Non-technical Summary
The late Miocene (11.5–5.5 million years ago) was a period of faunal change for small mammal communities. The evolution of several climatic parameters has greatly impacted faunas from Europe, and the surviving species also had to deal with the competitive pressure of new migrant species into Europe. In this context, mammal groups having high sensitivity to climatic parameters, such as temperature and humidity, show peculiar patterns of evolution. This is the case for the Erinaceidae (hedgehogs and gymnures) and the extinct family Dimylidae, well recorded in the fauna from the late Miocene of Slovakia. At least six Erinaceidae and two Dimylidae were present in Slovakia during that time, as shown by material extracted from the localities of Borský Svätý Jur, Krásno, Pezinok, Šalgovce, Studienka, and Triblavina. Both families were extremely abundant during the early part of the late Miocene, the Vallesian (11.5–9.0 million years ago), supporting the idea that central Europe played an important role in the preservation of high paleodiversity of insectivore species. However, the abundance of the Erinaceidae and Dimylidae strongly declined afterward, eventually leading to the extinction of the Dimylidae soon after the Vallesian. On a smaller scale, the material described from the late Miocene of Slovakia brings a lot of new information about the morphology, variability, and phylogeny of the identified species, namely ‘Schizogalerix’ voesendorfensis, Schizogalerix cf. S. moedlingensis, Lantanotherium sanmigueli, Atelerix cf. A. depereti, Atelerix aff. A. depereti, cf. Postpalerinaceus sp. indet., Erinaceinae gen. indet. sp. indet., Plesiodimylus chantrei, and Metacordylodon aff. M. schlosseri.
Introduction
The Vallesian (11.2–9.9 Ma) represents a period of major faunal turnover among mammals (Agustí and Moyà-Solà, Reference Agustí and Moyà-Solà1990; Van der Made et al., Reference Van der Made, Morales and Montoya2006; Daxner-Höck et al., Reference Daxner-Höck, Harzhauser and Göhlich2016). Within the order Eulipotyphla, the decline of Aragonian (17.2–11.2 Ma) taxa was partially counterbalanced by migratory waves into Europe with the return of warmer and more humid environments (Furió and Agustí, Reference Furió and Agustí2017). These migrations had their origins in Anatolia and western to eastern Asia (Ziegler, Reference Ziegler2006a; Van den Hoek Ostende et al., Reference Van den Hoek Ostende, Furió, Madern and Prieto2016; Cailleux et al., Reference Cailleux, Chaimanee, Jaeger and Chavasseau2020). As a result, the Vallesian is characterized by high abundance of insectivore taxa in Europe (Furió et al., Reference Furió, Van den Hoek Ostende, Agustí and Minwer-Barakat2017) and the overall success of forest-adapted taxa. Notably, Eulipotyphla were especially diversified in central Europe (e.g., Van Dam, Reference Van Dam2004; Van den Hoek Ostende et al., Reference Van den Hoek Ostende, Bilgin, Braumuller, Hír and Joniak2020), which is related to wetter environmental conditions (Furió et al., Reference Furió, Casanovas-Vilar and Van Den Hoek Ostende2011b). However, the end of the Vallesian is characterized by a deterioration of these conditions, favored by the progressive settlement of seasonality (Van Dam, Reference Van Dam2006).
From a biogeographical point of view, central Europe is a strategic area to study the record of newly arriving taxa and the pattern of faunal dynamics in Eulipotyphla during the Miocene because it constituted an important migration corridor. The evolution of Lake Pannon (Magyar et al., Reference Magyar, Geary and Müller1999) surely had an additional regional effect. Slovakia, strategically situated in the northern part of the Pannonian Basins System (PBS; Fig. 1), has preserved several vertebrate sites of major importance that help to understand the faunal evolution of central Europe, because it was affected during the late Miocene by uplift of the western Carpathians and the regression of the megalake (Joniak et al., Reference Joniak, Šujan, Fordinál, Braucher, Rybár, Kováčová and Kováč2020).
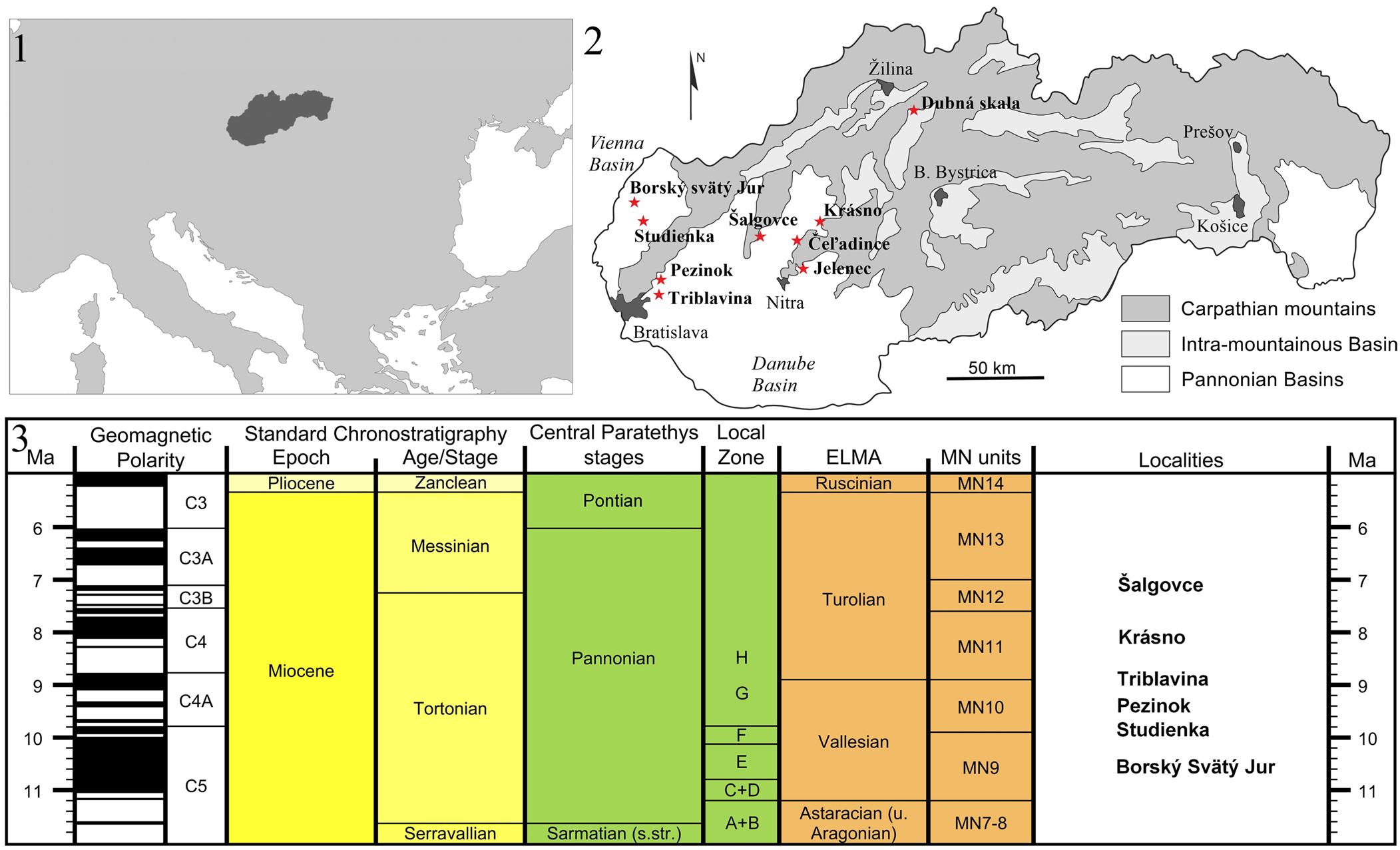
Figure 1. (1) Location of Slovakia within Europe. (2) Map of Slovakia showing the main geological structures and all of the late Miocene localities containing small mammals (red stars). (3) Preliminary stratigraphical correspondence of the localities studied in the present work (created with the software TimeScale Creator [v. 8.0, https://timescalecreator.org/]; MN units modified after Van Dam et al., Reference Van Dam, Mein, Garcés, van Balen, Furió and Alcalá2023).
Recent excavations have yielded a series of late Miocene localities, testifying the rich micromammal record of the area (Fig. 1). The first results on the rodents of these localities have already been published (e.g., Joniak, Reference Joniak2005, Reference Joniak2016; Joniak and Šujan, Reference Joniak and Šujan2020). However, whereas the late Miocene Eulipotyphla and Chiroptera from the Austrian part of the Danube and Vienna basins (northwestern part of the PBS; Fig. 1) have been fully described (e.g., Bachmayer and Wilson, Reference Bachmayer and Wilson1970, Reference Bachmayer and Wilson1978; Ziegler, Reference Ziegler2006b, Reference Ziegler2006a), no detailed work has been done on the Slovak material. As a first step toward understanding the late Miocene Eulipotyphla and Chiroptera from Slovakia, this work focuses on Erinaceidae and Dimylidae, two families frequently encountered in the early-late Miocene.
Geological setting
The Miocene sedimentary history of Slovakia is closely connected to the evolution of the western Carpathians and the Paratethys Sea. The sedimentary sequences of the large Danube Basin demonstrate well the coevolution of these structures (Kováč et al., Reference Kováč, Márton, Oszczypko, Vojtko and Hok2017). The formation of the extensive Danube Basin began in the early Miocene with the stretching of the forearc basins at the western margin of the Carpathians. The Vienna Basin and the Slovak parts of the Danube Basin constituted the northwesternmost part of the PBS (Fig. 1) (Kováč et al., Reference Kováč, Synak, Fordinal, Joniak and Toth2011), which was covered by a large lake system during the late Miocene (Harzhauser and Mandič, Reference Harzhauser and Mandič2008; Kováč et al., Reference Kováč, Synak, Fordinal, Joniak and Toth2011; Magyar et al., Reference Magyar, Radivojević, Sztanó, Synak, Ujszászi and Pócsik2013). The PBS was still connected to the eastern Paratethys Sea during the early Sarmatian (ca. 13.5 Ma, according to Magyar et al., Reference Magyar, Geary and Müller1999) until the middle–late Miocene transition when Lake Pannon emerged (ca. 12 Ma according to Magyar et al., Reference Magyar, Geary and Müller1999; Joniak and Šujan, Reference Joniak and Šujan2020). The progressive retreat of the Pannonian lake during the late Miocene (after ca. 9.5 Ma, according to Magyar et al., Reference Magyar, Geary and Müller1999) was characterized by the progradation of the paleo-Danube shelf margin from northwest to southeast, leading to the formation of a shift of different environments along Lake Pannon.
The large water mass of Lake Pannon reached its maximum extent in the early Vallesian, evidenced by the predeltaic deposits from Borský Svätý Jur (MN9) in the Slovak part of the Vienna Basin (Fig. 1) and by the slightly younger swamp deposits from Studienka (Sabol et al., Reference Sabol, Joniak., Bilgin, Bonilla-Salomón, Cailleux, Čerňanský, Malíková, Šedivá and Tóth2021). These facies are grouped in the Ivanka Formation, ending with the retreat of Lake Pannon from the Danube Basin (Magyar et al., Reference Magyar, Radivojević, Sztanó, Synak, Ujszászi and Pócsik2013).
The paleo-Danube River entered the PBS ca. 11–10 Ma from the western North Alpine Foreland Basin and initially went through the Vienna Basin (Šujan et al., Reference Šujan, Braucher, Kováč, Bourlčs, Rybár, Guillou and Hudáčková2016; Fig. 1). A new large deltaic structure in the northwestern part of the PBS was maintained by large water and sediment inputs from the paleo-Danube and other rivers. This period is represented in the Slovak Danube Basin by the Beladice Formation, containing the MN10 locality of Pezinok (Joniak, Reference Joniak2016; Fig. 1). At that time, the area was very humid, potentially due to orographic conditioned precipitation from the Alpine-Carpathian Arc (Utescher et al., Reference Utescher, Erdei, Hably and Mosbrugger2017) and a system of shallow lakes and channels formed on the deltaic shelf. Consistently, the Beladice Formation is predominantly composed of coastal, lagoonal, and deltaic facies, ending with a heterogeneous southeastern progradation of the lake (Joniak and Šujan, Reference Joniak and Šujan2020). This process was accompanied by the final decline of broadleaved evergreen vegetation in the northern part of the basin system (Utescher et al., Reference Utescher, Erdei, Hably and Mosbrugger2017).
The deltaic phase was followed by the alluvial deposition of the Volkovce Formation in the study area. This phase is characterized by a noticeable spatial heterogeneity in deposits, which were strongly influenced by river dynamics and sediment supply. This is well-documented in sediments including the MN11 locality of Triblavina (Fig. 1) where the Volkovce Formation represent a transition from poorly drained to well-drained floodplains (Joniak and Šujan, Reference Joniak and Šujan2020).
Krásno (Fig. 1), another MN11 small-mammal locality in Slovakia, yielded an abundant collection of material displaying a typical Turolian fauna. However, the depositional conditions in Krásno significantly differ from those in Triblavina. The fauna of Krásno was found within the Hlavina Member, which is characterized by the deposition of freshwater limestones alternating with calcareous silts and clays. Deposits of the Hlavina Member developed along active fault systems during uplift of the surrounding Carpathian massifs and continued at least until MN12, as documented by the fauna from Šalgovce (Sabol et al., Reference Sabol, Joniak., Bilgin, Bonilla-Salomón, Cailleux, Čerňanský, Malíková, Šedivá and Tóth2021; Fig. 1), the youngest locality included in this work.
Materials and methods
The material described here comprises 264 isolated teeth of Erinaceidae and 110 isolated teeth of Dimylidae. For Erinaceidae, we mostly follow the measurement methods of Prieto and Rummel (Reference Prieto and Rummel2009), as shown in Figure 2, and the dental terminology of Klietmann et al. (Reference Klietmann, Nagel, Rummel and Van den Hoek Ostende2014a). Additionally, we use the term ‘loph/lophid’ to designate the fusion of two crests, and thus imply a clear and continuous connection between two cusps/cuspids. This includes the ectoloph (postparacrista + premetacrista), the paralophid (postparacristid + preprotocristid), and the protolophid (postprotocristid + premetacristid). Moreover, when the protoconule is distinguishable, the term ‘preprotoconule crest’ is used to indicate the crest labial of the cuspule.
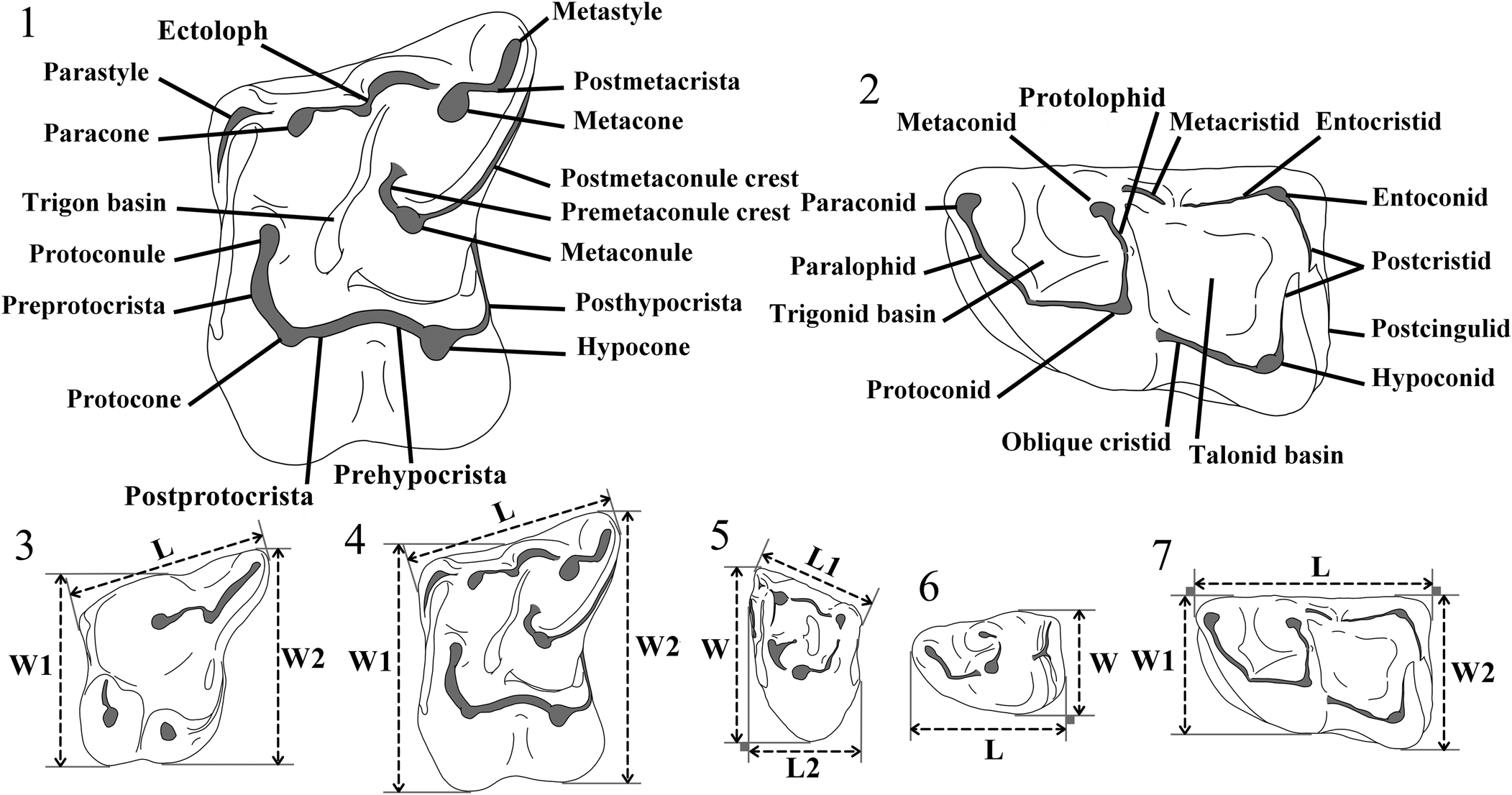
Figure 2. Terminology used for the M1 (1) and m1 (2) of Erinaceidae, and measurement protocols for P4 (3), M1 (4), M3 (5), p4 (6), and m1 (7). L = length; L1 = labial length; L2 = shortest anteroposterior length; W = width; W1 = anterior width; W2 = posterior width.
We follow the measurement methods and dental terminology of Klietmann et al. (Reference Klietmann, Nagel, Rummel and van den Hoek Ostende2014b) for Dimylidae (Fig. 3). The low bulge rarely observed near the paraconid is called ‘accessory cuspid.’

Figure 3. Terminology used for the M1 (1) and m1 (2) of Dimylidae, and measurement protocols for P4 (3), M1 (4), M3 (5), p4 (6), and m1 (7). L = length; W = width; W1 = anterior width; W2 = posterior width.
All measurements are given in millimeters (mm). They were measured using a digital measuring microscope with a mechanical stage and digital measuring clocks. Full data (collection numbers, identifications, and specimen measurements) are provided in Supplementary Data Set 1. Specimens in figures are represented in left orientation; reversed specimens are indicated by an underlined figure number. Unless otherwise noted, scanning electron micrographs (SEM) are shown in occlusal view. Drawings were obtained with a graphic tablet (Wacom Intuos Pro) and the software Autodesk SketchBook (v. 8.7.1, https://www.sketchbook.com). All of the described specimens are housed at the Department of Geology and Paleontology of Comenius University, Bratislava, Slovakia.
Abbreviations used in the text are: H = height; L = length; L1 = labial length; L2 = shortest anteroposterior length; N = number of specimens; W = width; W1 = anterior width; W2 = posterior width.
Repositories and institutional abbreviations
ICP = Institut Català de Paleontologia Miquel Crusafont, Sabadell, Spain; MAFI = Hungarian Geological Institute, Budapest, Hungary; NHMA = Natural History Museum of Augsburg, Germany; NHMV = Natural History Museum of Vienna, Austria; UWPI = Institute of Paleontology, University of Vienna, Austria.
Systematic paleontology
Order Eulipotyphla Waddell, Okada, and Hasegawa, Reference Waddell, Okada and Hasegawa1999
Family Erinaceidae Fischer, Reference Fischer1814
Subfamily Galericinae Pomel, Reference Pomel1848
Tribe Galericini Pomel, Reference Pomel1848
Genus Schizogalerix Engesser, Reference Engesser1980
Type species
Schizogalerix anatolica Engesser, Reference Engesser1980.
Other species
Schizogalerix zapfei (Bachmayer and Wilson, Reference Bachmayer and Wilson1970); S. moedlingensis (Rabeder, Reference Rabeder1973); ‘S.’ voesendorfensis (Rabeder, Reference Rabeder1973); S. pasalarensis Engesser, Reference Engesser1980; S. samartica (Lungu, Reference Lungu1981); S. sinapensis Sen, Reference Sen1990; S. macedonica Doukas in Doukas et al., Reference Doukas, van den Hoek Ostende, Theocharopoulos, Reumer and Schmidt-Kittler1995; S. duolebulejinensis Bi et al., Reference Bi, Wu, Ye, Meng, Yuanqing and Tao1999; S. intermedia Selänne, Reference Selänne, Fortelius, Kappelman, Sen and Bernor2003; and S. evae De Bruijn et al., Reference De Bruijn, Mayda, Van den Hoek Ostende, Kaya and Saraç2006.
Diagnosis
See Van den Hoek Ostende (Reference Van den Hoek Ostende2001, p. 686).
Occurrence
Early–late Miocene of Anatolia (Sen, Reference Sen1990; De Bruijn et al., Reference De Bruijn, Mayda, Van den Hoek Ostende, Kaya and Saraç2006) and Asia (Bi et al., Reference Bi, Wu, Ye, Meng, Yuanqing and Tao1999; Zijlstra and Flynn, Reference Zijlstra and Flynn2015); middle–late Miocene of Africa (Engesser, Reference Engesser1980; Cailleux, Reference Cailleux2021); late-middle and late Miocene of Europe (Bachmayer and Wilson, Reference Bachmayer and Wilson1970; Rabeder, Reference Rabeder1973; Mein, Reference Mein, Agustí, Rook and Andrews1999; Ziegler, Reference Ziegler2006a).
Remarks
The generic attribution of ‘Schizogalerix’ voesendorfensis is put in quotation marks considering the strong similarities found between this species and representatives of Parasorex Meyer, Reference Meyer1865, especially Parasorex socialis Meyer, Reference Meyer1865, as already mentioned by Prieto et al. (Reference Prieto, Angelone, Casanovas-Vilar, Gross, Hír, Van den Hoek Ostende, Maul and Vasilyan2014) and Van den Hoek Ostende et al. (Reference Van den Hoek Ostende, Furió, Madern and Prieto2016).
‘Schizogalerix’ voesendorfensis (Rabeder, Reference Rabeder1973)
Figure 4.1–4.22; Table 1
Holotype
Left M2, UWPI 1909/2/12.
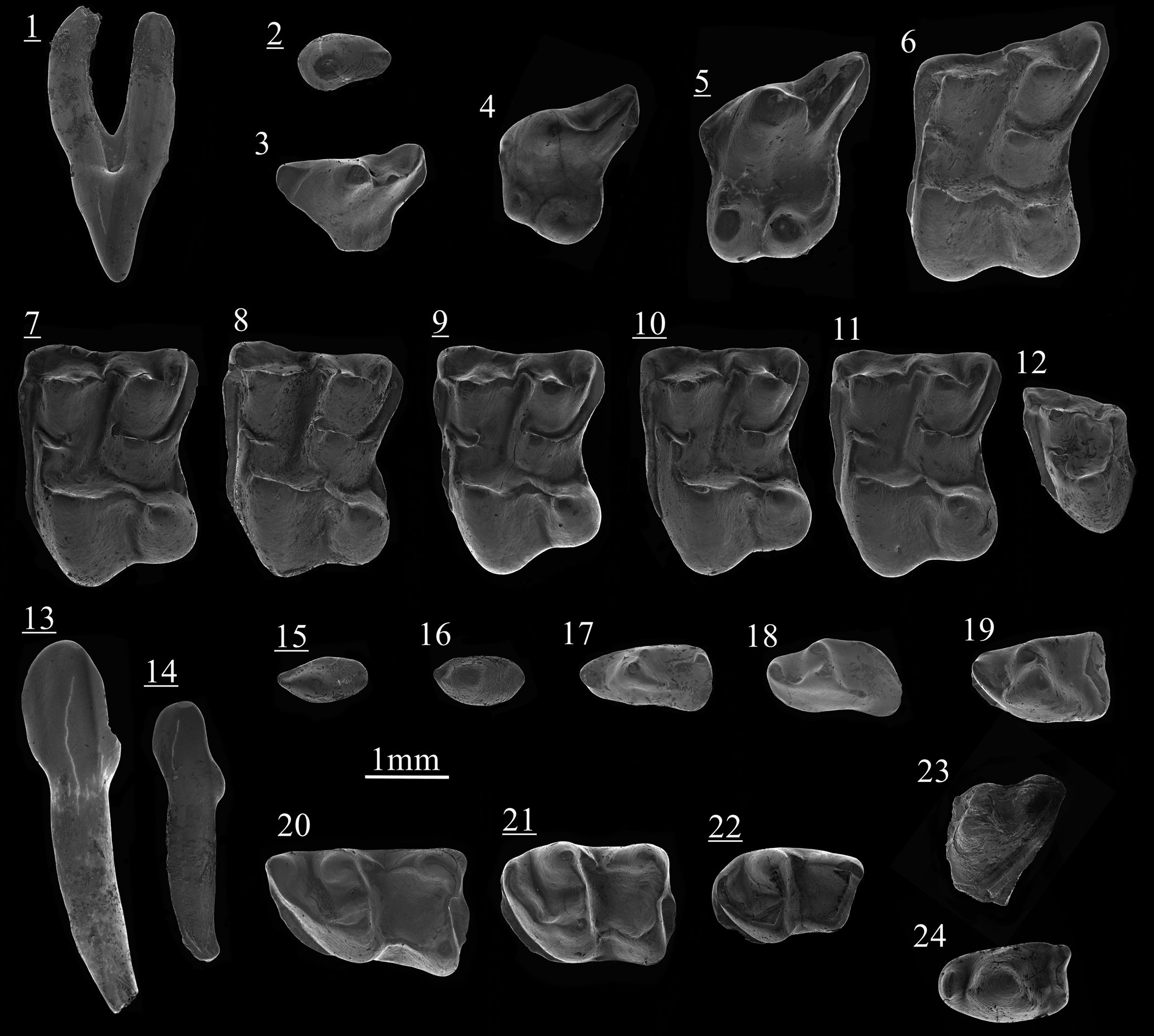
Figure 4. Scanning electron photomicrographs of ‘Schizogalerix’ voesendorfensis (Rabeder, Reference Rabeder1973) from Borský Svätý Jur (1–22) and Schizogalerix cf. S. moedlingensis (Rabeder, Reference Rabeder1973) from Krásno (23, 24): (1) C, BJ213053, labial view; (2) P2, BJ213057; (3) dP3, BJ213180; (4) P3, BJ213172; (5) P4, BJ213071; (6) M1, BJ213014; (7) M2, BJ213029; (8) M2, BJ213033; (9) M2, BJ213035; (10) M2, BJ213045; (11) M2, BJ213047; (12) M3, BJ213075; (13) i1/2, BJ213133, labial view; (14) i3, BJ213029, labial view; (15) p1, BJ213063; (16) p2, BJ213061; (17) dp3, BJ213083; (18) dp4, BJ213092; (19) p4, BJ213091; (20) m1, BJ213140; (21) m2, BJ213154; (22) m3, BJ213158; (23) M1, KR127020; (24) p3, KR127028. Images with underlined numbers are reversed.
Table 1. Measurements (in mm) of ‘Schizogalerix’ voesendorfensis (Rabeder, Reference Rabeder1973) from Borský Svätý Jur, Slovakia. L = length; L1 = labial length; L2 = shortest anteroposterior length; W = width; W1 = anterior width; W2 = posterior width.

Diagnosis
See Prieto et al. (Reference Prieto, Gross, Böhmer and Böhme2010, p. 108).
Occurrence
Late-middle to early-late Miocene (MN7+8, MN9) of central and western Europe (Rabeder, Reference Rabeder1973; Ziegler, Reference Ziegler2000; Prieto et al., Reference Prieto, Gross, Böhmer and Böhme2010, Reference Prieto, Angelone, Casanovas-Vilar, Gross, Hír, Van den Hoek Ostende, Maul and Vasilyan2014; Hír et al., Reference Hír, Venczel, Codrea, Angelone, Van den Hoek Ostende, Kirscher and Prieto2016, Reference Hír, Venczel, Codrea, Rossner, Angelone, Van den Hoek Ostende, Rosina, Kirscher and Prieto2017). The type locality is Vösendorf, Austria (MN9).
Description
The upper canine has two compressed roots and simple morphology. The main cusp is situated slightly anteriorly. There is no posterior shoulder. The P1 is a highly reduced tooth with no cingulum and a single bent cusp. The P2 has similar dimensions as the upper canine but has a less-sharp profile and an extended posterior bulge. An anteroposterior crest is present. The P3 is rounded and molarized. The postparacrista is a strong and moderately curved crest in which the metacone is included. This crest descends from the paracone with an anteroposterior orientation but ends at the posterolabial border of the tooth with an oblique orientation. The paracone is massive. There is no parastyle. The protocone is at the anterolingual border of the tooth and is situated more labial than the hypocone. The high protocone is somewhat compressed anteroposteriorly. The two lingual cusps are separated by a narrow valley. A hardly distinguishable crest starts from the low hypocone and leads to a cingulum running all along the posterior border. The dP3 is a subtriangular element with a paracone carrying a low posterior crest. The metacone and the metastyle are well differentiated. The parastyle is low and elongated. The lingual flange bears only a thin crest starting from the base of the paracone and ending as a minute bulge (protocone; see Fig. 4.3). The P4 is a solid premolar with a slight transverse elongation. The paracone is high and conical. A short parastyle is attached at its anterior base. The posterocrista is a large, short, bipartitioned crest. The postparacrista and premetacrista are merged into a single ridge (ectoloph) displaying an oblique orientation, creating an S-shaped labial margin. The metacone is usually absent (Fig. 4.5); in one specimen, a metacone is distinguishable, creating a slightly stronger carnassial notch. The protocone is an anteroposteriorly compressed cusp, reaching half the height of the paracone. The preprotocrista starts from the anterior side of the protocone and runs on the anterior border until the middle part of the tooth; it is not connected to the parastyle area. The conical hypocone is lower than the protocone. A wide posterior cingulum starts from the hypocone, reaches the posterolabial margin, and closes the posterolingual basin.
The M1 is a quadrangular molar with no oblique elongation. The metacone is a conical cusp higher than the paracone. From its posterior border runs a high and curved metastyle. The S-shaped ectoloph starts from the labial side of the metacone. It is usually complete (N = 11) but is sometimes divided (N = 5; Fig. 4.6). The paracone is subtriangular and always connected to a well-developed parastyle by a thin ridge. The preprotocrista of the protocone is large and always bears a robust protoconule that has a variable base depending on the division of the preprotoconule crest: usually triangular, sometimes circular. The preprotoconule crest is always interrupted by a notch. The protocone is connected to the lower, conical hypocone by a curved crest. The posthypocrista joins the posterior cingulum in 13 of 15 specimens. There is no clear protocone-metaconule connection. The crescentic metaconule is situated in a more anterior position than the metacone and the hypocone. The postmetaconule crest is well developed and connected to the labial part of the postcingulum, isolating it from the shorter lingual part. The premetaconule crest ends at the base of the metacone. A continuous anterior cingulum joins the parastyle. The labial cingulum is always discontinuous.
The M2 has an anterior side wider than the posterior side. The metacone is compressed labial-lingually and the metastyle is shorter and more angular than in M1. The ectoloph is always S-shaped (N = 13; Fig. 4.7–4.11). The parastyle is strong and always connected to the conical paracone (N = 18). The protoconule is strong and the preprotoconule is often divided (nine of 15 specimens). In one molar, the anterior branch of the preprotoconule is connected to the anterior cingulum. The protocone is the strongest cusp, connected to the hypocone by a reverse V-shaped crest. There is no posthypocrista. One of 20 specimens (Fig. 4.8) shows a clear protocone-metaconule connection. The postmetaconule-postcingulum connection is always present, separating the postcingulum into two parts.
The M3 is a small subtriangular tooth showing anteroposterior compression. The triangular paracone is higher than the conical metacone; they are connected by an irregular ectoloph. An elongated parastyle is connected to the base of the paracone. The protocone is the highest cusp. The protocone-metacone connection is continuous whereas the protocone-paracone connection is interrupted by a notch at the base of the paracone. The protoconule is usually not visible. A continuous anterior cingulum joins the parastyle. The posterior cingulum, when present, forms a short median extension (Fig. 4.12).
An edentulous fragment of a mandible has been found, preserving the alveola of m2 and m3, and a part of the ascending ramus. The main characteristics are the small size of the m3 alveoli compared to those of m2, the circular anterior and elliptical posterior alveola of m3, the constricted posterior part of the corpus mandibulae, and the strong ramus.
The i1 is spatulate and asymmetrical. The crown is slightly projected forward, following the curve of the compressed root. The anterior base of the crown is concave whereas the posterior base has a short and low distal shoulder. The i2 is a smaller tooth reaching half the height of i1. The crown is more projected, the mesial concavity fitting with the distal shoulder of the anterior tooth. In three of four specimens, a median furrow is found in the lingual face of the compressed root.
The p1 is a simple monocuspid tooth with an enlarged root in which a median furrow can be distinguished. The only cusp is narrow and in an anterior position. A small bulge is present at the posterior end of the tooth, separated from the cusp by a shallow slope. The p2 is an elliptical two-rooted tooth bearing one circular to subtriangular main cusp in an anterior position. An anterolingual bulge is present; a larger bulge is found at the posterior border of the premolar. The dp3 is a narrow tooth characterized by a low and isolated paraconid. The protoconid is subtriangular with a convex lingual flank. The posterior margin is the broadest part of the tooth. The thin posterior cingulid bears a cuspule. The p3 is a robust, double-rooted premolar with a low, usually independent paraconid. A curved paralophid is sometimes present, connecting the paraconid to the protoconid. The strong main cusp is flattened on its posterior side. A marked cingulid is present along its posterior margin. Rarely, a thin median crest is connected to the postcingulid. The dp4 presents the usual oblique elongation of Galericini. The paraconid is low and the paralophid short. The protoconid has an irregular outline. On its anterolingual side, a well-developed metaconid is attached, almost reaching the height of the protoconid. The posterior area is stretched: the labial part is longer than the lingual one (see Fig. 4.18). The postcingulid is curved. The p4 is a subtriangular tooth with two roots. The paraconid is included in a blade-like paralophid, separated from the triangular protoconid by a small notch. The metaconid is situated more anteriorly than the protoconid and reaches two-thirds its height. The posterior margin has a marked cingulid, higher in its median part. This creates a short basin open on the lingual and labial sides. There is a short anterolabial cingulid below the paralophid. In one of nine specimens, a cingulid is found between the paraconid and the metaconid.
The trigonid of the m1 is of similar length as the talonid. The paraconid is conical and connected to a subtriangular protoconid by a curved paralophid. The metaconid is situated more anteriorly than the protoconid. The trigonid basin is simple and narrow. The talonid area is characterized by two subtriangular cuspids. The entoconid is higher than the hypoconid. A high entocristid occupies the posterolingual border. The notch between the short metacristid and the entocristid is weak. The hypoconid is connected to the trigonid wall by an almost straight oblique cristid. The postcristid runs from the hypoconid, usually ending at the base of the entoconid or rarely reaching the posterior side of that cuspid. The posterior cingulid is strong and always connected to the entoconid, although it sometimes joins the postcristid before reaching the cusp. An anterolabial cingulid is always present, and rarely also a short ectocingulid.
The m2 differs from the m1 by its dimensions and more compressed trigonid (compare Fig. 4.20, 4.21). The paraconid is not differentiable from the curved and low paralophid. The subtriangular protoconid and the conical metaconid are of similar size. The talonid is somewhat longer than the trigonid. The entoconid is elongated and the entocristid high. The entoconid-metaconid connection is interrupted by a stronger notch than in the m1. The hypoconid is subtriangular, lower than the entoconid, and connected to the trigonid wall by a thin oblique cristid. The posterior cingulid is isolated (N = 2) or connected to the entoconid (N = 4). When connected, the posterior cingulid is fused with the curved postcristid. A narrow anterolabial cingulum is present. The ectocingulid is usually lacking, but one specimen bears a minute extension.
The m3 is a small two-rooted molar in which the trigonid is shorter than the talonid. The paraconid is included in the low curved paralophid. The conical metaconid is higher and situated in a more anterior position than the subtriangular protoconid. A strong elongated entoconid dominates the talonid. The metaconid-entoconid connection is weak but not interrupted. The short hypoconid is subtriangular and connected to the entoconid by a hardly distinguishable straight ridge. The anterolabial cingulid is present, but not the posterior one.
Materials
Borský Svätý Jur: one C, one P1, six P2, 13 P3, three dP3, 16 P4, 23 M1, 26 M2, 11 M3, two i1, four i2, one p1, eight p2, two dp3, 13 p3, two dp4, nine p4, 20 m1, 24 m2, 12 m3, one fragment of mandible. See Table 1 for measurements.
Remarks
Schizogalerix is a frequent member of late Miocene faunas in central Europe. Restricted to Anatolia during the early and most of the middle Miocene (Engesser, Reference Engesser1980; De Bruijn et al., Reference De Bruijn, Mayda, Van den Hoek Ostende, Kaya and Saraç2006), a European group emerged during the late-middle Miocene including several species: ‘Schizogalerix’ voesendorfensis (MN8, MN9), S. sarmatica (MN9), S. moedligensis (MN11, MN12), S. zapfei (MN10–MN12), and S. macedonica (MN13). A trend is observed through time with the progressive oblique elongation of P4 and upper molars, the division of the ectoloph, the complexification of M3, and the strength of the postcingulid-entoconid connection on the lower molars. It is worth noting that this group is not constituted by one lineage. The species from Borský Svätý Jur corresponds to the most basal morphological stage of this European group and displays all of the diagnostic features of ‘S.’ voesendorfensis. This species was first recorded in the Hungarian locality of Felsőtárkány 2/3 ca. 12.1 Ma (see Hír et al., Reference Hír, Venczel, Codrea, Angelone, Van den Hoek Ostende, Kirscher and Prieto2016) before its occurrences in the German locality of Gratkorn, ca. 12 Ma (see Prieto et al., Reference Prieto, Gross, Böhmer and Böhme2010). Borský Svätý Jur and Vösendorf represent the last occurrences of this basal Schizogalerix in central Europe. The material from Borský Svätý Jur is morphologically more advanced than the late-middle Miocene occurrences. We found a very similar form in the Spanish locality of Nombrevilla 2, dated 11.9 Ma. Much of the Spanish Vallesian and Turolian material has been identified as Parasorex ibericus (Mein and Martín-Suárez, Reference Mein and Martin-Suárez1993). The relationship between ‘S.’ voesendorfensis and the Iberian species needs to be illuminated, also to decide whether the central European species is best classified as a primitive Schizogalerix or rather indicates convergent evolution by an advanced species of Parasorex. In this respect, it is noteworthy, as Prieto et al. (Reference Prieto, Gross, Böhmer and Böhme2010) already observed, that there is a distinct morphological gap between ‘S.’ voesendorfensis and S. moedligensis, especially in the upper molars.
Schizogalerix cf. S. moedlingensis (Rabeder, Reference Rabeder1973)
Figure 4.23, 4.24
Diagnosis
See Rabeder (Reference Rabeder1973, p. 433).
Occurrence
Schizogalerix moedlingensis is known from its type locality, Eichkogel (MN11, Austria), and has been identified in Turolian localities from Greece, namely Pikermi and Pikermi-Chomateri (Doukas et al., Reference Doukas, van den Hoek Ostende, Theocharopoulos, Reumer and Schmidt-Kittler1995; Vasileiadou and Doukas, Reference Vasileiadou, Doukas and Vlachos2021).
Description
The two labial fragments of P3 have a robust shape. The paracone is conical. From it descends a low and slightly curved metastyle reaching the posterior cingulum at the posterolabial margin of the tooth. There is no distinct parastyle, although a short anterolabial cingulum is present. The labial outline of the P4 is slightly curved. The paracone is conical and connected to a strong bipartitioned metastyle ending in a more labial position than the paracone. The parastyle is well differentiated and is connected to both the base of the paracone and the anterior cingulum. The protocone is low and the hypocone well developed. Both cusps have a slight posterolabial orientation. A short and small crest running transversely from the protocone is distinguishable. Thin ridges are found between the two cusps and on both anterior and posterior margins.
The M1 is only represented by two fragments. The posterolabial fragment preserves a conical metacone connected to a metastyle by a short and curved crest. The anterior mesoloph is short and has a clear labial orientation (Fig. 4.23). A postmetaconule crest is present, but the metaconule is not preserved. The labial outline is curved with a median constriction. The second fragment corresponds to a protocone, a distinct protoconule, and a narrow anterior cingulum. A fragment of the triangular M3 shows a small and conical protocone connected to the base of a higher metacone by a low crest. A much lower crest is also distinguishable on the anterior margin of the molar. The paracone is missing in this specimen.
The p3 is a two-rooted tooth with an isolated, low, and pointed paraconid. The protoconid is conical and positioned in the middle of the tooth. Its posterior flank is slightly concave. The talonid consists mostly of a robust postcristid and a faint transverse ridge.
The trigonid and the talonid on m3 are of similar size. The paraconid is not distinguishable from the curved and low paralophid. The protoconid and metaconid are conical and connected by a thin but complete ridge. The trigonid basin is broad but shallow. The entoconid is strong and distinguishable from the high entocristid. The hypoconid is low. The postcingulid is well developed and connected to the entoconid. Only a slight ridge connects the two parts of the S-shaped postcristid.
Materials
Krásno: two fragments of P3 (L = 1.78, 1.79), three fragments of P4 (L = 2.22, 2.28, third too fragmented to measure), two fragments of M1, one fragment of M3, one p3 (L = 1.63, W = 0.94), one m3 (L = 1.91, W1 = 1.27, W2 = 1.16).
Remarks
The split ectoloph on the upper molars and the advanced development of the entoconid and postcingulid on the lower molars are significant features of Schizogalerix. The Schizogalerix from Krásno can be easily distinguished from the Moldovan S. sarmatica (MN9) by the much less oblique elongation of the P4. The derived stage of the ectoloph on M1 also excludes ‘S.’ voesendorfensis. Two species have been identified in MN11 of central Europe: S. zapfei and S. moedlingensis, both being recorded in Austria (Rabeder, Reference Rabeder1973; Ziegler, Reference Ziegler2006a). Schizogalerix zapfei and S. moedligensis share a similar dental pattern that can make their identification difficult based on scanty material. Nevertheless, based on the material from Kohfidisch, we noticed a more complex ectoloph morphology in S. zapfei, whereas S. moedlingensis has more reduced mesostyles. The labial margin of the metacone area is always fully convex in S. zapfei, but is more irregular in S. moedlingensis, as in our specimen. The m3 of S. zapfei shows a distinct transverse pattern with a straight, oblique paralophid and a well-developed entoconid-postcingulid connection. This is not found in our specimen, in which the paralophid is clearly curved and the postcristid not fully divided, as in S. moedlingensis. In view of the limited material from Krásno, we classify the galericine as S. cf. S. moedlingensis.
Tribe Incertae sedis
Genus Lantanotherium Filhol, Reference Filhol1888
Type species
Erinaceus sansaniensis Lartet, Reference Lartet1851.
Other species
Lantanotherium robustum (Viret, Reference Viret1940); L. sanmigueli Villalta and Crusafont, Reference Villalta and Crusafont1944; L. longirostre Thenius, Reference Thenius1949; L. sawini (James, Reference James1963); L. dehmi (James, Reference James1963); L. lactorense (Baudelot and Crouzel, Reference Baudelot and Crouzel1976); L. sabinae (Mein and Ginsburg, Reference Mein and Ginsburg2002); L. observatum (Korth and Evander, Reference Korth and Evander2016); L. anthrace Cailleux et al., Reference Cailleux, Chaimanee, Jaeger and Chavasseau2020.
Occurrence
Early to late Miocene of Europe (Villalta and Crusafont, Reference Villalta and Crusafont1944; Baudelot and Crouzel, Reference Baudelot and Crouzel1976; Mein and Ginsburg, Reference Mein and Ginsburg2002; Ziegler, Reference Ziegler2005b, Reference Ziegler2006b); middle to late Miocene of Asia and North America (James, Reference James1963; Engesser, Reference Engesser1972; Storch and Qiu, Reference Storch and Qiu1991; Korth and Evander, Reference Korth and Evander2016; Cailleux et al., Reference Cailleux, Chaimanee, Jaeger and Chavasseau2020).
Lantanotherium sanmigueli Villalta and Crusafont, Reference Villalta and Crusafont1944
Figures 5–7; Table 2
Table 2. Measurements (in mm) of Lantanotherium sanmigueli Villalta and Crusafont, Reference Villalta and Crusafont1944 from the late Miocene of Slovakia. L = length; L1 = labial length; L2 = shortest anteroposterior length; W = width; W1 = anterior width; W2 = posterior width.


Figure 5. Scanning electron photomicrographs of Lantanotherium sanmigueli Villalta and Crusafont, Reference Villalta and Crusafont1944 from Borský Svätý Jur (8, 12–14), Studienka A (1–7, 15, 16) and Krásno (9–11): (1) P3, ST214001; (2) P4 (fragment), ST214220; (3) M1, ST214003; (4) M1, ST214221; (5) M2, ST214000; (6) M2, ST214004; (7) M2, ST214222; (8) M3, BJ213000; (9) M3, KR127012; (10) M3, KR127013; (11) p4, KR127014; (12) m1, BJ213001; (13) m2, BJ213002; (14) m3, BJ213003; (15) m2, ST214006; (16) m3, ST214224. Images with underlined numbers are reversed.

Figure 6. Scanning electron photomicrographs of the dP4 of Lantanotherium sanmigueli Villalta and Crusafont, Reference Villalta and Crusafont1944: (1) BJ213220; (2) ST214021; (3) ST214022. Image with underlined number is reversed.
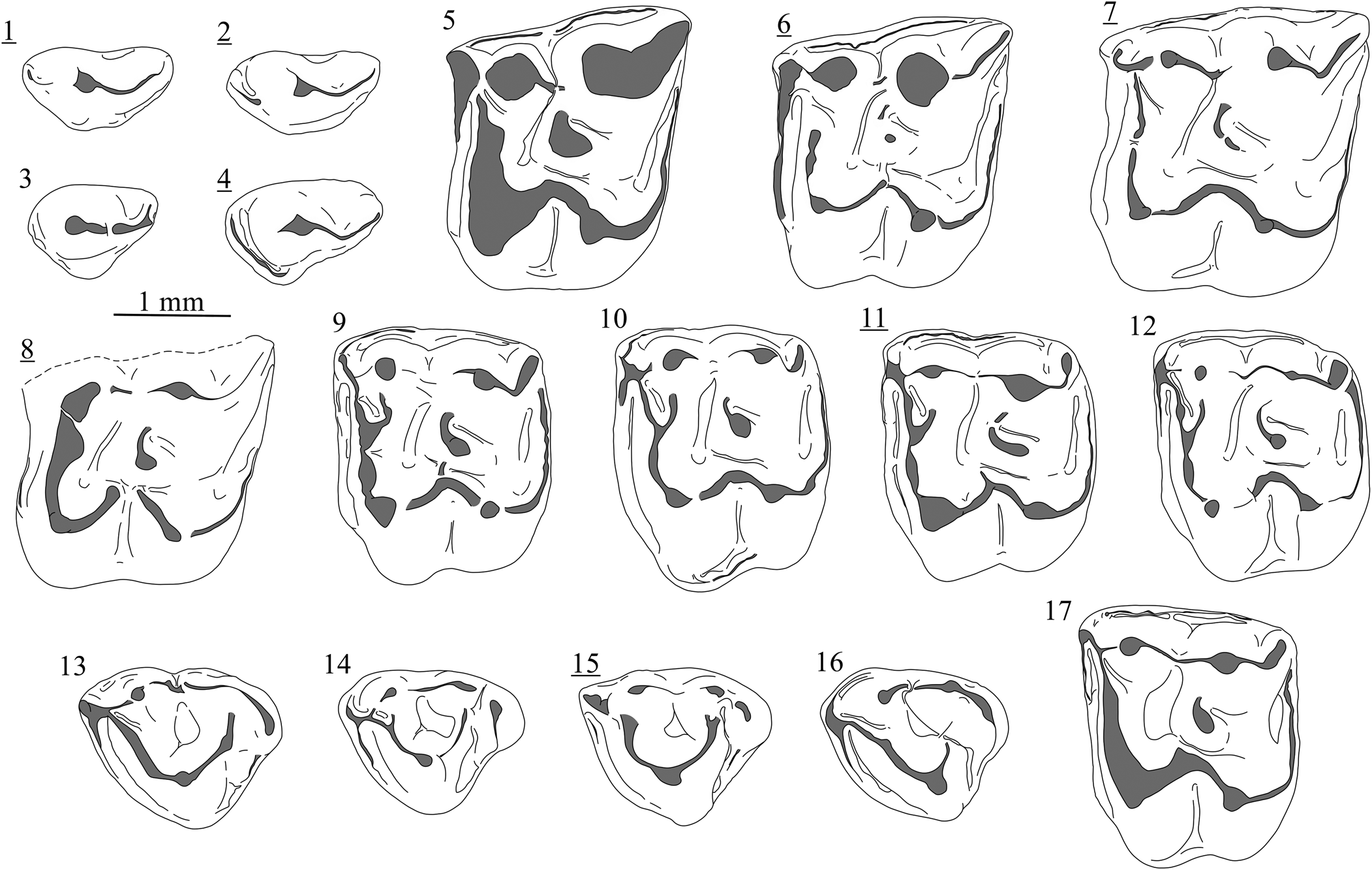
Figure 7. Variability of the dental elements of Lantanotherium sanmigueli Villalta and Crusafont, Reference Villalta and Crusafont1944 from Richardhof-Wald (1–4), Schernham (5–16), and Kohfidisch (17): (1) P3, Rh94/1-18A; (2) P3, Rh94/1-19A; (3) P3, Rh94/1-18B; (4) P3, Rh94/1-19C; (5) M1, SCH-3A; (6) M1, SCH-5C; (7) M1, SCH-2C; (8) M1, SCH-2B10; (9) M2, SCH-7D; (10) M2, SCH-4D; (11) M2, SCH-1F; (12) M2, SCH-8D; (13) M3, SCH-3H; (14) M3, SCH-1H; (15) M3, SCH-3I; (16) M3, SCH-2H; (17) M2, 2021/0049/0001. Images with underlined numbers are reversed.
Holotype
Fragment of left mandible with m1 and m2, unnumbered, ICP, Viladecavalls, Spain (Villalta and Crusafont, Reference Villalta and Crusafont1944).
Diagnosis
Small-sized Lantanotherium (m1 length of the holotype: 2.4 mm) characterized by the following combination of features: large I1; lack of diastema separating the upper and lower premolars; subtriangular P3 usually with a slight lingual extension; M1 and M2 with crescent metaconule; on M3, metacone and hypocone fused or distinct; usual presence of a reduced p1; narrow p4.
Occurrence
MN9, MN10, and MN11 of Europe (Villalta and Crusafont, Reference Villalta and Crusafont1944; Ziegler, Reference Ziegler2006a; Ménouret and Mein, Reference Ménouret and Mein2008; Jablonski et al., Reference Jablonski, Su, Flynn, Ji and Deng2014; Vasileiadou and Doukas, Reference Vasileiadou, Doukas and Vlachos2021); MN12 of Asia (Storch and Qiu, Reference Storch and Qiu1991).
Description
The P3 is a small, triple-rooted tooth. The conical paracone is situated in a slightly anterior position; it is connected to a low metacone by a concave crest in occlusal view. A clear lingual extension bearing a small protocone is present (Fig. 5.1). From this cusp descends a low crest closing the anterolingual margin of the tooth. The labial part of P4 shows a relatively low, conical paracone, connected to the distinct parastyle by a faint ridge. The lingual extension has a rectangular outline with straight margins. A low crest along the anterior margin is connected to the protocone. The low hypocone is connected to a distinct posterior crest following the slight curvature of the posterolingual corner. A short, barely visible crest is found at the labial flank of the hypocone. Similarly, a hardly distinguishable V-shaped crest connects the lingual base of the hypocone to the lingual base of the protocone. The dP4 is more gracile than the P4 (Fig. 6). The paracone is high and conical; a thin posterocrista connects it to the narrow posterior cingulum. The parastyle is small and independent from the paracone. The lingual area has two distinct cusps in anterior position. The protocone is smaller and in a less lingual position than the hypocone. The paracone is connected to the parastyle and the hypocone to the posterior cingulum.
The M1 is a sturdy, square-shaped molar with four roots. The metacone is wider than the paracone. A low metastyle is included in a short but broad postmetacrista connected to the metacone. The conical paracone is connected to the latter by a short, low, and straight ectoloph. From the anterior side of the paracone, a crest descends leading to a parastyle at the corner of the tooth. The protoconule is distinguishable within the protocone crest; it is not connected to the paracone, but to the paracone-parastyle crest at the base of the paracone. The protocone is as strong as the metacone and presents a robust triangular base; it is connected to a low and conical hypocone by a curved loph. From the posterior side of the hypocone descends a crest closing the posterior margin of the molar. The slightly crescent metaconule is isolated from the protocone and weakly connected to the metacone (Fig. 5.3, 5.4); it is higher than the hypocone. The inner basin thus corresponds to a valley compressed between the metaconule and the paracone. On the opposite side, the posterior basin is enlarged. The anterior cingulum is wide and starts from the base of the protocone. The labial cingulum is discontinuous. There is no cingulum on the lingual side.
The M2 is a compact and square tooth. The two lingual roots are fused at their bases. The postmetacrista is short and curvy. One of the specimens from Studienka A shows a posterolabial compression leading to an even shorter crest and a more rounded anterolabial corner (Fig. 5.6). The paracone is a conical cusp connected to the wider metacone by a low but sharp ectoloph; it is also connected to a small parastyle that veers labially to create a right anterolabial angle. The protocone is smaller than the metacone and the paracone and is connected to the hypocone by a curved crest. The protocone is connected to the paracone by a robust crest bearing a distinct protoconule. The posterior postprotoconule notch is strong. The protocone-metaconule connection is absent, except in one specimen (Fig. 5.7) in which a low connection can be distinguished. The metaconule, slightly higher than the hypocone, is anteriorly connected to the base of the metacone. Anterior and posterior cingulums are large. The labial cingulum is narrower but complete and the lingual cingulum is very faint.
The M3 is a strong subtriangular tooth constituted by four cusps. The paracone is the strongest cusp, connected by a high, curved crest to the protocone and by a low crest to the metacone. Descending from the protocone, the posterior crest is weaker and does not reach the top of the hypocone; this crest is straight in three specimens (e.g., Fig. 5.10) and curved in one (Fig. 5.9). In a similar way, the metacone and hypocone are fused as a crescent crest in three specimens and are slightly separated in one. Anterior and posterior cingulums are present.
The p1 is a one-rooted, subtriangular unicuspid with no cingulid. The main cusp is situated in the middle of the premolar and continues as a low, curved bulge to the anterolingual border of the tooth; a thin crest connects the cusp to a low bulge situated at the posterolabial border. The p4 has a low conical and isolated paraconid in an anterolingual position. The protoconid is a sturdy cusp situated in the middle of the tooth. A small crest descends lingually without reaching the talonid; it ends as a small bulge (metaconid) at the base of the cusp. A low posterior crest—higher in its central part—surrounds the talonid; a feeble transverse crest starts from the central part and fades out at the base of the protoconid.
The m1 is a narrow tooth with an elongated trigonid. The lowest cusp of the trigonid is the paraconid, included in a long, bipartitioned paralophid connected to the protoconid; the latter is a high triangular cusp, weakly connected to a medium-sized, rounded metaconid by a notched protolophid. The trigonid basin is very narrow, but still largely open on the labial side of the tooth. The square-shaped talonid consists of a deep, almost-closed basin. The entoconid is pointed and only slightly smaller than the metaconid. The entocristid is a short, high crest that slopes down abruptly. A short metacristid is present (Fig. 5.12). On the posterior side of the entoconid runs a crest that splits into two arms in the middle of the posterior margin of the tooth; the first one reaches the hypoconulid whereas the second connects to a postcingulid reaching the posterolabial corner of the tooth. The hypoconid is the lowest cusp of the tooth; from it descends a straight oblique cristid decreasing in size anteriorly. The anterolabial cingulid is well developed. A short, narrow anterolingual cingulid is distinguishable between the paraconid and the metaconid. The m2 differs from the m1 mainly by the compressed trigonid, leading to a shorter paralophid and deeper trigonid basin. The metaconid is higher than the protoconid. The talonid is longer in m2 than in m1 (compare Fig. 5.12, 5.13). The anterior crest of the low hypoconid is less transversly directed. The postcingulid is connected to a low postcristid in all the four specimens. The labial cingulid is continuous. The m3 has a trigonid longer and slightly wider than the talonid, with a conical metaconid and a more anteroposteriorly compressed protoconid. The paraconid-metaconid connection is variable. The crest descending from the paraconid touches the base of the metaconid but usually does not complete the closure of the trigonid basin. Rarely, a short oblique crest starts from the metaconid and ends in the middle of the trigonid. The valley appears as a hook. A strong entoconid and a short but high entocristid characterize the square-shaped talonid. The oblique cristid is almost parallel to the entocristid. A reduced posterior cingulid is present in one of the two specimens in which this character could be observed.
Materials
Borský Svätý Jur: one dP4, M3, one m1, one m2, two m3. Studienka A: one P3, one P4, two dP4, three M1, three M2, one M3, one c, two m1, four m2, three m3. Triblavina: one P4, one p1, one m3. Krásno: one M2, two M3, two p4, two fragments of m2, one m3. See Table 2 for measurements.
Remarks
The species name Lantanotherium sanmigueli has been used to designate all of the small Lantanotherium from the late Miocene. This is partly a consequence of limited type material and a vague original description, making the data available poorly suited for comparisons. Moreover, the type material is a fragment of a mandible with m1 and m2 (Villalta and Crusafont, Reference Villalta and Crusafont1944) that does not show any diagnostic features except a size smaller than other European species. Ziegler (Reference Ziegler2006b), for instance, considered L. sanmigueli a nomen dubium. It is still unclear whether all of the material assigned to L. sanmigueli represents a single species. As a result, this has led to seeing this species as conservative, whereas relatively large variation is distinguishable in the plentiful European material (Furió et al., Reference Furió, Casanovas-Vilar and Van Den Hoek Ostende2011b). Pending a study on the homogeneity of L. sanmigueli, we provide here an emended diagnosis sensu lato. The main distinctions among Lantanotherium spp. have been summarized by Cailleux et al. (Reference Cailleux, Chaimanee, Jaeger and Chavasseau2020; Table 1).
Lantanotherium sanmigueli is a very common taxon of the European late Miocene (Furió and Alba, Reference Furió, Alba, Pérez-García, Gascó, Gasulla and Escaso2011). Direct comparisons with material from Austria (Götzendorf, Richardhof-Golfplatz, Richardhof-Wald, Schernham, Eichkogel), France (Lo Fournas 1993, Montredon), and Spain (Can Llobateres 1) reveal relative homogeneity in size, but exceptions are found. This is the case for the relatively large specimens from the MN10 locality of Soblay and the more gracile elements from Borský Svätý Jur.
Lantanotherium sanmigueli is known for its wide morphological variability (Furió et al., Reference Furió, Alba, Carmona and Rifà2011a). The presence of a minute protocone on a P3 from Studienka A is a feature only mentioned in non-Eurasian members of the genus (see Cailleux et al., Reference Cailleux, Chaimanee, Jaeger and Chavasseau2020). P3 usually shows a slight or no lingual extension (Fig. 7.1–7.4). However, it is worth noting that no lacteal P3 of Lantanotherium is known, or at least has been identified as such. It is conceivable that the most reduced P3 of L. sanmigueli (e.g., Fig. 7.1, 7.2) represent decidual elements. This would be consistent with the overall smaller size and more reduced lingual region of the dP3 compared to the P3 in modern Erinaceinae and Hylomyinae (e.g., Butler, Reference Butler1948; Engesser and Jiang, Reference Engesser and Jiang2011; Voyta, Reference Voyta2017).
Three isolated elements from Borský Svätý Jur and Studienka A are attributed to the dP4 (Fig. 6), an element never described before in Lantanotherium. These teeth, for a long time unidentified in our material, share strong affinities with erinaceids. They are more gracile than in Erinaceinae and display a more reduced lingual configuration than in known dP4 of Galericini (e.g., Engesser, Reference Engesser1980; Ziegler, Reference Ziegler1983). On the other hand, they still preserve lingual cusps, unlike the dP3 of known Galericini. These premolars are smaller than the P3, P4, and dP4 of Schizogalerix voesendorfensis and do not show any oblique orientation or angular aspect. Moreover, L. sanmigueli is the only Erinaceidae identified in both Borský Svätý Jur and Studienka A. Noticeable similarities are found between this element and the dP4 of the Recent Hylomyini Neotetracus sinensis Trouessart, Reference Trouessart1909, of which the dental similarities with Lantanotherium were already noted (see Viret, Reference Viret1940; Engesser and Jiang, Reference Engesser and Jiang2011).
The protocone-hypocone loph on M1 is almost always complete and slightly curved, but the assemblage of Schernham yielded M1 with clearly curved loph. Within the 24 complete M1 from Schernham, two have a divided lophs (e.g., Fig. 7.8), which is a remarkable character only recorded in Lantanotherium robustum from La Grive (Mein and Ginsburg, Reference Mein and Ginsburg2002, fig. 25). One of the specimens of Studienka A shows a low protocone-metaconule connection (Fig. 5.7). This character has never been previously described in Lantanotherium. We also observed that same crest in three of 24 M1 from Schernham (Fig. 7.6) and a central incomplete crest was also observed in the specimen from Rudabánya figured by Ziegler (Reference Ziegler2005b, pl. 1, fig. 8, MAFI V20504). Despite the fact that the rare occurrence of this probably ancestral morphotype is thus part of the broad qualitative variability of Lantanotherium, the common lack of the protocone-metaconule connection is still an important character to differentiate Lantanotherium from extinct to extant species of gymnures, e.g., Neohylomys hainanensis Shaw and Wong, Reference Shaw and Wong1959, Neotetracus butleri Mein and Ginsburg, Reference Mein and Ginsburg1997, and Otohylomys megalotis (Jenkins and Robinson, Reference Jenkins and Robinson2002).
One M2 from Studienka A (Fig. 5.6) stands out by its more compressed outline. Such a morphology has already been recorded in Lantanotherium sanmigueli specimens from the type locality of Viladecavalls (Furió et al., Reference Furió, Casanovas-Vilar and Van Den Hoek Ostende2011b) and is also found in Richardhof-Wald and Schernham.
The fusion of the hypocone and metacone on the M3 of Lantanotherium sanmigueli (Fig. 7.13) is a feature frequently found during the Vallesian (e.g., Richardhof-Golfplatz, Studienka A), whereas these cusps are more clearly distinct in younger localities (e.g., Montredon, Krásno). The Schernham assemblage takes an intermediate position, with half of the M3 having a distinct hypocone and metacone (e.g., Fig. 7.14, 7.15). We observed that the lower molars from Borský Svätý Jur are narrow compared to the younger material from Studienka, Schernham, and Soblay. Such a shape is also found in the material of Lufeng (MN12; Storch and Qiu, Reference Storch and Qiu1991) and Eichkogel (MN11; Rabeder, Reference Rabeder1973), which could indicate intraspecific variability. The latter locality yielded a p4 attributed to L. cf. L. sanmigueli by Rabeder (Reference Rabeder1973, fig. 28), which, however, seems to belong to a vespertilionid bat.
Subfamily Erinaceinae Fischer, Reference Fischer1814
Genus Atelerix Pomel, Reference Pomel1848
Type species
Erinaceus albiventris Wagner, Reference Wagner, Schreber, Goldfuss and Wagner1841.
Other species
Atelerix frontalis (Smith, Reference Smith1831); Atelerix algirus (Lereboullet in Duvernoy and Lereboullet, Reference Duvernoy, Lereboullet and Levrault1842); Atelerix sclateri Anderson, Reference Anderson1895; Atelerix depereti Mein and Ginsburg, Reference Mein and Ginsburg2002; Atelerix rhodanicus Mein and Ginsburg, Reference Mein and Ginsburg2002; and Atelerix steensmai Van Dam, Mein, and Alcalá, Reference Van Dam, Mein and Alcalá2020.
Diagnosis
See Frost et al. (Reference Frost, Wozencraft and Hoffmann1991, p. 31).
Occurrence
Late-middle Miocene to late Miocene of Europe (Mein and Ginsburg, Reference Mein and Ginsburg2002; Ziegler, Reference Ziegler2006b, Reference Ziegler2006a); identified from the Plio-Pleistocene in Africa (Zouhri et al., Reference Zouhri, Benammi, Geraads and El Boughabi2017; Cailleux, Reference Cailleux2021). Crespo et al. (Reference Crespo, Sevilla, Montoya and Ruiz-Sanchez2020) recorded cf. Atelerix from the early Miocene (MN3) of Mas d'Antolino B3, but this identification is based on a single M3, which is insufficient to allow identification beyond the subfamily level. The inclusion of Mioechinus Butler, Reference Butler1948 into Atelerix would extend its geographical and temporal range (e.g., Qiu, Reference Qiu1996; Mein and Ginsburg, Reference Mein and Ginsburg1997; Li et al., Reference Li, Li, Xue, Li, Zhang and Yang2019).
Remarks
We consider here Miocene forms as Atelerix, but we are aware that such generic attribution is still uncertain. If extant Erinaceinae diversified during the late Miocene (Bannikova et al., Reference Bannikova, Lebedev, Abramov and Rozhnov2014), it is not surprising to see slight differences between Atelerix, Erinaceus Linnaeus, Reference Linnaeus1758, and Mioechinus. Because there are only a few derived dental features allowing differentiation of modern Erinaceus from modern Atelerix, pinpointing the emergence of Erinaceus is also hardly possible. The taxonomy of this subfamily is largely based on cranial elements, which are exceedingly rare in the fossil record. We follow Ziegler (Reference Ziegler2005a) rather than Mein and Ginsburg (Reference Mein and Ginsburg2002) in considering Mioechinus and Atelerix as separate taxa.
Atelerix cf. A. depereti Mein and Ginsburg, Reference Mein and Ginsburg2002
Figure 8.1, 8.2
Diagnosis
See Mein and Ginsburg (Reference Mein and Ginsburg2002, p. 14).

Figure 8. Scanning electron photomicrographs of Atelerix cf. A. depereti Mein and Ginsburg, Reference Mein and Ginsburg2002 (1, 2), Atelerix aff. A. depereti (3–7), and cf. Postpalerinaceus sp. indet. (8): (1) p3, BJ213006; (2) p4, BJ213006; (3) I2, KR127002, labial view; (4) P2, KR127000; (5) P2, KR127001; (6) i3, KR127007; (7) m3, SG198001; (8) I2, KR127006. Images with underlined numbers are reversed.
Occurrence
The type locality of Atelerix depereti is the MN7/8 fissure filling L5 from La Grive Saint-Alban (Mein and Ginsburg, Reference Mein and Ginsburg2002).
Description
Only the posterior part of the lower canine is preserved. The tooth is large and elliptical. From the main cusp descends a sharp crest reaching a small cuspule on the posterior border; from this cuspule runs a well-developed posterolingual cingulum. The posterolabial cingulum only corresponds to a small slope. The lower p3 is also only partially preserved (Fig. 8.1). This one-rooted tooth has an angular shape with one large but low main cusp. A bulge is present on the posterior border of the tooth, not connected to the main cusp. The posterior border is oblique. The fragment of p4 (Fig. 8.2) is characterized by a very narrow anterior part. The heavily worn paraconid is large; it is connected to the protoconid by a worn labial crest well differentiated from the narrow valley. There is no ectocingulid. The anterior face of the metaconid is robust.
The lower m2 has a well-developed trigonid basin. The paralophid and paraconid are fused into a single ridge, which bends at the lingual side and partly closes the trigonid basin. The latter cusp is strong and conical. A short, straight crest runs from its posterior part, reaching at least the middle part of the trigonid.
Materials
Borský Svätý Jur: one fragment of c (W = 1.89), one fragment of p3, one fragment of p4, one fragment of m2 (W1 = 2.22).
Remarks
Alone, the dental morphology of Erinaceinae has only feeble resolving power (Gould, Reference Gould2001). Van Dam et al. (Reference Van Dam, Mein and Alcalá2020) made a comprehensive summary of what we know—and what we do not know—about the paleodiversity of Erinaceinae during the Miocene. Five genera are defined in Europe: Amphechinus Aymard, Reference Aymard1850, Postpalerinaceus Crusafont and Villalta, Reference Crusafont and Villalta1947, Erinaceus, Atelerix, and Mioechinus, the last being included in Atelerix by Mein and Ginsburg (Reference Mein and Ginsburg2002). Amphechinus is an ancestral group that was not present in the late Miocene of central Europe (Gibert, Reference Gibert1975; Van Dam et al., Reference Van Dam, Mein and Alcalá2020; Cailleux, Reference Cailleux2021). This epoch was characterized by the success of Postpalerinaceus vireti in western Europe.
The form from Borský Svätý Jur is only known by fragments, whose dimensions correspond with a medium-sized species, e.g., Atelerix depereti. Our fragment of p4 is narrower than in Postpalerinaceus cingulatus Zeigler Reference Ziegler2005b and does not present any noticeable trace of an ectocingulid. It also differs by its deep trigonid basin from most species, with the noticeable exception of Atelerix rhodanicus, Atelerix depereti, and the indeterminate Erinaceinae from Petersbuch 31 (Ziegler, Reference Ziegler2005a, NHMA P31-137A3). The labial border of the p3 is transverse, which is a feature found in Atelerix depereti and in Atelerix aff. A. depereti from Spain (Mein and Ginsburg, Reference Mein and Ginsburg2002; Van Dam et al., Reference Van Dam, Mein and Alcalá2020). Direct comparison with ‘?cf. Atelerix depereti’ from Götzendorf (Ziegler, Reference Ziegler2006b) revealed strong similarities with our material. Because Götzendorf and Borský Svätý Jur are close in age and from the same basin, the two samples probably correspond to a single species. Additionally, Erinaceinae gen. indet. sp. indet. 1 from the MN9 Moldovan locality of Buzor-1 (Rzebik-Kowalska and Lungu, Reference Rzebik-Kowalska and Lungu2009) shows strong similarities with our material and could correspond to a closely related form.
Atelerix aff. A. depereti Mein and Ginsburg, Reference Mein and Ginsburg2002
Figure 8.3–8.7
Description
The I2 is a high, curved, one-rooted tooth; its crown and root are anteroposteriorly compressed. In the middle of the tooth, the only cusp has an elongated top with an anteroposterior orientation. The distal side is only composed of the strong slope of the main cusp whereas the mesial side is more complex. From the most posterior part of the cusp starts a curved crest strengthening the distal margin before ending on the anteromesial border of the tooth (Fig. 8.3). The oval I3 has a sturdy main cusp in an anterior position and an anteriorly elevated crown; from the cusp descends a ridge ending at the posterior border of the tooth. There is no distinct cingulid but the slope of the main cusp becomes smaller on the distal side of the specimen, leading to a more extended zone. The two roots show an oblique, backward orientation. The anterior root is smaller than the posterior one.
The posterior part of the upper canine presents a rounded outline. The main cusp is slightly inflated. A robust, central posterior crest is present, reaching the low, irregular posterior cingulum. The P2 has an ovoid to slightly elongated outline (Fig. 8.4, 8.5); the roots have not been preserved. The cusp is situated in an anterior position; it is included in a median crest crossing all of the premolar. At the most anterior margin, this crest turns lingually; at the back, it ends at the straight posterior margin of the tooth as a small bulge. The slight labial crest creates a concave posterolingual slope.
The i3 is an oval tooth similar in size to the P2 but with a more flattened crown. The main cusp is situated at the anterior margin. A crest crosses the incisor obliquely to reach the posterodistal border.
The broken m2 is only known by a lingual fragment. We mainly note a conical metaconid, from which a notched protolophid descends labially. A notch is found in the middle of the protolophid. The cuspid is enlarged. The complete m3 is elliptical. It mostly consists of the long paralophid in which the paraconid is not distinct. The metaconid is slightly stronger than the protoconid. They are set close together, implying a short protolophid. The trigonid basin is deep and widely open (Fig. 8.7). A narrow anterolabial cingulid is distinguishable.
Materials
Krásno: one I2 (L = 1.53, W = 1.46), one fragment of C, two P2 (L = 2.20, W = 1.53; L = 2.28, W = 1.38), one i3 (L = 2.06, W = 1.51). Šalgovce 5: one I3 (L ≃ 2.56, W = 1.66), one fragment of m2 and one m3 (L = 2.17, W = 1.73).
Remarks
All specimens described here correspond to a medium-sized erinaceine considered to represent a single species. The I2 from Krásno (KR127006) presents a shape found in Atelerix and is similar to the I2 of Atelerix aff. A. depereti described by Van Dam et al. (Reference Van Dam, Mein and Alcalá2020; ROM 7-259, from Masia de la Roma, Teruel Basin, central Spain), despite being larger and having a stronger cingulum. The I2 of Erinaceus samsonowiczi Sulimski, Reference Sulimski1959 from Maramena is even larger and more massive (unpublished data, F. Cailleux, Reference Cailleux2021). The I3 from Šalgovce 5 is easily distinguishable from the caniniform I3 of Amphechinus (see Viret, Reference Viret1938) and presents a more advanced flattening stage than Postpalerinaceus intermedius from La Grive (Mein and Ginsburg, Reference Mein and Ginsburg2002) and ‘Mioechinus’ tobieni Engesser, Reference Engesser1980 from Eskihisar (Engesser, Reference Engesser1980). Our specimen is similar in shape to the material of Atelerix depereti described by Mein and Ginsburg (Reference Mein and Ginsburg2002) but is slightly larger. Atelerix aff. A. depereti described from MN9-12 localities of Spain (Van Dam et al., Reference Van Dam, Mein and Alcalá2020) have an I3 which corresponds in size and displays an oblique orientation. The median crest of our specimen is, however, less marked. The size of the P2 from Krásno falls into the lowermost variability of Atelerix aff. A. depereti but in the center of the variability of Atelerix depereti, the latter having less massive teeth, like our specimens. The dimensions of the m3 are found in the upper variability of Atelerix depereti from La Grive (Mein and Ginsburg, Reference Mein and Ginsburg2002) and the lower variability of Atelerix aff. A. depereti from the Turolian of Spain (Van Dam et al., Reference Van Dam, Mein and Alcalá2020).
The material from Krásno and Šalgovce 5 fits well with Atelerix aff. A. depereti as described from MN9-12 localities in Spain (Van Dam et al., Reference Van Dam, Mein and Alcalá2020). This form overall shows a larger size and proportionally wider elements than the type material of Atelerix depereti. According to Van Dam et al. (Reference Van Dam, Mein and Alcalá2020), the development of the lingual extension on P2, leading to a width increase, occurred during the Atelerix depereti-Atelerix aff. A. depereti lineage.
Genus Postpalerinaceus Crusafont and Villalta, Reference Crusafont and Villalta1947
Type species
Postpalerinaceus vireti Crusafont and Villalta, Reference Crusafont and Villalta1947.
Other species
Postpalerinaceus intermedius (Gaillard, Reference Gaillard1899); Postpalerinaceus cingulatus Ziegler, Reference Ziegler2005b.
Occurrence
Postpalerinaceus is recorded from the late-middle Miocene and the late Miocene of Europe (Mein et al., Reference Mein, Moissenet and Adrover1990; Mein and Ginsburg, Reference Mein and Ginsburg2002; Ziegler, Reference Ziegler2005b, Van Dam et al., Reference Van Dam, Mein and Alcalá2020).
cf. Postpalerinaceus sp. indet.
Figure 8.8
Description
The I2 from Krásno is a rounded monocuspid tooth with only a slightly asymmetrical shape. The main cusp is included in a median crest. The distal side is inflated whereas the mesial one is anteriorly straight and posteriorly concave. The median crest ends posteriorly by joining a small posterodistal cingulum.
Materials
Krásno: one I2 (L = 1.41, W = 1.49).
Remarks
The structure of the I2 can be distinguished from the morphology of Amphechinus, Atelerix, and Mioechinus, in which the crown is more elevated and complex. Our specimen fits the configuration of Postpalerinaceus (see Crusafont and Villalta, Reference Crusafont and Villalta1947). The specimen from Krásno is smaller than the I2 of the holotype of Postpalerinaceus vireti but falls within the size variability of Postpalerinaceus cf. P. vireti from the Teruel Basin (Van Dam et al., Reference Van Dam, Mein and Alcalá2020) and in the lower part of the Postpalerinaceus intermedius variability from La Grive (Mein and Ginsburg, Reference Mein and Ginsburg2002). Morphologically, the Krásno tooth resembles either species. Postpalerinaceus is poorly recorded in central Europe. The only identified species, Postpalerinaceus cingulatus from the MN9 locality of Rudabánya (Ziegler, Reference Ziegler2005b), is distinctively smaller than western European species. We note the possible occurrence of Postpalerinaceus in the Austrian MN10 locality of Schernham, Austria (Ziegler, Reference Ziegler2006b), which suggests the presence of a medium-sized species, at least similar to Postpalerinaceus, during the late Vallesian and early Turolian of central Europe.
Erinaceinae gen. indet. sp. indet.
Description
A large fragment of an upper canine from Šalgovce 5 shows a main cusp with an inflated tip. A marked posterior crest starts from the top of the cusp and joins the posterior shoulder. A fainted posterior cingulum is present, broader on the lingual side.
Materials
Šalgovce 5: one fragment of C (W ≈ 2.49).
Remarks
The morphology of the canine fits with the usual morphology of upper canine in erinaceines. Its size, however, does not correspond with the known dimensions of Atelerix depereti or Atelerix aff. A. depereti, but rather fits with larger species, e.g., Postpalerinaceus vireti and Atelerix steensmai.
Family Dimylidae Schlosser, Reference Schlosser1887
Genus Plesiodimylus Gaillard, Reference Gaillard1897
Type species
Plesiodimylus chantrei Gaillard, Reference Gaillard1897.
Other species
Plesiodimylus huerzeleri Müller, Reference Müller1967; Plesiodimylus crassidens Engesser, Reference Engesser1980; Plesiodimylus bavaricus Schötz, Reference Schötz1985; Plesiodimylus helveticus Bolliger, Reference Bolliger1992; Plesiodimylus johanni Kälin and Engesser, Reference Kälin and Engesser2001; Plesiodimylus gaillardi Mein and Ginsburg, Reference Mein and Ginsburg2002; Plesiodimylus similis Fejfar and Sabol, Reference Fejfar and Sabol2009; and Plesiodimylus ilercavonicus Crespo et al., Reference Crespo, Furió, Ruiz-Sánchez and Montoya2018.
Diagnosis
See Fejfar and Sabol (Reference Fejfar and Sabol2009, p. 612), translation from Müller (Reference Müller1967).
Occurrence
Early to late Miocene of Europe and Anatolia (Engesser, Reference Engesser1980; Doukas, Reference Doukas1986; Fejfar and Sabol, Reference Fejfar, Sabol, Van den Hoek Ostende, Doukas and Reumer2005, Reference Fejfar and Sabol2009; Ziegler, Reference Ziegler2006a; Klietmann et al., Reference Klietmann, Nagel, Rummel and van den Hoek Ostende2014b; Van den Hoek Ostende and Fejfar, Reference Van den Hoek Ostende and Fejfar2015; Crespo et al., Reference Crespo, Furió, Ruiz-Sánchez and Montoya2018).
Plesiodimylus chantrei Gaillard, Reference Gaillard1897
Figure 9.1–9.15; Table 3
Table 3. Measurements (in mm) of Plesiodimylus chantrei Gaillard, Reference Gaillard1897 from the late Miocene of Slovakia. L = length; W = width; W1 = anterior width; W2 = posterior width.

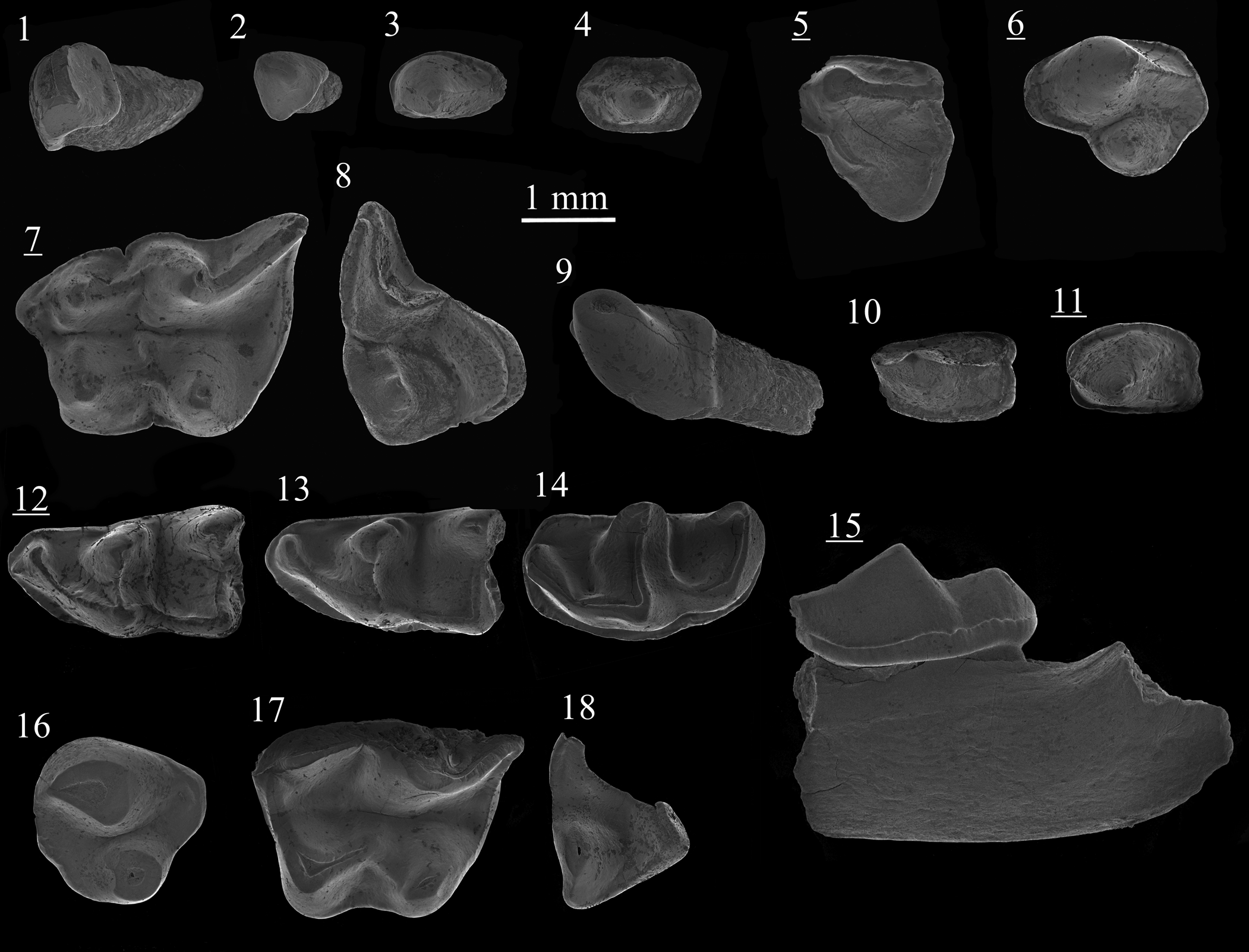
Figure 9. Scanning electron photomicrographs of Plesiodimylus chantrei Gaillard, Reference Gaillard1897 (1–15) and Metacordylodon aff. M. schlosseri (Andreae, Reference Andreae1904) (16–18) from Studienka A (1–4, 6–18) and Studienka E (5): (1) I1, ST214093; (2) I2/3, ST214094; (3) C, ST214102; (4) P1, ST214095; (5) DP4, ST214211; (6) P4, ST214107; (7) M1, ST214060; (8) M2, ST214087; (9) i1, ST214122; (10) p1, ST214125; (11) p4, ST214129; (12) m1, ST214115; (13) m1, ST214119; (14) m2, ST214134; (15) mandible with m2, ST214140; (16) P4, ST214052; (17) M1, ST214050; (18) M2, ST214051. Images with underlined numbers are reversed.
Holotype
Fragment of left mandible with p2–m2, La Grive 208b, La Grive Saint-Alban, France (Gaillard, Reference Gaillard1899).
Diagnosis
See Fejfar and Sabol (Reference Fejfar and Sabol2009, p. 612).
Occurrence
Early to late Miocene of Europe (Gaillard, Reference Gaillard1897; Rzebik-Kowalska, Reference Rzebik-Kowalska1996; Ziegler, Reference Ziegler2005b, Reference Ziegler2006b; Klietmann et al., Reference Klietmann, Nagel, Rummel and van den Hoek Ostende2014b). The type locality of Plesiodimylus chantrei is La Grive-Saint-Alban (MN7+8, France). Gaillard (Reference Gaillard1897) did not mention the fissure from which the holotype was extracted, but the work of Chantre and Depéret at the end of the nineteenth century was focused on the Peyre et Beau quarry (Mein and Ginsburg, Reference Mein and Ginsburg2002).
Description
I1/I2 is a simple triangular tooth bearing no cingulum. It has a pointed cusp. The mesial and posterior faces are flat and the anterodistal is convex. The root is almost straight, semicircular in cross section with a flat mesial face. I3 is a small monocuspid tooth that is labiolingually compressed. The subtriangular crown bears a short median crest. Two short extensions are found on either side, the lingual one running at a lower level than the labial one. The rounded root is posteriorly orientated.
The upper canine is a two-rooted, almond-shaped tooth with a caniniform cusp. The posterior crest connects the cusp with the posterior border of the narrow but complete cingulum. The P1 has a subhexagonal outline (Fig. 9.3). The cusp is situated in the center of the tooth. Low anterior and posterior crests descend from it, reaching the complete cingulum at the anterior and posterior border, respectively; these crests have a slightly labial position. Two thin but well-separated roots are present with a posterior orientation. The P4 is a three-rooted tooth with an elongated labial margin. The large and oval paracone is in an anterolabial position. From it descends a robust but low crest that reaches a straight oblique wall at the posterior margin of the tooth. The protocone is conical, situated on the lingual side in a more central position than the paracone; its posterior slope is extended, leading to a small and narrow basin. The P4 is surrounded by an almost complete, thick cingulum, interrupted at the base of the protocone. The dP4 is distinguishable by its more irregular outline (Fig. 9.5). The paracone is weaker than in the P4 and connected to a blade-like protocone. Due to the wide posterolingual extension, the lingual basin is larger than in P4.
The M1 is a massive subrectangular tooth with five roots and three basins. The large metacone is connected to an indistinct metastyle by a wide postmetacrista. The postparacrista reaches the flank of the metacone. Together with the protocone, the parastyle, which is connected to the base of the paracone, forms a small anterior basin. The protocone is almost as large as the hypocone and connected to it by a low crest. The prehypocrista is shorter and weaker than the postprotocrista. The hypocone has a more lingual position than the protocone. On its posterior side, a deep basin is bordered by a robust cingulum reaching the base of the metastyle. Thin cingula are distinguishable all along the anterior margin of the tooth, and between the protocone and the hypocone. In occlusal view, a short central notch is usually observed on both labial and lingual cingulums. The three-rooted M2 displays a wide and isolated protocone, occupying a significant part of the occlusal surface. A valley separates the protocone from the other cusps. The metacone is not differentiable from the straight premetacrista and the curved postmetacrista. Mesostyles are only slightly divided on the most unworn specimen. The paracone is more distinct than the metacone because the postparacrista and the preparacrista are relatively reduced. The first crest is S-shaped when reaching the parastyle. A short cingulum is present lingual to the parastyle. A shorter one is distinguishable between the mesostyle and the parastyle.
The lower canine is a robust, almond-shaped, one-rooted tooth with a strong cuspid in an anterior position. A small bulge is found on the anterior face of this cuspid. The posterior part only bears a faint cingulum. The posterolabial slope is reaching lower than the posterolingual one. The p1 is an elliptical, two-rooted tooth with a relatively wide posterior part; it is the largest lower premolar. The main cuspid is placed in a more anterior position; from it descends an anterior crest that turns lingually, and a weaker posterior crest that reaches the middle of the posterior margin. A continuous cingulid is present. The one-rooted p2/p3 is wider than long, with two lobes. The cuspid is found in an anterior position and is included in a crest reaching the anterior border. An almost complete cingulid is present, except for on the most posterior part of the tooth, at which the outline is slightly concave. The p4 is slightly shorter than p1 (compare Fig. 9.11, 9.10) and has an angular shape. The main cuspid has a subtriangular base and from its top descends a short anterior crest but no posterior crest. A cingulid is present all around the premolar except below the anterior crest. In one out of three specimens, the cingulum is connected to this crest.
The m1 is a robust tooth with a narrow trigonid. The valley of the trigonid basin is weak and oblique. The low blade-like paraconid is transversly oriented and connected to the protoconid by a bipartitioned paracristid. In two of seven specimens, a low accessory cuspid is attached to the paracristid (Fig. 9.12). The protoconid is a stout, bulbous cuspid. The metaconid has the same height but is less wide. The cuspids are connected to each other by a short, straight protocristid. The talonid basin is longer and wider than the trigonid one; it is less lingually opened. The entoconid is high with no anterior crest, whereas the hypoconid is lower and wider. A robust oblique cristid runs from the hypoconid; this straight crest reaches the base of the protoconid. A postcingulid is connected to the postcristid or to the entoconid. The cingulid is continuous except between the metaconid and the entoconid. In two of three specimens, a second short cingulid is distinguishable. In the m2, deep valleys separate high ridges in which cuspids are sometimes not distinguishable. The paraconid corresponds to a compressed bulge in the high, transverse paracristid. The metaconid and the protoconid are similar in morphology and are smaller than the protoconid; they are connected to each other by a high protocristid. The trigonid basin is longer than in the m1 and the talonid basin is much more rounded. The two talonid cuspids are fused with the oblique cristid as a single long, convex crest. The cingulid runs from the base of the metaconid to the center of the talonid posterior margin. A bulge is situated on the anterolingual side of the cingulid, near the paraconid.
Materials
Studienka A: one I1/2, one I3, eight C, five P1, 10 P4, 20 M1, 13 M2, two c, four p1, one p2/p3, two p4, nine m1, nine m2. See Table 3 for measurements. Studienka BC: two C (L = 1.28, W = 0.77; L = 1.29, W = 0.76), one P4 (L = 1.97), three M1, three M2 (L = 1.86, 1.89, 1.94), one p2/p3 (L = 0.62, W = 0.77). Studienka E: one dP4 (L = 1.70), one P4 (L = 2.00), one p4 (L = 1.61, W = 0.99), three m1 (L = 2.77, W1 = 1.18, 1.33), three m2. Pezinok A: two m1 (L = 2.58, W1 = 1.40; W2 = 1.56), one m2 (L = 2.73, W 1 = 1.42, W2 = 1.19). Triblavina: one M1 (W = 2.30).
Remarks
The described P1 are large and exceed the upper canine in size. The variability of this element was not previously mentioned in the literature. No P3 are recognized in our material. Similarly, Ziegler (Reference Ziegler2006b) did not report any P3 nor P1 from the rich Austrian localities. P3 are similar to P1 in size and shape but can be distinguished by the more anterior position of the cuspid and the reduction of the central anterior crest. However, this observation is based on the early Miocene material described by Klietmann et al. (Reference Klietmann, Nagel, Rummel and van den Hoek Ostende2014b), which could present dissimilarities with younger forms. The recognition of some antemolar elements appears hardly possible without complete material (e.g., Viret, Reference Viret1931; Seeman, Reference Seemann1938; Engesser, Reference Engesser2009).
Plesiodimylus chantrei is one of the most common Miocene insectivores because of both its high abundance and longevity, ranging from MN4 to MN11 (Klietmann, Reference Klietmann2013; Van den Hoek Ostende et al., Reference Van den Hoek Ostende, Bilgin, Braumuller, Cailleux, Skandalos, Casanovas-Vilar, van den Hoek Ostende, Janis and Saarinen2023), which also seems related to a generalist paleoecology. Its remarkable stratigraphical distribution led several workers to investigate the size and morphological homogeneity of Plesiodimylus chantrei, which did not support the division of this taxon (e.g., Engesser, Reference Engesser1972; Klietmann et al., Reference Klietmann, van den Hoek Ostende, Nagel and Rummel2015). Nevertheless, the various populations present a wide range of morphological and size variability. Ziegler (Reference Ziegler2006b) identified the material from Eichkogel as Plesiodimylus cf. P. chantrei, and the material from Götzendorf, Richardhof-Golfplatz, Richardhof-Wald, and Schernham as Plesiodimylus aff. P. chantrei. The use of the latter open nomenclature is related to the larger dimensions of the specimens from Austria, that almost reach the dimensions of the material from Nebelbergweg attributed to Plesiodimylus johanni by Kälin and Engesser (Reference Kälin and Engesser2001). Plesiodimylus johanni displays several morphological peculiarities, none of which are present in the Austrian and Slovak material (see Ziegler, Reference Ziegler2006b). Moreover, the morphometric variability of Plesiodimylus chantrei and that of Plesiodimylus johanni are not perfectly separated (Kälin and Engesser, Reference Kälin and Engesser2001, fig. 18). In the case of the Slovak material, there are no clear outliers as in Richardhof-Wald and Richardhof-Golfplatz, despite the mean measurements being higher than the usual mean measurements of Plesiodimylus chantrei. As in the case of Rudabánya (Ziegler, Reference Ziegler2005b), the use of open nomenclature for our sample is considered unjustified.
Rzebik-Kowalska (Reference Rzebik-Kowalska1996) highlighted that there is no clear pattern in the size evolution of Plesiodimylus chantrei. This is in line with the model of Klietmann (Reference Klietmann2013), in which Plesiodimylus chantrei corresponds to a uniform, widespread taxon in which separated populations have emerged, resulting in new local species. Nevertheless, the central European late Miocene record (e.g., Ziegler, Reference Ziegler2005b, Reference Ziegler2006b; Fejfar and Sabol, Reference Fejfar and Sabol2009; this work) does show a tendency to larger variety. In that sense, despite some similarities with the older Plesiodimylus crassidens, Plesiodimylus johanni can be seen as a local MN9 morphological variant of an already large-sized population of Plesiodimylus chantrei. This hypothesis is still coherent with the model of Klietmann (Reference Klietmann2013) and the size dimorphism observed in the Plesiodimylus material from the MN7/8 fissure fillings of Petersbuch by Ziegler (Reference Ziegler2005a).
Genus Metacordylodon Schlosser, Reference Schlosser1911
Type species
Cordylodon schlosseri Andreae, Reference Andreae1904, by monotypy.
Occurrence
Late-early Miocene to early-late Miocene of Europe (Andreae, Reference Andreae1904; Wegner, Reference Wegner1913; Viret, Reference Viret1931; Crusafont-Pairó and Golpe, Reference Crusafont-Pairó and Golpe1972; Fahlbusch, Reference Fahlbusch1989; Ziegler, Reference Ziegler2000, Reference Ziegler2005a, Reference Zieglerb; Fejfar and Sabol, Reference Fejfar, Sabol, Van den Hoek Ostende, Doukas and Reumer2005).
Metacordylodon aff. M. schlosseri (Andreae, Reference Andreae1904)
Figure 9.16–9.18
Description
The P4 is a robust ovoid tooth with three roots. A short crest starts on the posterior side of the massive paracone and ends abruptly on the posterior wall. A low posterior crest constitutes the straight anterolabial margin of the teeth. The large, conical protocone is situated in a more anterolingual position than the paracone. A posterolabical extension is present between the paracone and the posterior margin and consists of a weak slope.
The M1 is a massive molar with two basins. The labial side is not preserved (Fig. 9.17). The protocone is a massive subtriangular cusp connected by an anterior arm to a parastyle that lies in an anterolabial position. The anterior basin is deep and circular. The protocone is connected to the hypocone by a reversed V-shaped crest. The prehypocrista is weaker than the postprotocrista. The hypocone is smaller than the protocone and has an oblique orientation. The posterior cingulum is narrow, bordering a posterior basin less deep and larger than the anterior one. A short lingual cingulum is present between the protocone and the hypocone. The incomplete M2 has a subtriangular outline. The protocone is wide, anteroposteriorly compressed, and separated from the other cusps by a wide valley. A short crest descends from the lingual side of the protocone, leading to a small bulge on the anterolingual corner of the tooth. The postmetacristra is straight and connects the base of the protocone to a low metacone. The latter cusp creates a right angle at the posterolingual corner. The premetacrista is higher and larger than the postmetacrista. A short cingulum is partly preserved on the posterolabial margin.
Materials
Studienka A: one P4 (L = 1.87, W = 1.80), one broken M1 (L > 2.94), one broken M2 (L = 1.46).
Remarks
Metacordylodon is a very rare member of late Miocene fauna. Mainly recorded during the middle Miocene, this genus was also recorded in the early Miocene of Sandelzhausen and Franzensbad (Ziegler, Reference Ziegler2000). The late Miocene records include Schernham, Richardhof-Golfplatz (Ziegler, Reference Ziegler2006b), and Rudabánya (Ziegler, Reference Ziegler2005b). At the latter locality, the material was attributed to a small form named Metacordylodon aff. M. schlosseri. Our material is significantly smaller than middle Miocene material but only slightly smaller than the P4 (MAFI V20525) and the M1 (MAFI V20524) specimens described from Rudabánya. Our P4 (Fig. 9.16) is significantly smaller than the one from Richardhof-Golfplatz (Ziegler, Reference Ziegler2006b; RH-A2-C11) and does not present a distinct anterior cuspule. There are no morphological differences between Slovak and Hungarian samples. Because of this and considering the geographic and chronologic proximity between Rudabánya and Studienka A, our material is attributed to a similar, still unnamed form.
Discussion
Our study has yielded several new insights on the spatiotemporal distribution of late Miocene, central European Erinaceidae and Dimylidae. The late MN9 locality of Borský Svätý Jur evidences the first co-occurrence of Lantanotherium sanmigueli and ‘Schizogalerix’ voesendorfensis (Table 4). The latter species is only identified in the MN7/8 and the earliest MN9 (e.g., Gratkorn, Felsőtárkány 2/3, Vösendorf), whereas L. sanmigueli is recorded during the MN9 in slightly younger localities (e.g., Can Ponsic I, Götzendorf, Rudabánya). This supports a late extinction of ‘S.’ voesendorfensis in central Europe, after the apparent migration of L. sanmigueli from Asia (Cailleux et al., Reference Cailleux, Chaimanee, Jaeger and Chavasseau2020). This also suggests that Borský Svätý Jur has an intermediate position between Vösendorf and Götzendorf, the former being the last occurence of S. voesendorfensis and the latter being the first occurence of L. sanmigueli in central Europe. Ziegler (Reference Ziegler2005b) described a small trigonid of a m3 (MAFI V20510) from Rudabánya (MN9), with a protolophid structure that could indicate a basal Schizogalerix. The co-occurrence of these two genera was also identified in the Ukrainian MN9 locality of Grytsiv, where Schizogalerix sp. indet. and Lantanotherium sp. indet. were recognized (Nesin and Kovalchuk, Reference Nesin and Kovalchuk2021). However, whereas ‘S.’ voesendorfensis is found in western and central European localities during the MN9, eastern European localities were apparently occupied by another species, S. sarmatica.
Table 4. Composition of the Erinaceidae and Dimylidae from the late Miocene of Slovakia (in N of identified specimens).

The absence of Plesiodimylus chantrei at Borský Svätý Jur (Table 4) is surprising knowing its usual richness in deposits and its attested presence in the MN9 Austrian localities of the same basin (Ziegler, Reference Ziegler2006b). The Dimylidae were otherwise extremely abundant during the Vallesian, representing > 30% of the Eulipotyphlan material in Studienka, Richardhof-Wald, and Schernham (Daxner-Höck et al., Reference Daxner-Höck, Harzhauser and Göhlich2016, supplementary data). Their extremely fast decline during the earliest Turolian in the Pannonian region, as shown by the single element found from both Triblavina and Eichkogel, mirrors the overall rapid extinction of Dimylidae in the earliest Turolian.
The last occurrences of Lantanotherium in Europe are recorded during MN11, e.g., in Ambérieu 2 (Mein, Reference Mein, Agustí, Rook and Andrews1999), Dorn-Dürkheim (Ziegler, Reference Ziegler2006a), and possibly Csákvár under the name Galerix hipparionum Kretzoi, Reference Kretzoi1954 (see Kretzoi, Reference Kretzoi1954; Zijlstra and Flynn, Reference Zijlstra and Flynn2015). Bachmayer and Wilson (Reference Bachmayer and Wilson1978) identified in the extremely rich locality of Kohfidisch (MN 11) a single lower molar of Lantanotherium that is here identified as a m3 of L. cf. L. sanmigueli. An inspection of the unpublished insectivore collection housed at NHMV revealed the presence of only one other specimen, a gracile, complete left M2 (Fig. 7.17; collection number 2021/0049/0001) also identified as L. cf. L. sanmigueli. On the other hand, the material from the MN11 of Krásno contains a high proportion of L. sanmigueli. This supports a slightly older age for Krásno or a spatial heterogeneity in the distribution of the last populations of L. sanmigueli. We noticed the presence of a single specimen of Dimylidae in Triblavina, but also in Eichkogel (Daxner-Höck et al., Reference Daxner-Höck, Harzhauser and Göhlich2016, supplementary data), whereas the latter locality is considered slightly older than Kohfidisch and Krásno where dimylids are no longer recorded.
The material described here is characterized by a moderate diversity of erinaceids and dimylids in Slovakia, which was an expected result because central Europe is known as yielding a high overall diversity of insectivores (Furió et al., Reference Furió, Alba, Pérez-García, Gascó, Gasulla and Escaso2011; Van den Hoek Ostende et al., Reference Van den Hoek Ostende, Bilgin, Braumuller, Hír and Joniak2020). The Erinaceidae are dominated by the Galericinae, which are frequently encountered during the late Miocene. We observed the re-entrance of Schizogalerix in the Pannonian basin based on the MN11 fauna of Krásno and Eichkogel, leading to an increase in the regional abundance of Galericinae as noticed at Kohfidisch.
The history of Schizogalerix in the late Miocene of Europe can be split into two phases. The two MN9 species, ‘Schizogalerix’ voesendorfensis and S. sarmatica are found in clearly separated regions, i.e., central Europe for ‘S.’ voesendorfensis and eastern Europe for S. sarmatica. They share few morphological similarities because S. sarmatica is more advanced in the acquisition of the oblique dental pattern; they likely represent two different lineages. The Turolian is represented by S. zapfei and S. moedligensis, two species widely spread across Europe even if poorly recorded. Schizogalerix moedlingensis is found in Austria and Greece (Rabeder, Reference Rabeder1973; Doukas et al., Reference Doukas, van den Hoek Ostende, Theocharopoulos, Reumer and Schmidt-Kittler1995) whereas S. zapfei is found in Austria and France (Bachmayer and Wilson, Reference Bachmayer and Wilson1970; Mein, Reference Mein, Agustí, Rook and Andrews1999). The northern Carpathians probably played a very minor role in this distribution because the reliefs surrounding the Danube and Vienna basins were still low. Both Turolian species share closer similarities in their morphological grade with S. sarmatica and likely belong to the same lineage. Thus, they could have an eastern European origin.
The diversity of central European Erinaceinae was low during the late Miocene. The few taxa (tentatively) identified in Austria and Slovakia are not significantly different from the few identified by Van Dam et al. (Reference Van Dam, Mein and Alcalá2020) in Spain. Moreover, the highest diversity of Erinaceidae during the late Miocene of Slovakia is recorded in the MN11 of Krásno. A similar peak is observed in Spain during the MN11. This supports the suggestions of Van Dam et al. (Reference Van Dam, Mein and Alcalá2020) about a pan-European diversity phenomenon. Namely, the presence of few but widely spread species in Europe. Such a phenomenon seems to have persisted until the Pliocene before the entry of modern genera.
Conclusions
The collection of Eulipotyphla from the late Miocene of Slovakia indicates a moderate diversity but a high abundance of Erinaceidae and Dimylidae during the Vallesian. The material is dominated by ‘Schizogalerix’ voesendorfensis. The occurrence of S. cf. S. moedlingensis in Krásno (MN11) supports the early Turolian re-emergence of Schizogalerix in central Europe, likely descending from a lineage that originated in eastern Europe. The dimylid Plesiodimylus chantrei is frequent in late MN9 and MN10 deposits. Rare specimens support its survival to early MN11. The marked decline in abundance of the erinaceids and dimylids between the Vallesian and the Turolian of Slovakia reflects a wider faunal and trophic turnover in eulipotyphlan fauna, coinciding with the regression of Lake Pannon.
Acknowledgments
We would like to thank J. van Dam, L. Voyta, editor J. Calede, and managing editor J. Kastigar for their valuable comments that significantly improved the manuscript. We are also very grateful to U.B. Göhlich, W. Wessels, and E. Robert for their kind help in providing comparative material from the paleontological collections in Vienna, Utrecht, and Lyon. This research was supported by the grants UK/27/2022 and UK/221/2023 from Comenius University, the Scientific Grant Agency of the Ministry of Education, Science, Research, and Sport of the Slovak Republic and Slovak Academy of Sciences (VEGA) under the contract VEGA 1/0533/21, the Slovak Research and Development Agency (project APVV-20-0120), and the Austrian Science Funds (FWF) under the project P-15724-N06.
Declaration of competing interests
The authors declare none.
Accessibility of supplemental data
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.79cnp5j1t.
















