates in both groups (87.1%, n = 27 lithium; 83%, n = 25 placebo, non-significant). Supplementary Fig. 1 displays the study flow chart (CONSORT diagram) indicating the number of completers in each treatment group after 1, 2, 3 and 4 years of follow-up. Patients in the lithium group had mean serum levels of 0.39 mEq/L (s.d. 0.08) during the double-blind phase, and of 0.41 mEq/L (s.d. = 0.10) during the extension phase of the trial.
Discontinuations occurred for several reasons, but mostly unrelated to the ongoing treatment. In the first year of the trial, four patients dropped out (two from each group): one patient was withdrawn from the lithium group after having an ischemic stroke, and another patient in the placebo group died from sepsis secondary to pneumonia; two participants discontinued the trial for personal reasons (one in each group). In the second year of follow-up, three patients in the placebo group were diagnosed either with prostate cancer, severe hypertension or ventricular arrhythmia, and were therefore advised to withdraw; one patient in the lithium group required hospital admission because of a delirious state (unrelated to lithium toxicity) and another had clinical symptoms of lithium intolerance (tremor and nausea), also therefore being excluded from the trial.
In the extension phase of the study (years 3 and 4), the attrition rate was considerably higher (nine individuals dropped out from each group), resulting in 55.7% (n = 40) overall completion rate (58%, n = 18 lithium; 53%, n = 16 placebo). It is noteworthy that all discontinuations in this phase occurred in the third year of follow-up, largely from participants' decisions at the beginning of year 3. Of these, nine participants withdrew consent after completion of the double-blind phase (six of whom were in the lithium group), and nine were excluded because of medical reasons such as: recurrent falls (1.6%, n = 1); the need for diuretic use (3.3%, n = 2); supraventricular arrhythmia (3.3%, n = 2); recent diagnosis of cancer (3.3%, n = 2); and referral for major surgery (3.3%, n = 2). These clinical events were not statistically associated with allocation in either treatment group.
Patients in the lithium group were cognitively and functionally stable over 24 months, whereas patients in the placebo group displayed mild, but statistically significant cognitive and functional decline as shown by total ADAS-Cog and CDR-SoB scores (Table 1). Baseline-to-end-point changes in test scores indicated that lithium treatment was beneficial, although the magnitude of the differences between the two groups was small.
Table 1 Cognitive and functional changes after 2 yearsa

NS, non-significant differences.
a. Cognitive and functional changes calculated as end-point (2 years) minus baseline scores.
b. Independent sample t-test (d.f.) = 59.
c. Negative values indicate improvement on test performance.
d. Negative values indicate decline on test performance.
Figure 1 illustrates the longitudinal changes in clinical outcome variables during the double-blind phase. We found significant effects of treatment group allocation, favouring the lithium group, on the dissociation of CDR-SoB (Fig. 1(a)) and ADAS-Cog (Fig. 1(b)) scores over time, as well as in delayed recall (Fig. 1(c)) and figure recall (Fig. 1(d)). This effect was also observed in trail making test-A (F = 7.19, P<0.01), but not in other attention tests such as sequence of letters and numbers, and trail making test-B. The changes in the figure recall test displayed interaction between time and group allocation. Except for the aforementioned differences in memory subscores, no statistically significant differences between lithium and placebo groups were found in other ADAS-Cog subdomains (naming objects and fingers, commands, constructional and ideational praxis, orientation, word recognition, language, comprehension and word finding).

Fig. 1 Cognitive and functional changes according to group allocation (lithium versus placebo) in 2 years of follow-up.
Figure 2 displays Kaplan–Meier curves of lithium-treated and placebo groups during the full length of follow-up, starting with a CDR-SoB score <1 (i.e. CDR-SoB = 0 or CDR-SoB = 0.5) and censored by the characterisation of a CDR-SoB score ≥1 in any follow-up reassessment i.e., indicating a functional status compatible with the diagnosis of dementia. Five patients in the lithium group (16%) and nine in the placebo group (30%) converted from MCI to dementia during follow-up, but the statistical significance of this difference (P = 0.06) was marginally above the pre-defined threshold of 0.05.
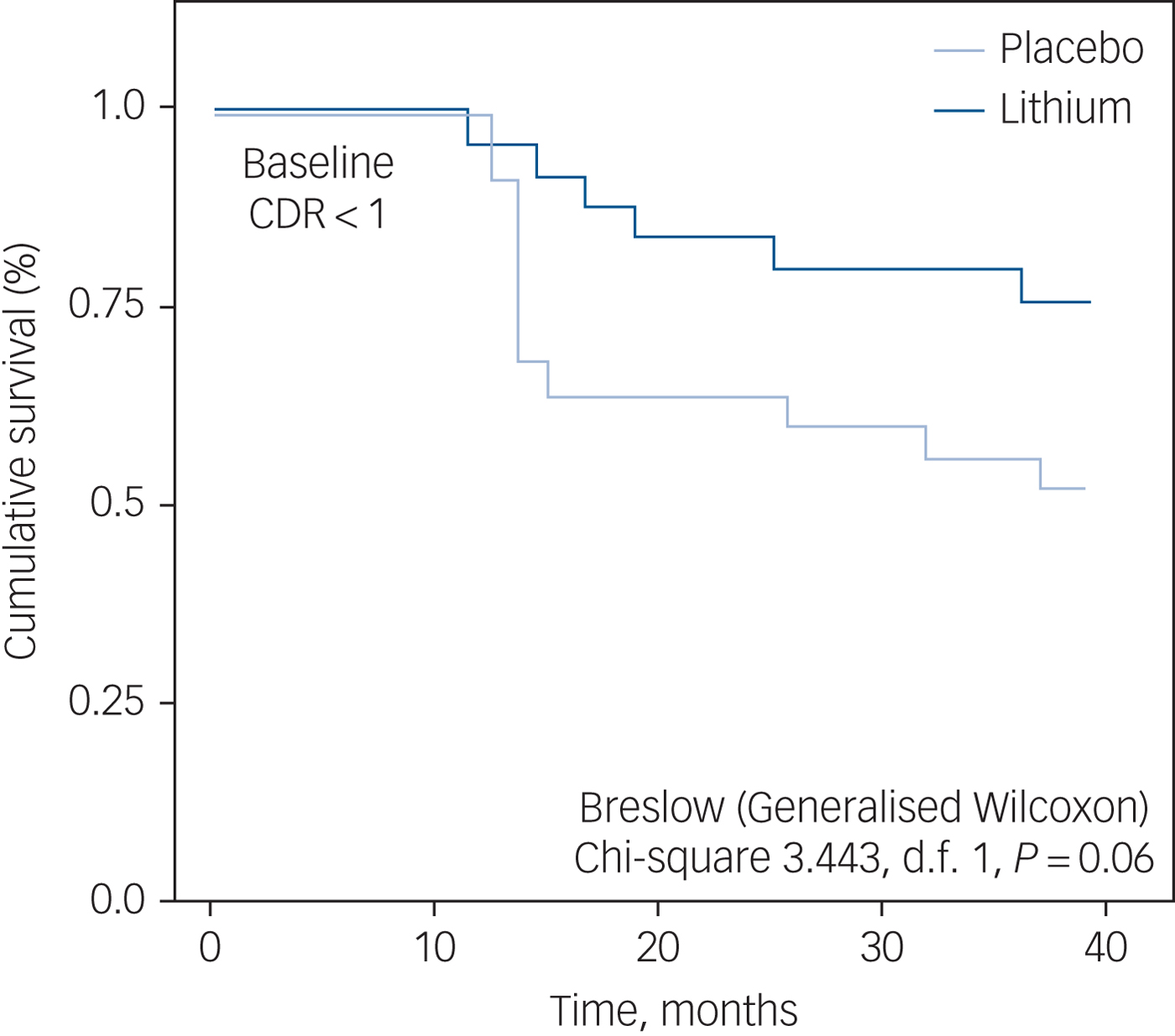
Fig. 2 Kaplan–Meier curves indicating the rate of conversion from mild cognitive impairment (MCI) to dementia, according to exposure or not to lithium treatment. All patients with MCI had baseline scores on the Clinical Dementia Rating Scale (CDR) <1.
Figure 3(a) shows a statistically significant increase in CSF concentration of Aβ1−42 after 3 years of continuous lithium use. No such effect was observed after 12 months of treatment, suggesting an effect of group allocation plus time. No statistically significant effects were observed in the concentrations of total or phosphorylated tau at this end-point.
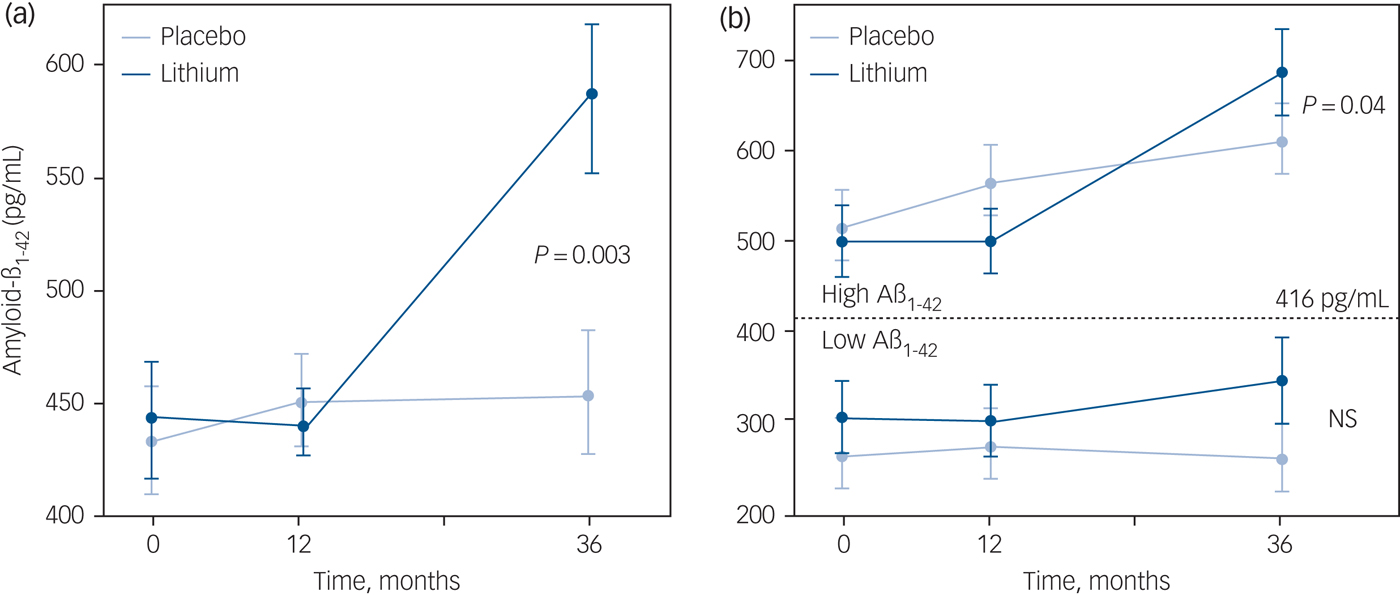
Fig. 3 Longitudinal changes in cerebrospinal fluid (CSF) concentrations of amyloid-β peptide (Aβ1−42) in lithium-treated and control groups.
The subsample of patients with CSF data available at baseline and after 36 months was split into two subgroups according to their profile of CSF Aβ1−42 prior to lithium treatment. Patients were categorised as ‘low-’ or ‘high CSF Aβ1−42’ using the threshold of 416 pg/mL to separate these subgroups,Reference Forlenza, Radanovic, Talib, Aprahamian, Diniz and Zetterberg23 according to which lower concentrations of the biomarker subsume higher intracerebral amyloid burden and, therefore, more severe Alzheimer's disease pathology. This subanalysis indicated that the significant increments in CSF Aβ1−42 after 36 months of lithium treatment were restricted to the participants with higher baseline concentrations of the peptide (P = 0.04), whereas non-significant changes were observed among lithium recipients with low-CSF Aβ1−42 at baseline (Fig. 3(b)).
Discussion
Main findings
Lithium is believed to modulate important intracellular signalling systems implicated in neurotrophic responses and in mechanisms related to neurodegeneration. These include downregulation of apoptosis, upregulation of autophagy, protection against excitotoxic and ischaemic damage to the brain, and stimulation of trophic responses, ranging from the secretion of growth factors to neurogenesis.Reference Chen, Rajkowska, Du, Seraji-Bozorgzad and Manji24,Reference Forlenza, De-Paula and Diniz25
The present study was designed to warrant a controlled observation of the effects of long-term, low-dose, lithium treatment on a set of clinical and biological outcome variables, in a sample of clinically healthy older adults with amnestic MCI. The overall results are in line with our preliminary findings from our interim analysis after 12 months,Reference Forlenza, Diniz, Radanovic, Santos, Talib and Gattaz13 and reinforces the notion that chronic lithium use may be associated with a lesser deterioration of cognitive abilities and with a relative preservation of functional capacity. The magnitude of the differences between treated and untreated groups was small, and both groups (as expected) displayed a mild deterioration over time. Nonetheless, the present findings suggest that chronic lithium use was beneficial to patients with amnestic MCI. The analysis of primary outcome variables, i.e. global cognitive (ADAS-Cog) and functional (CDR-SoB) state, along with the performance on neuropsychological tests (memory and attention subscores), indicate that participants in the lithium group performed better than those in the placebo group after 2 years of treatment.
It is noteworthy that the differences in global cognitive and functional performance observed at the end of the trial were already detectable after 12 months; however, differences in memory and attention scores favouring the lithium group after 2 years were not statistically significant in the interim analysis. This suggests a subtle, cumulative, benefit of chronic lithium treatment on such cognitive functions. In addition, differences in memory and attention scores were apparently not as a result of improved performance per se, but rather because of the stabilisation of these functions among lithium users, along with mild (slowly progressive) deterioration in the placebo group. The number of conversions from MCI to dementia was smaller among lithium users – albeit the statistical analysis indicated only a trend toward statistical significance of the cumulative survival across groups. Anyhow, there was a clear dissociation of the two slopes suggesting a distinct pattern of the survival curves over time for lithium and placebo groups. This tendency was already observed in the interim analysis after 12 months of lithium treatment in a smaller sample of patients with MCI, and apparently continued to build in the same direction in the 4-year outcome with the complete sample. Possibly, the separation of the survival curves will eventually result in statistical significance with an even longer follow-up.
Methodological considerations
We acknowledge a few methodological aspects that may have been relevant to the present results, starting with the actual study design. Because of the difficulties in maintaining the double-blind procedures for a period as long as 4 years, the study was subdivided into an initial, 2 year, double-blind phase followed by an extension phase of 2 additional years, in which participants (but not raters) were made aware of group allocation and entitled to decide whether or not to continue in the trial. For this reason, the assessment of primary outcome variables (global cognitive and functional status) and a subset of secondary outcome variables (neuropsychological test scores) was restricted to the double-blind phase of the study. Nonetheless, the assessment of the conversion status (i.e. clinical diagnosis of incident dementia) and the analysis of longitudinal changes in Alzheimer's disease-related CSF biomarkers were based on variables obtained in both phases of trial. We believe that these variables may not have been critically affected by the awareness of group allocation by participants in the extension phase of the study.
Another relevant methodological aspect is the therapeutic window of lithium utilised in the trial. Lithium treatment was administered at doses sufficient to yield serum levels of 0.25–0.5 mM/L. This decision was taken for the sake of safety and tolerability, given the high discontinuation rate in previous studies of lithium in Alzheimer's disease using therapeutic doses.Reference Macdonald, Briggs, Poppe, Higgins, Velayudhan and Lovestone11 Also, preparatory studies conducted in our laboratory indicated that lithium use within this subtherapeutic range was associated with good availability of lithium in the brain, as shown by magnetic spectroscopy, and with a 50% inhibition of glycogen synthase kinase 3-beta (GSK3β) activity in platelets drawn from peripheral blood (data not shown). The inhibition of GSK3β is a possible mechanism associated with the neuroprotective effects of lithium in Alzheimer's disease, since overactive GSK3β has been implicated in the pathophysiology of Alzheimer's disease and lithium is a potent inhibitor of its enzymatic activity.Reference Phiel, Wilson, Lee and Klein26 Nonetheless, it is likely that the effects of lithium on other molecular targets, or the combination of its pharmacodynamics properties, may better account for the clinical and biological changes observed in this trial.
We found a distinct pattern of change in Alzheimer's disease-related CSF biomarkers after 3 years of treatment: patients in the lithium group had a statistically significant, 30% increase in the CSF concentrations of Aβ1−42. This effect may depend on a longer exposure to lithium, because no such effect was noted after 12 months. We interpret this as a possible indication that long-term lithium treatment may promote mechanisms related to the clearance of the Aβ peptide from the brain. The subanalysis of CSF data according to the degree of amyloid burden of participants at baseline indicate that those who had higher CSF concentrations of Aβ1−42 (therefore, less intracerebral accumulation) prior to receiving lithium treatment were more prone to respond with increment in CSF levels of Aβ1−42 after 3 years. It is noteworthy that the putative lithium-induced decrement in CSF concentrations of phosphorylated tau observed after 12 months of treatmentReference Forlenza, Diniz, Radanovic, Santos, Talib and Gattaz13 did not withstand at this late end-point (i.e. was not observed after 36 months). However, the present evidence of changes in CSF concentrations of Aβ1−42 upon long-term lithium treatment, if confirmed by other experimental and clinical models, may warrant the chronic use of lithium at low doses as an approach to enhance amyloid clearance. In addition, it seems possible that, depending on the biological target and on the stage of the disease process, some patients may have a good response to lithium treatment, whereas others may not respond at all.
Strengths and limitations
A limitation of the study is the relatively small sample size, compared with multicentre trials. Nonetheless, as a single-centre study, one could argue the final sample enrolled to the trial is substantial, particularly in the light of the long-term follow-up and the frequent monitoring based on clinical and biological parameters. The present set of data, in addition to other recent clinical observations and evidence drawn from experimental models, may warrant the design of large-scale trials involving experts from different groups and including a wider array of outcome variables. Another important methodological improvement would be to define intervention groups according to biological variables at baseline (for example Alzheimer's disease-related CSF biomarkers and/or amyloid and tau imaging with positron–emission tomography).The characterisation of individuals with ‘prodromal Alzheimer's disease’ or even ‘pre-clinical Alzheimer's disease’ upon enrolment, along with the exclusion of those with cognitive impairment unrelated to Alzheimer's disease pathology, might be a better approach to identify the clinical conditions that might benefit most from lithium.
Likewise, these subanalyses may also help determine the time window of lithium use to deliver disease-modifying effects, as tentatively shown in our study addressing the magnitude of Alzheimer's disease pathology according to CSF biomarkers at baseline. We are still uncertain about the best therapeutic range of working lithium concentrations to target such biological effects within good safety–tolerability limits. Several lines of evidence (mostly from experimental models and epidemiological studies) suggest that chronic exposure to lithium at much lower doses – ranging from micromolar down to nano- or even picomolar concentrations – may deliver effects associated with modification in pathophysiological mechanisms, which in the long run may reduce the overall prevalence of dementia.Reference Kessing, Gerds, Knudsen, Jørgensen, Kristiansen and Voutchkova8,Reference Fajardo, Fajardo, LeBlanc and MacPherson9 Nonetheless, controlled data from the present trial indicate that long-term lithium treatment at subtherapeutic doses may be safe and well tolerated by older adults,Reference Aprahamian, Santos, Santos, Talib, Diniz and Radanovic22 supporting its use in patients with cognitive deficits to target neuroprotection and disease modification in Alzheimer's disease.
Funding
The present work was supported by Conselho Nacional de Pesquisa Científica (CNPq, Project 554535/2005-0), Alzheimer's Association (NIRG-08-90688) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Project 2009/52825-8). The Laboratory of Neuroscience (LIM-27) receives financial support from Associação Beneficente Alzira Denise Hertzog da Silva (ABADHS). It represents an investigator's initiative, i.e. devoid of any financial support or other forms of influence by pharmaceutical or biotechnological companies.
Acknowledgements
We are indebted to Drs Franklin S. Santos and Breno S. Diniz for the support in data acquisition and analysis.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2019.76







eLetters
No eLetters have been published for this article.