Hypertension is one of the most important risk factors for CHD, heart failure, cerebrovascular disease and chronic kidney disease. The prevalence of hypertension is increasing worldwide, both in developed and developing countries(Reference Pereira, Lunet and Azevedo1). Up to 30 % of the world's adult population was estimated to have hypertension in 2000(Reference Kearney, Whelton and Reynolds2). Any reductions in blood pressure, however small, are meaningful; a systolic blood pressure (SBP) reduction of 9 mmHg and a diastolic blood pressure reduction of 5 mmHg reduce the risk of stroke by 35–40 % and CHD by 20–25 %(Reference Mensink, Aro and Den Hond3).
Besides pharmacological therapy, lifestyle and nutritional factors play a significant role in the prevention and treatment of hypertension and related disorders. Low saturated fat and Na intake and increased consumption of K, Ca and soluble fibre positively affect blood pressure(Reference Krousel-Wood, Muntner and He4). Plant sterols and stanols (also called as phytosterols and -stanols) have well-established cholesterol-lowering effects. The consumption of 2 g plant sterols per d has been shown to lower both total and LDL-cholesterol concentrations by approximately 10 % in human subjects in different population groups(Reference Katan, Grundy and Jones5, Reference AbuMweis, Barake and Jones6), but does not seem to decrease blood pressure as such in humans(Reference Tapola, Lyyra and Karvonen7, Reference Hallikainen, Lyyra-Laitinen and Laitinen8). In addition, both epidemiological and intervention studies suggest that the consumption of low-fat dairy products is inversely related to the risk of hypertension(Reference McCarron, Morris and Henry9–Reference Toledo, Delgado-Rodríguez and Estruch11). The beneficial effect of milk on blood pressure is attributed to its high Ca and K content, and also to specific peptide sequences, which are cleaved from milk protein during gastrointestinal digestion, fermentation of milk with proteolytic starter cultures or by enzymic hydrolysis(Reference Korhonen12). Milk products fermented with Lactobacillus helveticus contain casein-derived tripeptides isoleucine-proline-proline (Ile-Pro-Pro) and valine-proline-proline (Val-Pro-Pro), which have been shown to possess antihypertensive effects in human subjects(Reference Pripp13, Reference Xu, Qin and Wang14) and in experimental models of hypertension(Reference Nakamura, Yamamoto and Sakai15–Reference Jauhiainen, Pilvi and Cheng19).
The effect of tripeptides or fermented milk products containing them without or with plant sterols has been studied using young spontaneously hypertensive rats (SHR)(Reference Sipola, Finckenberg and Korpela16, Reference Jäkälä, Pere and Lehtinen17, Reference Jauhiainen, Collin and Narva20, Reference Jäkälä, Turpeinen and Rajakari21), young high-salt-fed Goto–Kakizaki rats(Reference Jäkälä, Hakala and Turpeinen18) and double transgenic rats harbouring human renin and angiotensinogen genes(Reference Jauhiainen, Pilvi and Cheng19). In these studies (duration 3–16 weeks), the long-term intake of tripeptides has been found to attenuate the SBP increase of the rats by 8 to 21 mmHg depending on the study, strain and milk product. Older SHR with established hypertension have been used only in studies investigating the acute effect of tripeptides on blood pressure(Reference Nakamura, Yamamoto and Sakai15, Reference Masuda, Nakamura and Takano22). Accordingly, there are no published data on the possible long-term antihypertensive effects of tripeptides Ile-Pro-Pro and Val-Pro-Pro in rats that already have established hypertension.
Blood vessels contribute to blood pressure regulation by controlling vascular resistance. Due to ageing, increased blood pressure or other pathophysiological factors, arteries stiffen and gradually lose their ability to adjust to blood pressure changes(Reference Ghiadoni, Bruno and Stea23). Endothelial dysfunction is often observed in the presence of CVD and risk factors, such as hypertension(Reference Taddei, Virdis and Ghiadoni24). Impaired endothelium-dependent relaxation, a measure of endothelial dysfunction, is observed in various experimental models of hypertension, such as SHR(Reference Mäkynen, Kähönen and Arvola25–Reference Bagnost, Berthelot and Bouhaddi27), angiotensin II-induced hypertension(Reference Dal-Ros, Bronner and Schott28, Reference Kane, Etienne-Selloum and Madeira29), double transgenic rats(Reference Mervaala, Cheng and Tikkanen30) and salt-loaded type 2 diabetic Goto–Kakizaki rats(Reference Jäkälä, Hakala and Turpeinen18, Reference Cheng, Vaskonen and Tikkanen31). Besides the beneficial effects on blood pressure, tripeptides or fermented milk products containing them have shown to have vasoprotective effects on the endothelial function of rat arteries in some previous studies(Reference Jäkälä, Hakala and Turpeinen18, Reference Jäkälä, Jauhiainen and Korpela32).
The mechanisms behind the effects of tripeptides Ile-Pro-Pro and Val-Pro-Pro on blood pressure and vascular function have been at least partly related to the renin–angiotensin system (RAS) and, more precisely, to angiotensin-converting enzyme (ACE) inhibition(Reference Jäkälä, Hakala and Turpeinen18, Reference Nakamura, Yamamoto and Sakai33, Reference Lehtinen, Jauhiainen and Kankuri34). However, the clinical studies in particular have not been able to consistently prove this(Reference Jauhiainen, Vapaatalo and Poussa35–Reference Usinger, Ibsen and Linneberg37). Thus, to obtain more insight into the mechanistic questions, we applied DNA microarray technology to detect the possible changes in aortic gene expression after long-term treatment with a tripeptide-containing milk product.
Based on the above-mentioned findings, our hypothesis was that long-term treatment with a fermented milk product containing ACE-inhibitory tripeptides and plant sterols could lower already increased blood pressure and improve endothelial function in SHR with established hypertension. We hypothesised that tripeptides and plant sterols combined could offer additional benefit to the treatment of experimental hypertension. We also hypothesised that alterations in the expression of the genes related to the RAS and vascular inflammation could be observed in the rat aorta and could explain the known beneficial cardiovascular effects of the tripeptide-containing milk products.
Methods
Experimental protocol
The protocol was approved by the National Animal Experimentation Committee according to EC Directive 86/609/EEC and the Finnish Experimental Animal Act 62/2006. A total of twelve male SHR were obtained from Charles River Laboratories (Sulzfeld, Germany) at the age of 14 weeks. The rats were housed three to a cage in a standard experimental animal laboratory (illuminated from 07.00 to 19.00 hours; temperature 22 ± 2°C; humidity 55 ± 15 %). The rats had free access to standard rat pellets (2018 Teklad Global 18 % Protein Rodent Diet; Harlan Laboratories, Madison, WI, USA) and water.
After 1 week of adaptation, baseline blood pressure measurements were performed. The systolic blood pressure (SBP) of the rats was assessed by the tail-cuff method using an Apollo 2AB Blood Pressure Analyzer (model 179-2AB; IITC Life Science, Woodland Hills, CA, USA). Rats were placed in restrainer tubes and warmed in a heated chamber (32–34°C) for 15–20 min to make the pulsations of the tail artery detectable. After obtaining three consecutive and successful recordings without disturbance of the signal, the average of the values was regarded as the SBP. Thereafter, 15-week-old rats were randomised into two groups (six animals per group) based on SBP values and body weights to receive either: (1) a milk product (‘milk’) or (2) a milk product containing tripeptides (18 mg/l) and plant sterols (0·8 g/100 g) (‘active milk’) (for details, see below) as the drinking fluid ad libitum for 6 weeks. Drinking bottles were changed and fluid consumption was monitored daily. Feed consumption was monitored weekly.
During the experiment, the SBP of the rats was measured every week at the same time of day by the same researcher and in random order. To confirm that the stable phase of hypertension was reached, a similar experimental protocol was performed with age-matched SHR (n 6), which received only water as the drinking fluid.
Study products
Study products were provided by Valio Ltd (Helsinki, Finland). The active milk was a yogurt-like milk product, which was fermented by L. helveticus and to which proline-specific endoprotease (DSM, Heerlen, Netherlands), standardised peptide powder and a plant sterol mixture were added during preparation. The plant sterol mixture (Cognis, Monheim, Germany) was obtained by esterification of non-esterified plant sterols with fatty acids obtained from vegetable oil (5·1 g SFA, 7·6 g MUFA and 27·3 g PUFA per 100 g), and contained mainly β-sitosterol (69 %), campesterol (15 %), β-sitostanol (8 %) and brassicasterol (3 %). The concentration of tripeptides in the active milk is determined by the manufacturing process. Only minor fluctuations in different batches are observed. The amount of plant sterols added to the product was based on our previous studies(Reference Jäkälä, Pere and Lehtinen17, Reference Jäkälä, Hakala and Turpeinen18) and the generally recommended dose of plant sterols for human use for lowering high LDL-cholesterol (portion size of 250 g giving 2 g plant sterols)(Reference Grundy, Cleeman and Merz38).
‘Milk’ was a fermented milk product containing neither tripeptides nor plant sterols in detectable amounts.
Both products were sweetened by sucrose (8 %, w/w) and were low-fat ( < 1 %) and low-lactose ( < 1 %) containing 222 kJ/100 g (milk) and 272 kJ/100 g (active milk) energy.
The contents of energy nutrients, minerals, tripeptides Ile-Pro-Pro, Val-Pro-Pro and Leu-Pro-Pro and plant sterols were analysed by Valio Ltd, R&D (Helsinki, Finland). Tripeptides were analysed with the Waters Aquity UPLC–Micromass ZQ2000 Mass Spectrometry System (Waters, Milford, MA, USA) and total plant sterol content by the method of Laakso(Reference Laakso39) with minor modifications as described by Jäkälä et al. (Reference Jäkälä, Hakala and Turpeinen18).
Collection of the samples
After a 6-week treatment period, rats were rendered unconscious with CO2–O2 (30:70; AGA, Riihimäki, Finland) and decapitated. The hearts, left kidneys and adrenal glands were excised, washed with ice-cold saline, blotted dry and weighed. The left ventricle was separated and weighed. Aortas were excised, placed in a sterile saline solution and cleaned of adherent connective tissue. Sections of 1 cm were cut, stored in RNA Stabilization Reagent (RNAlater; Qiagen, Helsinki, Finland) and maintained at 4°C for 24 h, and then stored at − 20°C. Superior mesenteric arteries were excised and placed in ice-cold oxygenated Krebs buffer (pH 7·4–7·6, composition in mmol/l: NaCl, 119·0; NaHCO3, 25·0; glucose, 11·1; CaCl2, 1·6; KCl, 4·7; KH2PO4, 1·2; MgSO4, 1·2) for vascular reactivity studies.
Vascular reactivity measurements
Mesenteric arteries were carefully cleaned of adherent connective tissue. Then a 5 mm section from the proximal end of the mesenteric artery–aorta junction was cut off and the following 3 mm section was used in the experiments. The endothelium-intact rings (one from each rat) were placed between stainless-steel hooks (diameter 0·15 mm) and mounted in a standard organ bath chamber (volume 10 ml) in Krebs solution (composition, as above, 37°C), and oxygenated with O2–CO2 (95:5; AGA). The rings were initially equilibrated for 1 h with a resting tension of 1·0 g. The force of contraction was measured with an isometric force-displacement transducer (EMKA Technologies, Paris, France).
The vascular reactivity of the mesenteric arteries was measured by adding vasoactive agents directly into the chambers either cumulatively (acetylcholine (ACh), sodium nitroprusside (SNP)) or as a single dose (phenylephrine (PE), KCl). The concentrations henceforth are the final concentrations in the organ chamber. The rings were equilibrated for 20–30 min between different experiments and washed two or three times with Krebs solution.
After the initial equilibration period, arterial rings were exposed to a high-K+-containing Krebs solution (60 mm) until reproducible contractile responses were obtained. The high-K+ solution was prepared by equimolar substitution of NaCl by KCl. Thereafter, arteries were pre-contracted with 1 μm-PE, and the relaxation to 1 μm-ACh was determined to check the presence of functional endothelium.
Endothelium-dependent relaxation of the mesenteric arteries was studied by pre-contracting the arterial rings with 1 μm-PE and constructing a concentration-response curve to ACh (1 nm–10 μm) in the absence of inhibitors and in the presence of: (1) cyclo-oxygenase (COX) inhibitor diclofenac (3 μm); (2) diclofenac and NO synthase (NOS) inhibitor N G-nitro-l-arginine methyl ester (l-NAME) (100 μm); and (3) diclofenac, l-NAME, apamin (0·1 μm) and 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM-34) (1 μm), inhibitors of small- and intermediate-conductance Ca-activated K channels, SKCa and IKCa, respectively. Arterial rings were exposed to different inhibitors 20 min before the PE pre-contraction.
Endothelium-independent relaxation of the mesenteric arteries was assessed by pre-contracting the arteries with 1 μm-PE and constructing a concentration–response curve to SNP (1 nm–10 μm).
ACh- and SNP-induced relaxations are calculated as the percentage of PE-induced (1 μm) pre-contraction. The vascular contractile responses are expressed in grams.
Compounds
Acetylcholine chloride, diclofenac sodium salt, l-NAME, (R)-( − )-phenylephrine hydrochloride, SNP and TRAM-34 were purchased from Sigma-Aldrich (St Louis, MO, USA). Apamin was from Latoxan (Valence, France). Apamin and diclofenac were dissolved in distilled water and TRAM-34 in dimethyl sulfoxide, while all the other compounds were dissolved in Krebs solution.
Statistical analysis
All data are presented as mean values with their standard errors. Statistical analysis (except for microarray data) was performed using GraphPad Prism® software (version 4.02; GraphPad Software, Inc., La Jolla, CA, USA). One-way ANOVA followed by Bonferroni's multiple-comparison test were used to compare groups. When the data consisted of repeated observations at successive drug concentrations, ANOVA for repeated measurements was applied in order to investigate between-group differences. Differences were considered significant when P < 0·05.
RNA isolation, cDNA preparation and hybridisation
The GeneChip® Rat Gene 1.0 ST Array (Affymetrix, Santa Clara, CA, USA) was applied to produce DNA microarray gene chips from aortic samples of studied rats (n 3 per group). Frozen aortic samples were homogenised and total RNA was prepared by TRIzol® (Invitrogen, Carlsbad, CA, USA) and purified using an RNeasy Mini Kit (Qiagen). Total RNA was measured by a NanoDrop® Spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE, USA) and sample quality was assessed by an Agilent 2100 Bioanalyzer (Agilent Technologies Inc., Santa Clara, CA, USA). A quantity of 100 ng of total RNA was used to generate single-stranded cDNA by the Ambion® WT Expression Kit (Applied Biosystems, Austin, TX, USA). Thereafter, the Gene Chip WT Terminal Labeling Kit (Affymetrix) was used for sample fragmentation, labelling and hybridisation for cartridge arrays. Arrays were washed, stained and scanned according to the manufacturer's instructions on the GeneChip® Rat Gene 1.0 ST Array (Affymetrix) covering over 27 000 genes.
Microarray data analysis
The data analysis was done using Anduril(Reference Ovaska, Laakso and Haapa-Paananen40). Data were normalised with the robust multichip average (RMA) method(Reference Irizarry, Bolstad and Collin41). The median value of three sample replicates was used to calculate differentially expressed genes. P values were produced by two-sided Student's t tests. P values were corrected for multiple hypotheses by the false discovery rate method(Reference Benjamini and Hochberg42). Pathway analysis was performed using signalling pathway impact analysis, which combines enrichment and pathway perturbation analysis(Reference Tarca, Draghici and Khatri43). Enrichment analysis counts the amount of differentially expressed genes in a given pathway. Perturbation analysis captures the topology of the pathway and the position of the gene in that pathway. Differentially expressed genes, where the fold change (fc) was < 0·833 or > 1·2 and P < 0·05, were included in the signalling pathway impact analysis.
Results
Intake of tripeptides, plant sterols and minerals
At the beginning of the experiment, rats in the milk, active milk and water groups weighed 321 (sem 3), 326 (sem 5) and 305 (sem 5) g on average, respectively. The weight gain of the rats during the study was 47 (sem 4), 41 (sem 2) and 39 (sem 7) g in the milk, active milk and water groups, respectively (NS).
Daily intakes of feed, drinking fluid, tripeptides, plant sterols and minerals are presented in Table 1. Rats in the water group ingested significantly more feed and consumed significantly less drinking fluid than the rats in the milk and active milk groups. Also Ca, K and Na intakes were significantly lower in the water group compared with the other groups. However, there were no statistically significant differences between the milk and active milk groups in any of these parameters besides tripeptide and plant sterol intake (Table 1).
Table 1 Daily intake of feed, drinking fluid, tripeptides, plant sterols and minerals in spontaneously hypertensive rats after 6 weeks of treatment with milk or active milk (containing tripeptides and plant sterols)†
(Mean values with their standard errors)
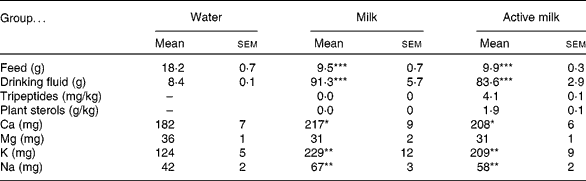
Mean value was significantly different from that of the group that received water: *P < 0·05, **P < 0·01, ***P < 0·001.
† All variables except plant sterols and tripeptides are presented as mg or g per rat per d. Intakes of tripeptides and plant sterols are calculated as mg or g per kg body weight per d. Mineral intakes are presented as feed + drinking fluid in total.
Blood pressure
Long-term treatment (6 weeks) either with milk or active milk decreased the SBP of the SHR (Fig. 1). The decrease was statistically significant after 2 weeks of treatment (P < 0·001; milk and active milk v. water). Although both the milk and active milk decreased SBP, the decline was stronger with the active milk, which contained tripeptides and plant sterols (P < 0·05). An upward trend back towards the baseline SBP values was seen in the milk group. At the end of the experiment, SBP was 16 mmHg lower in the active milk group compared with the water group.
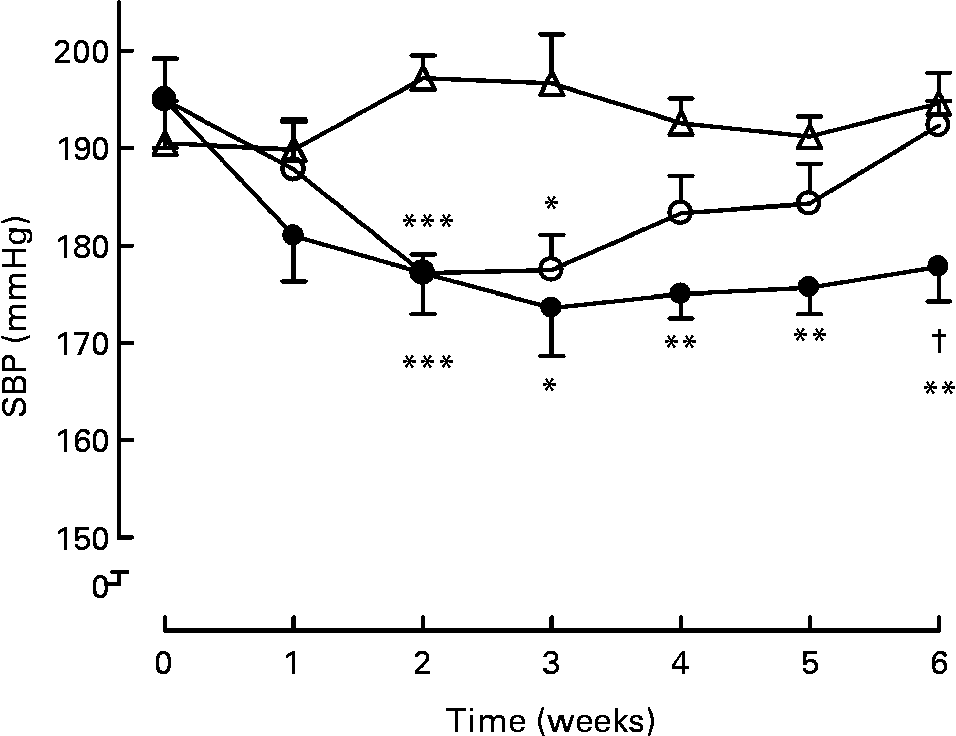
Fig. 1 Systolic blood pressure (SBP) during the 6-week treatment with milk (○), active milk (●) or water (△). Values are means (n 6 per group), with standard errors represented by vertical bars. Mean value was significantly different from that of the group that received water: *P < 0·05, **P < 0·01, ***P < 0·001. † Mean value was significantly different from that of the group that received milk (P < 0·05).
The heart rate of the rats was unaffected by the 6-week treatment with the milk or active milk compared with the water group (data not shown).
Vascular tone
The endothelial function of the mesenteric arteries, assessed by ACh-induced endothelium-dependent relaxation, was impaired in all SHR (Fig. 2(a)). However, treatment with active milk partly normalised this. The mesenteric arteries of rats treated with active milk for 6 weeks showed significantly better relaxation to ACh compared with the milk and water groups (P < 0·01 and P < 0·05, respectively). COX inhibition with diclofenac improved the ACh-induced relaxation in all groups; however, the active milk group was still superior to the other groups (P < 0·05, active milk v. milk; P < 0·001, active milk v. water) (Fig. 2(b)). NOS inhibition with l-NAME along with COX inhibition greatly attenuated the ACh-induced responses, demonstrating that relaxation was mediated mainly via NO, with endothelium-derived hyperpolarising factor (EDHF) playing only a minor role (Fig. 2(c)). However, the mesenteric arteries of rats from the active milk group still showed some relaxation whereas the response was almost abolished in the other groups, suggesting that the active milk treatment was associated with amelioration in EDHF-dependent responses (P < 0·05, active milk v. water; P < 0·01, active milk v. milk). Finally, apamin and TRAM-34, inhibitors of small- and intermediate-conductance Ca-activated K channels SKCa and IKCa, totally abolished the relaxation in all groups (Fig. 2(d)).

Fig. 2 Acetylcholine (ACh)-induced endothelium-dependent relaxations of spontaneously hypertensive rat mesenteric arteries treated with milk (○), active milk (●) or water (Δ) for 6 weeks. Values are means (n 5–6 per group), with standard errors represented by vertical bars. (a) Relaxation without inhibitors. *P < 0·05 active milk v. water; ††P < 0·01 active milk v. milk. (b) Relaxation after 20 min pre-incubation with diclofenac (3 μm). ***P < 0·001 active milk v. water; †P < 0·05 active milk v. milk. (c) Relaxation after 20 min pre-incubation with N G-nitro-l-arginine methyl ester (l-NAME) (100 μm) and diclofenac. *P < 0·05 active milk v. water; ††P < 0·01 active milk v. milk. (d) Relaxation after 20 min pre-incubation with diclofenac, l-NAME, apamin (0·1 μm) and 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM-34) (1 μm). There were no significant differences.
SNP-induced endothelium-independent relaxation reached 100 % in all groups (Fig. 3). The mesenteric arteries of rats from the water group had decreased sensitivity to SNP compared with those of the rats in the active milk group (P < 0·05). The difference between the milk and water groups was not statistically significant (NS).

Fig. 3 Sodium nitroprusside (SNP)-induced endothelium-independent relaxations of spontaneously hypertensive rat mesenteric arteries treated with milk (○), active milk (●) or water (Δ) for 6 weeks. Values are means (n 6 per group), with standard errors represented by vertical bars. *P < 0·05 active milk v. water.
PE- or KCl-induced contractile responses did not differ between the groups (data not shown).
Gene expression
To investigate whether the 6-week intake of tripeptides and plant sterols changed the gene expression in SHR aorta, DNA microarray data from the rats that received either milk or active milk (containing tripeptides and plant sterols) were compared. In total, twenty-seven genes were up-regulated and eighty-two down-regulated. To obtain more information about the mechanisms behind the antihypertensive and vasoprotective effects of tripeptides, changes in the expressions of genes linked to the RAS, vascular function and inflammation were first analysed and are presented in Table 2. Although changes in the gene expressions were small, ACE and IL-13 receptor were less expressed in the active milk group (fc 0·86, P = 0·047 and fc 0·71, P = 0·0314, respectively). Other genes filling the fc criteria (fc < 0·833 or > 1·2) but not reaching statistical significance (P < 0·05) were angiotensin II type 1A receptor (fc 0·82), chymase (fc 0·82), connexin-40 (fc 0·82), endothelial NO synthase (eNOS) (fc 0·65), endothelin-1 (fc 0·50), intermediate-conductance K-activated Ca channel (IKCa) (fc 0·78) and COX-2 (fc 0·78).
Table 2 Changes in selected genes linked to the renin–angiotensin system, vascular function and inflammation detected in the aorta of spontaneously hypertensive rats after 6 weeks of treatment with milk or active milk (containing tripeptides and plant sterols)

nd, Not detected.
* Fold change in the active milk group compared with the milk group; mean of three samples.
† Significant differences between the active milk and milk groups were evaluated by the two-sided Student's t test.
Thereafter, all differentially expressed genes (fc < 0·833 or > 1·2 and P < 0·05) were subjected to pathway analysis. In total, twenty signalling pathways, presented in Table 3(Reference Kanehisa, Goto and Furumichi44), were changed in the active milk group compared with the milk group. The most changed pathways were the hedgehog signalling pathway, chemokine signalling pathway and the leucocyte transendothelial migration pathway.
Table 3 Results for signalling pathway impact analysis (SPIA)

KEGG, Kyoto Encyclopedia of Genes and Genomes; NDE, number of differentially expressed genes on the pathway; pG, combination of pPERT (probability of observing a more intense perturbation than a perturbation factor by chance) and pNDE (P value to observe NDE number of genes on the pathway by chance).
Organ weights
Certain organs were dissected and weighed at the end of the experiment. Heart weight:body weight and left ventricle:body weight ratios were higher in the rats in the active milk group compared with the rats in the water group (P < 0·01) (Table 4). The rats in the milk group had higher adrenal gland weight compared with those in the water group (P < 0·05).
Table 4 Organ weight:body weight ratios (g/g ×1000)
(Mean values with their standard errors)
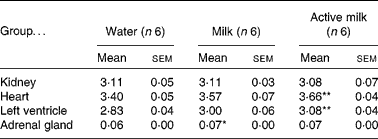
Mean value was significantly different from that of the group that received water: *P < 0·05, **P < 0·01.
Discussion
The present study investigated the long-term effects of a fermented milk product containing tripeptides and plant sterols (active milk) on blood pressure, vascular tone and aortic gene expression in SHR with established hypertension. The main finding, not previously described, was that the long-term treatment with active milk decreases the SBP of SHR with established hypertension. In addition, endothelial function is significantly ameliorated by active milk involving both NO- and EDHF-mediated mechanisms. Interestingly, milk without these active constituents also lowers blood pressure, although to a lesser degree.
In clinical studies, tripeptide-containing fermented milk products have been found to decrease SBP and diastolic blood pressure by approximately 5 and 2 mmHg, respectively, and the effect has been shown to be stronger in clearly hypertensive subjects than in subjects with mild hypertension(Reference Pripp13, Reference Xu, Qin and Wang14). Previous experimental studies have shown that long-term treatment of young SHR with tripeptide-containing milk products attenuates the development of hypertension(Reference Sipola, Finckenberg and Korpela16, Reference Jäkälä, Hakala and Turpeinen18, Reference Jauhiainen, Collin and Narva20, Reference Jäkälä, Turpeinen and Rajakari21, Reference Nakamura, Masuda and Takano45), and that SBP may be transiently lowered by 22 to 26 mmHg by a single dosing of the tripeptides(Reference Nakamura, Yamamoto and Sakai15, Reference Masuda, Nakamura and Takano22). The present study demonstrated for the first time that established high blood pressure may be constantly lowered by a fermented milk product enriched with tripeptides and plant sterols as well; active milk decreased SBP by 16 mmHg in SHR, which were aged 21 weeks at the end of the experiment.
In the present study, the active milk also contained plant sterols. Plant sterols and stanols effectively lower LDL-cholesterol, but do not seem to decrease blood pressure as such in human subjects(Reference Tapola, Lyyra and Karvonen7, Reference Hallikainen, Lyyra-Laitinen and Laitinen8). Therefore, we do not assume that plant sterols contributed to the antihypertensive effect observed in the active milk group. In previous studies, plant sterols have rather increased than decreased blood pressure of rats(Reference Chen, Gruber and Swist46). When plant sterols and tripeptides have been combined together, the attenuating effect on the development of blood pressure has been less than with tripeptides alone(Reference Jäkälä, Pere and Lehtinen17).
Milk, as such, also decreased blood pressure in the present study, although not to a similar degree as the active milk containing tripeptides and plant sterols. An inverse association with milk consumption and blood pressure has been demonstrated in several intervention studies in human subjects(Reference Appel, Moore and Obarzanek10, Reference Hilary Green, Richards and Bunning47–Reference Van Beresteijn, Van Schaik and Schaafsma49). Although acute antihypertensive effects in SHR have been shown by several isolated or synthesised milk peptides(Reference Nurminen, Sipola and Kaarto50–Reference Miguel, Gómez-Ruiz and Recio52) and by different casein or whey hydrolysates(Reference Costa, Almeida and Netto53, Reference Geerlings, Villar and Hidalgo Zarco54), long-term intervention studies using pure milk or fermented milk are scarce. Miguel et al. (Reference Miguel, Muguerza and Sánchez55) reported that milk fermented by L. delbrueckii and Streptococcus thermophilus (without known antihypertensive peptides) attenuated the development of hypertension in SHR during an 8-week period slightly but significantly. In contrast, Sipola et al. (Reference Sipola, Finckenberg and Korpela16) did not observe any effect on SBP by a 14-week skimmed milk treatment. The fermentation process itself may partly explain why some of the fermented milk products decrease blood pressure, as it is possible that other, still unknown antihypertensive peptide sequences are cleaved from the milk protein during milk processing. Milk is rich in minerals (Ca, K), which most probably also contribute to the antihypertensive effect. For example, the antihypertensive effect of Ca has been shown quite uniformly in SHR(Reference Mäkynen, Kähönen and Arvola25, Reference Pörsti, Arvola and Wuorela56, Reference Tolvanen, Mäkynen and Wu57).
Active milk significantly improved the endothelium-dependent relaxation of mesenteric arteries. Typically, from the age of 10 to 12 weeks, SHR show marked endothelial dysfunction(Reference Bernatova, Conde and Kopincova58). There seems to be no or little alteration in the production of NO. However, a marked attenuation in the EDHF-mediated component is observed in SHR resistant arteries, where endothelium-derived hyperpolarisations participate in endothelium-dependent relaxations(Reference Onaka, Fujii and Abe59–Reference Michel, Man and Man61). Therefore, NO and possibly prostanoids to some extent are mostly responsible for the relaxation. This was seen also in the present study, as the relaxations were almost abolished after blocking COX and NOS. Nonetheless, the use of active milk improved the impaired EDHF-mediated responses. This has been observed also with antihypertensive drugs, most significantly with RAS inhibitors(Reference Goto, Fujii and Kansui62). Thus, the beneficial effect of active milk on endothelial function was shown to be related to both NO and EDHF.
Both the active milk and milk increased the sensitivity of the mesenteric arteries to the endothelium-independent vasodilator SNP (although only the active milk significantly). In previous studies, fermented milk products containing tripeptides and plant sterols have not had any effect on SNP-induced vasorelaxation(Reference Jäkälä, Pere and Lehtinen17, Reference Jäkälä, Hakala and Turpeinen18). In the present study the animals were older and thus arterial dysfunction may have been further progressed, so the positive effect of fermented milk products on smooth muscle function is more evident. Fermented milk products are high in l-cysteine(Reference Chandan and Chandan63), which has been shown to augment endothelium-independent relaxation of isolated arteries(Reference Arvola, Pörsti and Vuorinen64). Thus, the presence of l-cysteine in the active milk and milk could possibly explain the increased sensitivity of the mesenteric artery to SNP.
DNA microarray analysis showed that treatment with tripeptides and plant sterols produced only mild changes in aortic gene expression. There are not many studies describing diet-induced changes in aortic gene expression, but a few studies in which drugs have been used have been published(Reference Wang, Lee and Brazeau65, Reference Abd Alla, Langer and Elzahwy66). In the present study, the ACE gene was slightly down-regulated, which is in line with previous findings considering the ACE-inhibitory activity of the tripeptides(Reference Jäkälä, Pere and Lehtinen17, Reference Jäkälä, Hakala and Turpeinen18, Reference Nakamura, Yamamoto and Sakai33, Reference Lehtinen, Jauhiainen and Kankuri34). Treatment with ACE-inhibitor drugs has also been shown to decrease ACE expression in SHR(Reference Linz, Jessen and Becker67, Reference Miguel-Carrasco, Zambrano and Blanca68).
Yamaguchi et al. (Reference Yamaguchi, Kawaguchi and Yamamoto69) investigated the effects of tripeptides on aortic gene expression by giving pure tripeptides to SHR for 5 d. Changes in gene expression were not large in this study either, but significant up-regulation of eNOS and connexin-40 genes were detected. We observed quite opposite effects, as both these two genes were rather down- than up-regulated in the present study. However, there may be distinct differences in gene expression profiles as regards short- and long-term treatment (5 d v. 6 weeks). In some studies, the up-regulation of aortic eNOS has been associated with the improvement of endothelial function(Reference Rodriguez-Rodriguez, Herrera and de Sotomayor70) and reduction of blood pressure in SHR(Reference Li, Witte and August71). However, there are also studies where no changes in eNOS expression have been detected despite improved endothelial function(Reference Rush, Quadrilatero and Levy72). Also, it must be taken into account that there are a considerable number of differences in gene expression profiles between SHR from different sources, not to speak of other models of experimental hypertension(Reference Okuda, Sumiya and Iwai73). As in the present study the active milk treatment affected the NO-dependent relaxation slightly but significantly, it is possible that it either improved NO bioavailability or increased eNOS activity instead of directly affecting eNOS gene expression.
Although treatment with the active milk did not cause many changes in the gene expression, it clearly lowered blood pressure of the rats and improved endothelial function. It may be that several smaller changes cause the beneficial effects observed with tripeptides if they occur in parallel. The affected signalling pathways involved down-regulation of several genes that are linked to inflammatory processes (chemokine receptor, cell adhesion molecules, IL receptor). This is interesting, as the importance of chronic, low-grade inflammation in the pathogenesis of hypertension and vascular diseases has been recently acknowledged(Reference Marchesi, Paradis and Schiffrin74). Long-term treatment with an ACE inhibitor captopril has been shown to decrease and increase the gene expression of pro- and anti-inflammatory cytokines, respectively, in SHR left ventricles along with blood pressure reduction as well(Reference Miguel-Carrasco, Zambrano and Blanca68). The role of tripeptides in vascular inflammation has not been studied before and perhaps also deserves further attention.
As was observed in a kinetic study with normotensive Sprague–Dawley rats(Reference Jauhiainen, Wuolle and Vapaatalo75), tripeptides seem to accumulate in tissues (for example, aorta, kidney). Therefore, it has been suggested that the local RAS would perhaps be more important as regards the antihypertensive and vasoprotective effects of tripeptides, especially as tripeptides have short plasma half-lives(Reference Foltz, Meynen and Bianco76). Although we were able to obtain tissue samples and thus reach the components of the local RAS in the present study, the effects of the active milk treatment on the expression levels of genes linked to the RAS and vascular function were mild. It may be that more changes in the gene expression levels would have been observed by using some other organ, for example, the kidney. In addition to tripeptides, the active milk also contained plant sterols, which may have influenced the gene profile. In the study of Chen et al. (Reference Chen, Gruber and Swist46), 5 weeks of plant sterol or stanol treatment elevated mRNA levels of neuronal NOS or ACE1 and eNOS, respectively, in the kidney of stroke-prone SHR. This suggests that tripeptides and plant sterols may have partly opposing actions and that, despite clear antihypertensive and vasoprotective effects observed with the active milk in the present study, changes in the genes regulating these effects were not large enough to be detected in the analyses.
In conclusion, we describe for the first time that long-term intervention with a fermented milk product enriched with bioactive tripeptides and plant sterols can decrease already established, severe hypertension in addition to the prevention of the development of experimental hypertension. Whether this is related to improved endothelial function and vasodilatation remains open.
Acknowledgements
The study was carried out within the Valio Ltd Milk Protein Project funded by the Finnish Funding Agency for Technology and Innovation (Tekes).
Elina Lausvaara (Valio Ltd) is thanked for the preparation of the study products, Outi Monni's group (Institute of Biomedicine, Medical Biochemistry and Developmental Biology, Genome-Scale Biology Research Program, University of Helsinki) for the microarray work and Lilli Saarinen (Computational Systems Biology Laboratory, Institute of Biomedicine and Genome-Scale Biology Research Program, University of Helsinki) for the microarray data analysis.
The authors' responsibilities were as follows: all authors contributed to the planning, design and decision making of the study; P. I. E. and A. S. K. conducted the research; P. I. E. analysed the data and wrote the manuscript; P. I. E. and H. V. had primary responsibility for the final content. All authors read and approved the final manuscript.
As regards conflicts of interest, A. M. T. is an employee of the Finnish dairy company, Valio Ltd. There are no other conflicts of interest.









