Osteoporosis is a chronic condition that results in reducing bone density and increasing bone fragility( 1 ). Osteoporosis is generally diagnosed with bone mineral density (BMD) measurement by dual-energy X-ray absorptiometry (DXA)( Reference Kanis, Melton and Christiansen 2 ). The World Health Organization( 3 ) defines osteoporosis as BMD T-score equal to or lower than −2·5( 3 ). In most patients, osteoporosis has no symptoms until a fracture occurs. Osteoporosis causes nearly nine million fractures annually worldwide and more than half of them occur in America and Europe( Reference Johnell and Kanis 4 ). On the basis of the severity and location of fractures, many complications, including significant disability, increased dependency, reduced quality of life and increased economic burden of healthcare costs, may occur( Reference Johnell and Kanis 4 – Reference Richmond, Aharonoff and Zuckerman 7 ).
Age, sex, ethnicity, family history, fracture history, some diseases and disorders (such as hypo-gonadal states, endocrine disorders, malnutrition, rheumatologic disorders, renal insufficiency and haematologic disorders), alcohol consumption, tobacco smoking and lack of physical activity are some of the risk factors for osteoporosis and fractures( Reference Anagnostis, Karagiannis and Kakafika 8 – Reference Berg, Kunins and Jackson 15 ). Dietary and nutritional factors have been identified as having a role in the prevention and incidence of osteoporosis and fractures. On the basis of previous studies, low intake of Ca, P, Mg, Zn, B, Fe, fluoride, Cu, vitamins A, K and D and high intake of Na, animal protein and soft drinks may result in osteoporosis and fractures( Reference Ilich and Kerstetter 16 – Reference Tucker, Morita and Qiao 18 ). Vitamin C is one of the dietary components that affect BMD. Vitamin C affects collagen synthesis and osteoblast genesis. Earlier studies have shown an inverse relationship between vitamin C intake and the risk of fracture or osteoporosis( Reference Sun, Li and Xie 19 – Reference Kim and Lee 21 ). However, one cohort study of 4367 people aged 39–79 years reached no significant association( Reference Finck, Hart and Lentjes 22 ). In addition, increased vitamin C intake was associated with higher BMD at different sites( Reference Kim, Cho and Park 23 – Reference Hall and Greendale 29 ). Other studies have failed to find any significant association between vitamin C intake and BMD( Reference Casale, von Hurst and Beck 30 , Reference Leveille, LaCroix and Koepsell 31 ).
Given the conflicting findings, this study aimed to systematically review available data on the association between vitamin C intake and BMD, as well as risk of fractures and osteoporosis, and to summarise this information by performing a meta-analysis.
Methods
This systematic review and meta-analysis was performed based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines( Reference Moher, Liberati and Tetzlaff 32 ) and has been recorded in PROSPERO by 42017055780 ID number.
Search strategy
Previous observational studies of vitamin C intake in relation to BMD or risk of fracture or osteoporosis were selected through searching PubMed, ISI Web of Science and Google Scholar before February 2017 by two reviewers independently (H. M., S. S.-b.). We used the following keywords in the search: (‘vitamin C’[tiab] OR ‘ascorbic acid’[tiab] OR ‘acid ascorbic’[tiab] OR ascorbate[tiab] OR ‘ascorbic acid’[MeSH]) AND (‘bone mineral density’[tiab] OR ‘bone mass density’[tiab] OR ‘bone density’[tiab] OR BMD[tiab] OR fracture[tiab] OR osteoporosis[tiab] OR ‘bone density’[MeSH] OR ‘fractures, bone’[MeSH] OR osteoporosis[MeSH]). No limitation was applied during the search. The reference lists of retrieved papers were also examined to avoid missing any published data.
Inclusion criteria
Publications that fulfilled the following criteria were eligible for inclusion: (1) all studies, conducted on humans, that examined the relationship between vitamin C intake and BMD, risk of fractures and osteoporosis; (2) studies that were of cross-sectional or case–control or cohort design; (3) those that reported OR or hazards ratios (HR) along with 95 % CI for fracture and osteoporosis; and (4) those that reported correlation coefficient for BMD.
Studies were excluded because of the following reasons: (1) those that were letters, comments, reviews, meta-analyses, ecological studies, animal studies and clinical trial studies; (2) studies that did not report any estimates for the association between vitamin C intake and the intended outcomes; and (3) studies that examined the relationship in children and adolescents. When we found more than one published report based on the same study population( Reference New, Bolton-Smith and Grubb 33 ), only the most comprehensive publication was included in this meta-analysis( Reference Macdonald, New and Golden 34 ).
Data extraction
From each eligible study, the following information was extracted: first author, year of publication, study design, country, age range, sex, sample size, number of cases, duration of follow-up, exposure variable, assessment of exposure, outcome variable, assessment of outcome, relevant effect sizes (OR or HR and 95 % CI, correlation coefficient), methods of fracture or BMD quantification and covariates adjusted.
Quality assessment
The quality of included studies was examined using the Newcastle–Ottawa Scale (NOS) by two reviewers independently (H. M. and S. S.-b.)( Reference Wells, Shea and O’Connell 35 ). The NOS assigns a maximum of nine points to each study: four for selection, two for comparability and three for assessment of outcomes and exposures. In the current analysis, when a study got more than median stars, it was considered as relatively high quality; otherwise, it was deemed to have low quality. All included studies were agreed upon.
Statistical methods
The effect sizes that we used in this analysis were HR and OR and their 95 % CI for risk of fracture and osteoporosis in the highest v. the lowest category of vitamin C intake. In addition, the correlation coefficient between vitamin C intake and BMD was used. For correlation analysis, Fisher’s Z and sample size was calculated and used to conduct the meta-analysis. A fixed-effect model was used to calculate pooled risk estimates by generic inverse variance method by the user-written ‘metan’ command in Stata (version 14) software( Reference Egger, Smith and Altman 36 ). Heterogeneity was assessed using Cochrane’s Q test, and I 2 statistic provided the relative amount of variance of the summary effect( Reference Higgins, Thompson and Deeks 37 ). In cases with heterogeneity, random-effects model (DerSimonian–Laird) was used. To evaluate the predefined sources of heterogeneity, subgroup analyses were conducted using a fixed-effect model with the user-written ‘metan’ command (‘by option’) in Stata (version 14) software( Reference Egger, Smith and Altman 36 ). Publication bias was assessed by visual inspection of funnel plots. Tests for funnel plot asymmetry were done with the user-written ‘metabias’ command in Stata (version 11) software 31. Statistical analyses were carried out by the use of STATA, version 14.0 (StataCorp). P values that were <0·1 for heterogeneity test and <0·05 in others were considered statistically significant.
Results
Findings from a systematic review
In total, 1462 articles were found in our initial search. After screening titles and abstracts, 1423 articles were excluded. After these exclusions, thirty-eight articles remained for systematic review and twelve studies were included in the meta-analysis. The details of the study selection process are shown in Fig. 1.
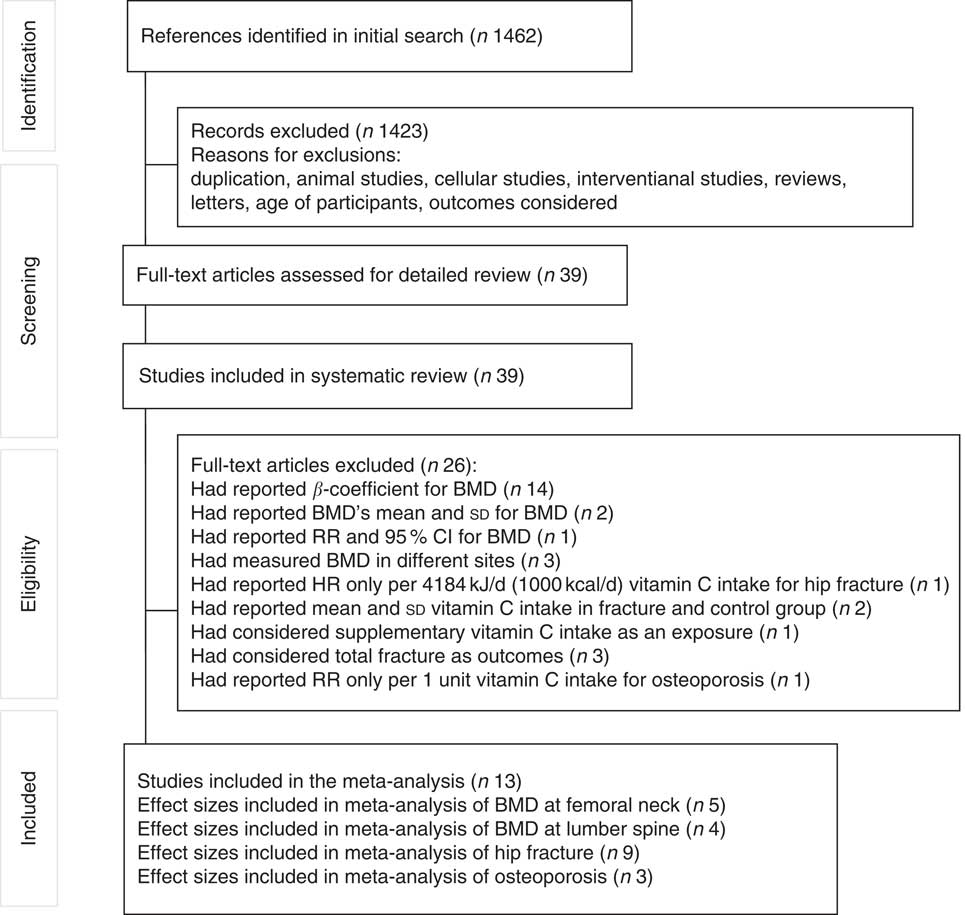
Fig. 1 Flow chart of article searching. BMD, bone mineral density; RR, relative risk; HR, hazard ratio.
We included thirty-eight studies (ten cohort( Reference Sahni, Hannan and Gagnon 20 , Reference Finck, Hart and Lentjes 22 , Reference Macdonald, New and Golden 34 , Reference Chan, Woo and Leung 38 – Reference White, Atchison and Gornbein 44 ), seven case–control( Reference Sun, Li and Xie 19 , Reference Park, Heo and Park 26 , Reference Lumbers, New and Gibson 45 – Reference Zhang, Munger and West 49 ) and twenty-one cross-sectional( Reference Kim and Lee 21 , Reference Kim, Cho and Park 23 – Reference Kim, Kim and Lim 25 , Reference Prynne, Mishra and O’Connell 27 – Reference Leveille, LaCroix and Koepsell 31 , Reference Hernández-Avila, Stampfer and Ravnikar 50 – Reference Zhang, Zhang and Shi 61 )) in the systematic review (Tables 1–3). These studies involved 106 741 individuals aged 20–103 years. All studies were published between 1988 and 2016. In all, fourteen studies were conducted in USA( Reference Sahni, Hannan and Gagnon 20 , Reference Ilich, Brownbill and Tamborini 28 , Reference Hall and Greendale 29 , Reference Leveille, LaCroix and Koepsell 31 , Reference Holbrook, Barrett-Connor and Wingard 39 , Reference Simon and Hudes 42 , Reference White, Atchison and Gornbein 44 , Reference Nieves, Grisso and Kelsey 48 – Reference Hernández-Avila, Stampfer and Ravnikar 50 , Reference Morton, Barrett-Connor and Schneider 52 , Reference Sahni, Hannan and Gagnon 55 , Reference Wang, Villa and Marcus 58 , Reference Wolf, Cauley and Pettinger 59 ), thirteen in East Asian countries and New Zealand( Reference Sun, Li and Xie 19 , Reference Kim and Lee 21 , Reference Kim, Cho and Park 23 – Reference Park, Heo and Park 26 , Reference Casale, von Hurst and Beck 30 , Reference Chan, Woo and Leung 38 , Reference Sugiura, Nakamura and Ogawa 43 , Reference Sasaki and Yanagibori 56 , Reference Sugiura, Nakamura and Ogawa 57 , Reference Yang and Kim 60 , Reference Zhang, Zhang and Shi 61 ) and eleven in European countries( Reference Finck, Hart and Lentjes 22 , Reference Prynne, Mishra and O’Connell 27 , Reference Macdonald, New and Golden 34 , Reference Key, Appleby and Spencer 40 , Reference Samieri, Ginder Coupez and Lorrain 41 , Reference Lumbers, New and Gibson 45 – Reference Michaelsson, Holmberg and Mallmin 47 , Reference Kaptoge, Welch and McTaggart 51 , Reference New, Robins and Campbell 53 , Reference Rivas, Romero and Mariscal-Arcas 54 ). Two studies were conducted on males( Reference Chan, Woo and Leung 38 , Reference Yang and Kim 60 ), fourteen on both males and females( Reference Sun, Li and Xie 19 – Reference Kim and Lee 21 , Reference Liu, Leung and Wong 24 , Reference Prynne, Mishra and O’Connell 27 , Reference Holbrook, Barrett-Connor and Wingard 39 – Reference Simon and Hudes 42 , Reference White, Atchison and Gornbein 44 , Reference Martínez-Ramírez, Pérez and Delgado-Martínez 46 , Reference Zhang, Munger and West 49 , Reference Morton, Barrett-Connor and Schneider 52 , Reference Sahni, Hannan and Gagnon 55 ) and others on females( Reference Kim, Cho and Park 23 , Reference Kim, Kim and Lim 25 , Reference Park, Heo and Park 26 , Reference Hall and Greendale 29 – Reference Leveille, LaCroix and Koepsell 31 , Reference Macdonald, New and Golden 34 , Reference Lumbers, New and Gibson 45 , Reference Michaelsson, Holmberg and Mallmin 47 , Reference Nieves, Grisso and Kelsey 48 , Reference Hernández-Avila, Stampfer and Ravnikar 50 , Reference New, Robins and Campbell 53 , Reference Rivas, Romero and Mariscal-Arcas 54 , Reference Sugiura, Nakamura and Ogawa 57 – Reference Wolf, Cauley and Pettinger 59 , Reference Zhang, Zhang and Shi 61 ). Three studies had considered osteoporosis as the outcome( Reference Kim and Lee 21 , Reference Sugiura, Nakamura and Ogawa 43 , Reference Zhang, Zhang and Shi 61 ); twelve studies examined fracture as the outcome( Reference Sun, Li and Xie 19 , Reference Sahni, Hannan and Gagnon 20 , Reference Finck, Hart and Lentjes 22 , Reference Holbrook, Barrett-Connor and Wingard 39 – Reference Samieri, Ginder Coupez and Lorrain 41 , Reference White, Atchison and Gornbein 44 – Reference Zhang, Munger and West 49 ); and twenty-two studies assessed BMD as the outcome( Reference Kim, Cho and Park 23 – Reference Kim, Kim and Lim 25 , Reference Prynne, Mishra and O’Connell 27 – Reference Leveille, LaCroix and Koepsell 31 , Reference Macdonald, New and Golden 34 , Reference Chan, Woo and Leung 38 , Reference Hernández-Avila, Stampfer and Ravnikar 50 – Reference Yang and Kim 60 ). One study had reported both BMD and fracture as the main outcomes( Reference Simon and Hudes 42 ) and two studies had reported both osteoporosis and BMD as the main outcomes( Reference Park, Heo and Park 26 , Reference Zhang, Zhang and Shi 61 ). The duration of follow-up in cohort studies was between 4( Reference Chan, Woo and Leung 38 , Reference Sugiura, Nakamura and Ogawa 43 ) and 20 years( Reference White, Atchison and Gornbein 44 ). Four studies used supplementary vitamin C intake as exposure( Reference Sahni, Hannan and Gagnon 20 , Reference White, Atchison and Gornbein 44 , Reference Morton, Barrett-Connor and Schneider 52 , Reference Sahni, Hannan and Gagnon 55 ), four studies used total dietary and supplementary vitamin C intakes as exposure( Reference Sahni, Hannan and Gagnon 20 , Reference Leveille, LaCroix and Koepsell 31 , Reference Hernández-Avila, Stampfer and Ravnikar 50 , Reference Wolf, Cauley and Pettinger 59 ) and in other studies dietary vitamin C intakes have been used as exposure( Reference Sun, Li and Xie 19 – Reference Leveille, LaCroix and Koepsell 31 , Reference Macdonald, New and Golden 34 , Reference Chan, Woo and Leung 38 – Reference Sugiura, Nakamura and Ogawa 43 , Reference Lumbers, New and Gibson 45 – Reference Kaptoge, Welch and McTaggart 51 , Reference New, Robins and Campbell 53 – Reference Zhang, Zhang and Shi 61 ). In seven studies, 24-h dietary recall for dietary assessment( Reference Kim and Lee 21 , Reference Kim, Kim and Lim 25 , Reference Holbrook, Barrett-Connor and Wingard 39 , Reference Simon and Hudes 42 , Reference Lumbers, New and Gibson 45 , Reference Rivas, Romero and Mariscal-Arcas 54 , Reference Yang and Kim 60 ), in four studies food records( Reference Finck, Hart and Lentjes 22 , Reference Prynne, Mishra and O’Connell 27 , Reference Ilich, Brownbill and Tamborini 28 , Reference Kaptoge, Welch and McTaggart 51 ), questionnaire for three studies( Reference White, Atchison and Gornbein 44 , Reference Morton, Barrett-Connor and Schneider 52 , Reference Wang, Villa and Marcus 58 ), in one diet history( Reference Sasaki and Yanagibori 56 ) and in other studies validated FFQ( Reference Sun, Li and Xie 19 , Reference Sahni, Hannan and Gagnon 20 , Reference Kim, Cho and Park 23 , Reference Liu, Leung and Wong 24 , Reference Park, Heo and Park 26 , Reference Hall and Greendale 29 – Reference Leveille, LaCroix and Koepsell 31 , Reference Macdonald, New and Golden 34 , Reference Chan, Woo and Leung 38 , Reference Key, Appleby and Spencer 40 , Reference Samieri, Ginder Coupez and Lorrain 41 , Reference Sugiura, Nakamura and Ogawa 43 , Reference Martínez-Ramírez, Pérez and Delgado-Martínez 46 – Reference Hernández-Avila, Stampfer and Ravnikar 50 , Reference New, Robins and Campbell 53 , Reference Sahni, Hannan and Gagnon 55 , Reference Sugiura, Nakamura and Ogawa 57 , Reference Wolf, Cauley and Pettinger 59 , Reference Zhang, Zhang and Shi 61 ) were used to record exposure.
Table 1 Characteristics of studies that reported the relationship between vitamin C intake in relation to bone mineral density (BMD) (β-Coefficients with their standard errors; mean values and standard deviations; odds ratios and 95 % confidence intervals)
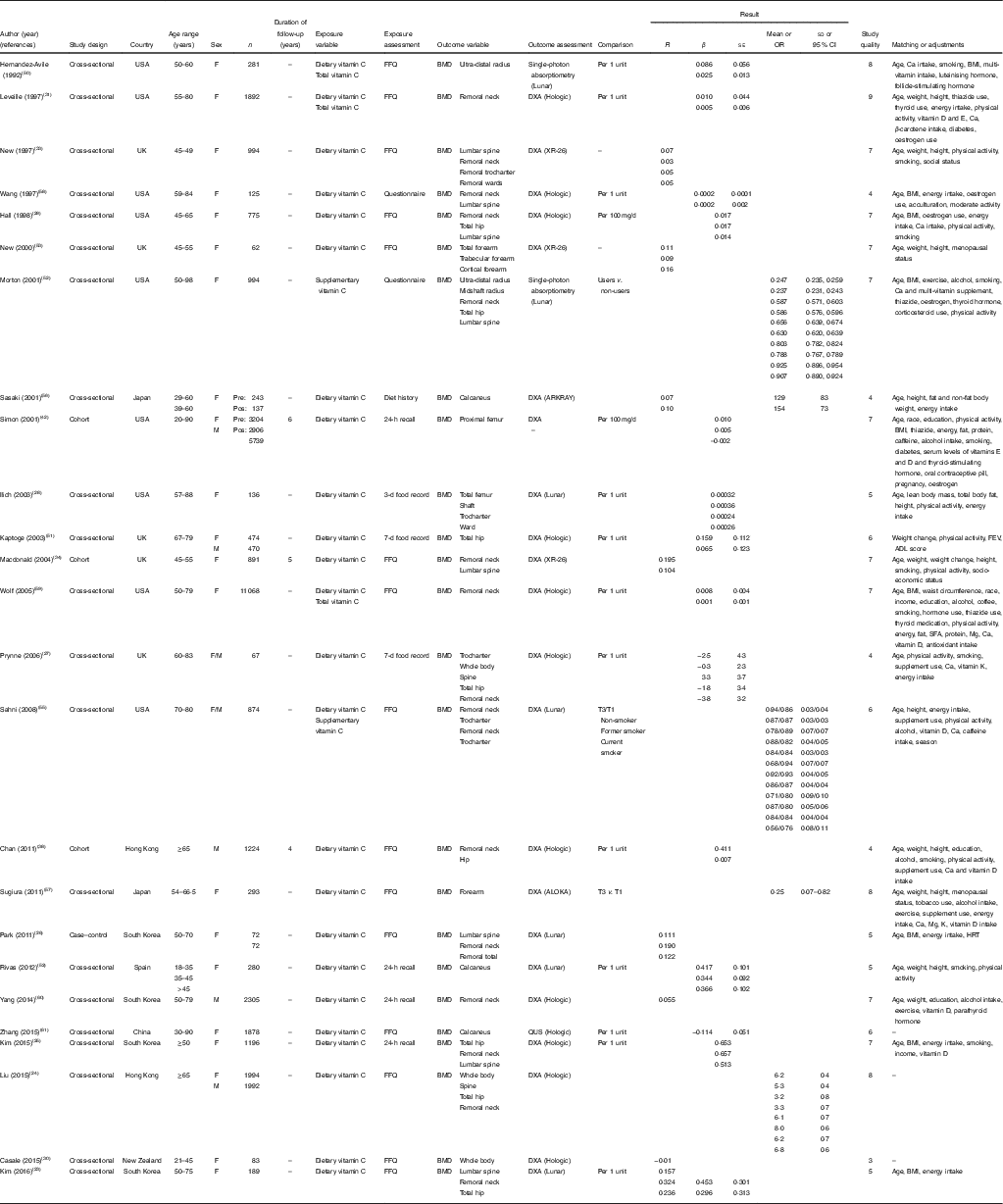
DXA, dual-energy X-ray absorptiometry; F, female; M, male; HRT, hormone replacement therapy; QUS, qualitative ultrasound.
Table 2 Characteristics of studies that reported the relationship between vitamin C intake and risk of fracture (Odds ratios, relative risks (RR) and 95 % confidence intervals; mean values and standard deviations)
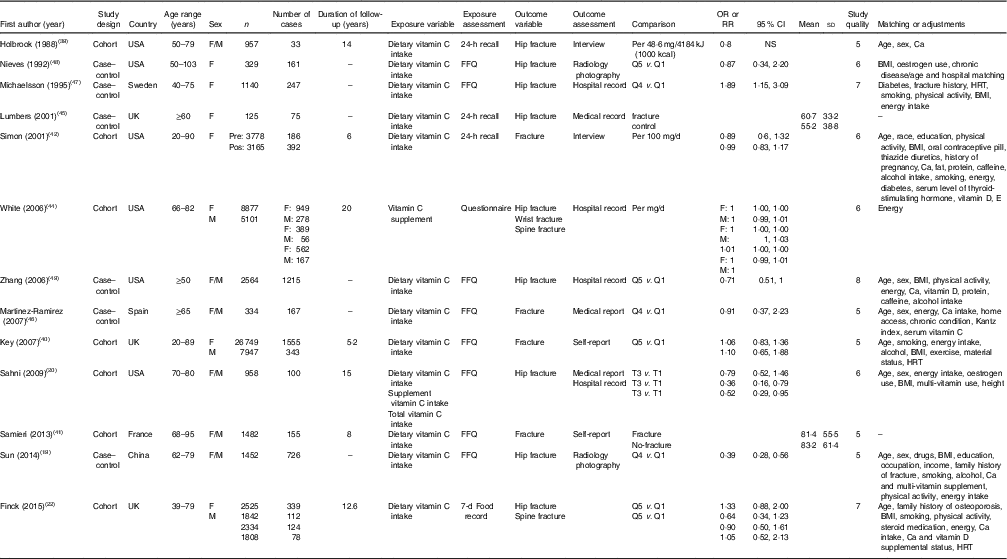
F, female; M, male; Q, quantiles; T, tertiles; HRT, hormone replacement therapy.
Table 3 Characteristics of studies that reported the relationship between vitamin C intake and risk of osteoporosis (OR, relative risk (RR) and 95 % confidence intervals; β-coefficients with their standard errors)
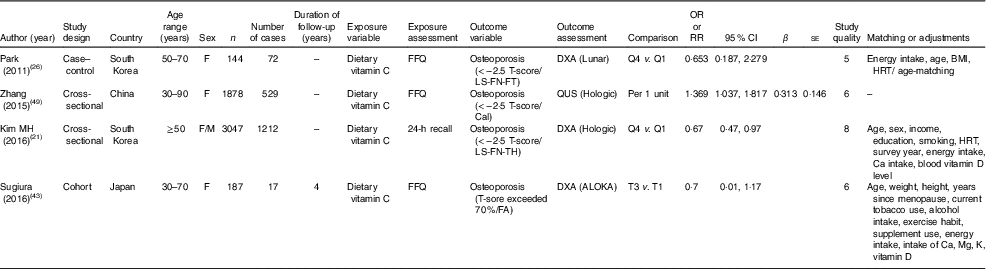
F, female; M, male; LS, lumber spine; FN, femur neck; FT, femoral total; Q, quantiles; HRT, hormone replacement therapy; TH, total hip; CAL, calcaneus; FA, forearm.
BMD was measured at different sites in included studies; fourteen studies have quantified BMD in femoral neck( Reference Kim, Cho and Park 23 – Reference Prynne, Mishra and O’Connell 27 , Reference Hall and Greendale 29 , Reference Leveille, LaCroix and Koepsell 31 , Reference Macdonald, New and Golden 34 , Reference Chan, Woo and Leung 38 , Reference Morton, Barrett-Connor and Schneider 52 , Reference Sahni, Hannan and Gagnon 55 , Reference Wang, Villa and Marcus 58 – Reference Yang and Kim 60 ), seven at lumbar spine( Reference Kim, Cho and Park 23 , Reference Kim, Kim and Lim 25 , Reference Park, Heo and Park 26 , Reference Hall and Greendale 29 , Reference Macdonald, New and Golden 34 , Reference Morton, Barrett-Connor and Schneider 52 , Reference Wang, Villa and Marcus 58 ), nine at total hip( Reference Kim, Cho and Park 23 – Reference Kim, Kim and Lim 25 , Reference Prynne, Mishra and O’Connell 27 , Reference Hall and Greendale 29 , Reference Macdonald, New and Golden 34 , Reference Chan, Woo and Leung 38 , Reference Kaptoge, Welch and McTaggart 51 , Reference Morton, Barrett-Connor and Schneider 52 ) and three at trochanter( Reference Prynne, Mishra and O’Connell 27 , Reference Ilich, Brownbill and Tamborini 28 , Reference Sahni, Hannan and Gagnon 55 ). BMD was also measured in ultra-distal radius( Reference Hernández-Avila, Stampfer and Ravnikar 50 , Reference Morton, Barrett-Connor and Schneider 52 ), calcaneus( Reference Rivas, Romero and Mariscal-Arcas 54 , Reference Sasaki and Yanagibori 56 , Reference Zhang, Zhang and Shi 61 ), total forearm, trabecular forearm, cortical forearm( Reference New, Robins and Campbell 53 ), midshaft radius( Reference Kaptoge, Welch and McTaggart 51 ), proximal femur( Reference Simon and Hudes 42 ), total femur, shaft, ward( Reference Ilich, Brownbill and Tamborini 28 ), whole body( Reference Prynne, Mishra and O’Connell 27 , Reference Casale, von Hurst and Beck 30 ), forearm( Reference Sugiura, Nakamura and Ogawa 57 ) and femoral total( Reference Park, Heo and Park 26 ). All studies used DXA for measuring BMD, except two studies that used single-photon absorptiometry( Reference Hernández-Avila, Stampfer and Ravnikar 50 , Reference Morton, Barrett-Connor and Schneider 52 ) and one that used qualitative ultrasound (QUS)( Reference Zhang, Zhang and Shi 61 ). To assess BMD, eleven studies applied Hologic scanner( Reference Liu, Leung and Wong 24 , Reference Kim, Kim and Lim 25 , Reference Prynne, Mishra and O’Connell 27 , Reference Hall and Greendale 29 – Reference Leveille, LaCroix and Koepsell 31 , Reference Chan, Woo and Leung 38 , Reference Kaptoge, Welch and McTaggart 51 , Reference Wolf, Cauley and Pettinger 59 – Reference Zhang, Zhang and Shi 61 ), five applied Lunnar scanner( Reference Kim, Cho and Park 23 , Reference Park, Heo and Park 26 , Reference Ilich, Brownbill and Tamborini 28 , Reference Rivas, Romero and Mariscal-Arcas 54 , Reference Sahni, Hannan and Gagnon 55 ), two studies applied XR-26 scanner( Reference Key, Appleby and Spencer 40 , Reference New, Robins and Campbell 53 ), one study applied ARKRAY scanner( Reference Sasaki and Yanagibori 56 ), one study applied ALOKA scanner( Reference Sugiura, Nakamura and Ogawa 57 ) and one did not report the measurement tool( Reference Simon and Hudes 42 ). In terms of site of fracture, nine studies considered hip( Reference Sun, Li and Xie 19 , Reference Sahni, Hannan and Gagnon 20 , Reference Finck, Hart and Lentjes 22 , Reference Holbrook, Barrett-Connor and Wingard 39 , Reference White, Atchison and Gornbein 44 , Reference Lumbers, New and Gibson 45 , Reference Michaelsson, Holmberg and Mallmin 47 – Reference Zhang, Munger and West 49 ), four considered total fracture( Reference Key, Appleby and Spencer 40 , Reference Samieri, Ginder Coupez and Lorrain 41 , Reference Simon and Hudes 42 , Reference Martínez-Ramírez, Pérez and Delgado-Martínez 46 ), two studies considered spine( Reference Finck, Hart and Lentjes 22 , Reference White, Atchison and Gornbein 44 ) and one study considered wrist fracture( Reference White, Atchison and Gornbein 44 ). In terms of osteoporosis, three studies measured BMD T-score by DXA( Reference Kim and Lee 21 , Reference Park, Heo and Park 26 , Reference Sugiura, Nakamura and Ogawa 43 ) and one study measured BMD T-score by QUS( Reference Zhang, Zhang and Shi 61 ).
In terms of BMD, increased vitamin C intake was associated with higher BMD at different sites in sixteen studies ( Reference Kim, Cho and Park 23 – Reference Hall and Greendale 29 , Reference Macdonald, New and Golden 34 , Reference Simon and Hudes 42 , Reference Martínez-Ramírez, Pérez and Delgado-Martínez 46 , Reference Morton, Barrett-Connor and Schneider 52 , Reference Rivas, Romero and Mariscal-Arcas 54 , Reference Sahni, Hannan and Gagnon 55 , Reference Sugiura, Nakamura and Ogawa 57 , Reference Yang and Kim 60 , Reference Zhang, Zhang and Shi 61 ). However, no significant association was seen between vitamin C intake and BMD at different sites in other studies( Reference Casale, von Hurst and Beck 30 , Reference Leveille, LaCroix and Koepsell 31 , Reference Chan, Woo and Leung 38 , Reference Kaptoge, Welch and McTaggart 51 , Reference New, Robins and Campbell 53 , Reference Sasaki and Yanagibori 56 , Reference Wang, Villa and Marcus 58 , Reference Wolf, Cauley and Pettinger 59 ). Higher vitamin C intake was associated with a reduced risk of fracture in two studies( Reference Sun, Li and Xie 19 , Reference Rivas, Romero and Mariscal-Arcas 54 ). No significant association was found in other studies( Reference Finck, Hart and Lentjes 22 , Reference Holbrook, Barrett-Connor and Wingard 39 , Reference Key, Appleby and Spencer 40 , Reference Simon and Hudes 42 , Reference White, Atchison and Gornbein 44 , Reference Martínez-Ramírez, Pérez and Delgado-Martínez 46 – Reference Zhang, Munger and West 49 ). Osteoporosis was directly associated with dietary vitamin C intake in one study( Reference Zhang, Zhang and Shi 61 ) and inversely associated with dietary vitamin C intake in another study( Reference Kim and Lee 21 ). However, two studies did not find any significant association between dietary vitamin C intake and the risk of osteoporosis( Reference Park, Heo and Park 26 , Reference Sugiura, Nakamura and Ogawa 43 ).
Findings of the meta-analysis
Vitamin C intake and bone mineral density
Out of twenty-four studies that were included in the systematic review, we excluded twenty studies because of the following reasons: those that reported results as beta regression coefficient( Reference Liu, Leung and Wong 24 , Reference Kim, Kim and Lim 25 , Reference Prynne, Mishra and O’Connell 27 – Reference Hall and Greendale 29 , Reference Leveille, LaCroix and Koepsell 31 , Reference Chan, Woo and Leung 38 , Reference Simon and Hudes 42 , Reference Hernández-Avila, Stampfer and Ravnikar 50 , Reference Kaptoge, Welch and McTaggart 51 , Reference Rivas, Romero and Mariscal-Arcas 54 , Reference Wang, Villa and Marcus 58 , Reference Wolf, Cauley and Pettinger 59 , Reference Zhang, Zhang and Shi 61 ); those that reported BMD’s means and standard deviations in categories of vitamin C intake( Reference Sahni, Hannan and Gagnon 55 ) or users vitamin C supplement v. non-users( Reference Morton, Barrett-Connor and Schneider 52 ); those that reported relative risks (RR) and 95 % CI in tertiles of vitamin C intake( Reference Sugiura, Nakamura and Ogawa 57 ); and those that measured BMD at calcaneus( Reference Sasaki and Yanagibori 56 ), total forearm, trabecular forearm, cortical forearm( Reference New, Robins and Campbell 53 ) and whole body( Reference Casale, von Hurst and Beck 30 ). Finally, four studies( Reference Kim, Cho and Park 23 , Reference Park, Heo and Park 26 , Reference Macdonald, New and Golden 34 , Reference Yang and Kim 60 ) that examined the correlation between dietary vitamin C intake and BMD at femoral neck (four effect sizes obtained from four studies) and lumbar spine (three effect sizes obtained from three studies) were included in the meta-analysis. Studies that reported correlation coefficient between dietary vitamin C intake and BMD included 3529 individuals in total. The meta-analysis of four effect sizes obtained from four studies at the femoral neck indicated that greater dietary vitamin C intake was significantly associated with BMD (Fisher’s Z: 0·18; 95 % CI 0·06, 0·30, P 0·003). Although between-study heterogeneity was statistically significant (I 2=87·6 %; P heterogeneity<0·001), we did not perform subgroup analysis to find the source of heterogeneity owing to the low number of publications. Combining three effect sizes obtained from three studies at lumbar spine indicated that greater dietary vitamin C intake was significantly associated with BMD (Fisher’s Z=0·14; 95 % CI 0·06, 0·22, P 0·001). Fisher’s Z and 95 % CI that was calculated in meta-analysis is equal to the correlation coefficient and 95 % CI. No evidence of between-study heterogeneity was found (I 2=30·7 %; P heterogeneity=0·236) (Fig. 2). Begg’s test (P=0·53) and Egger’s test (P=0·07) showed no publication bias.
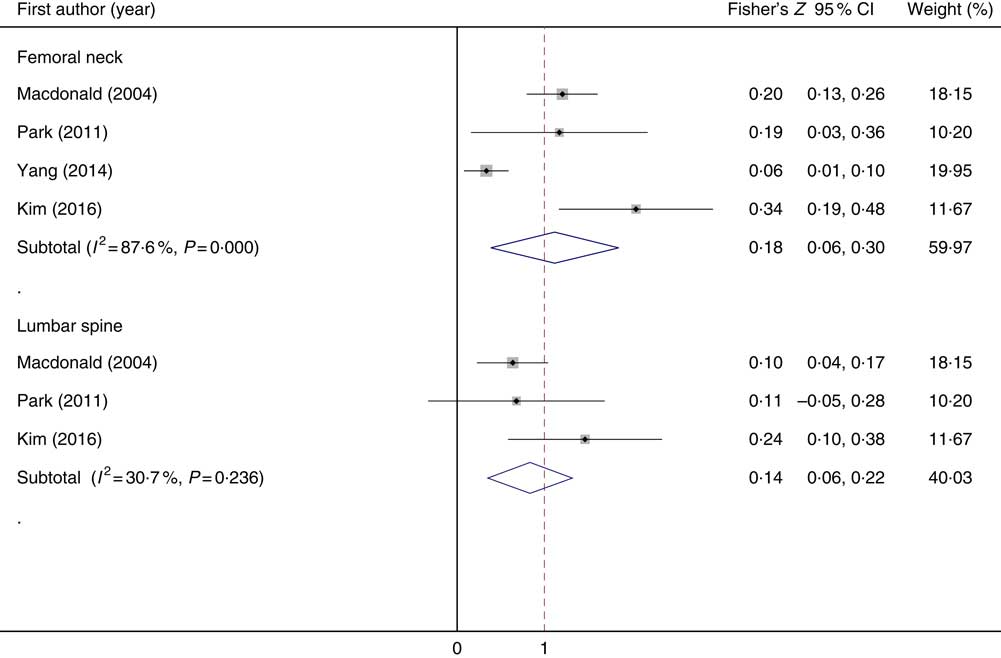
Fig. 2 Forest plot of correlation coefficient in bone mineral density at the femoral neck and lumbar spine and dietary vitamin C intake.
Vitamin c intake and risk of fracture
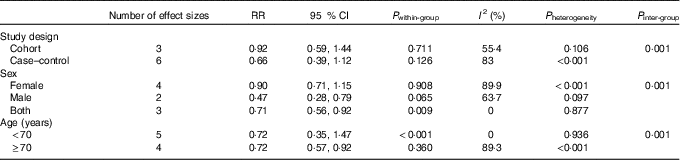
Of thirteen studies that were in the systematic review, we excluded seven studies from the meta-analysis: those that considered total fracture as the outcome( Reference Key, Appleby and Spencer 40 , Reference Simon and Hudes 42 , Reference Martínez-Ramírez, Pérez and Delgado-Martínez 46 ), supplementary vitamin C as an exposure( Reference White, Atchison and Gornbein 44 ), mean and standard deviation of vitamin C intake in fracture and control groups( Reference Samieri, Ginder Coupez and Lorrain 41 , Reference Lumbers, New and Gibson 45 ) and those that reported the risk of hip fracture for vitamin C consumption per 4184 kJ/d (1000 kcal/d)( Reference Holbrook, Barrett-Connor and Wingard 39 ). Finally, six studies that considered hip fracture were included in the meta-analysis( Reference Sun, Li and Xie 19 , Reference Sahni, Hannan and Gagnon 20 , Reference Finck, Hart and Lentjes 22 , Reference Michaelsson, Holmberg and Mallmin 47 – Reference Zhang, Munger and West 49 ). Publications that examined the association between vitamin C intake and risk of fracture included 10 810 individuals with 2898 cases of hip fractures. In this meta-analysis, nine effect sizes, obtained from six studies, indicated that high levels of vitamin C intake were not significantly associated with risk of hip fractures (overall RR=0·74; 95 % CI 0·51, 1·08, P 0·001) (Fig. 3). Significant between-study heterogeneity was found (I 2=79·1 %; P heterogeneity<0·001). Subgroup analysis was performed to find the potential sources of heterogeneity. This analysis indicated that study design, sex and age were the main sources of between-study heterogeneity (Table 4). In cohort and case–control studies, non-significant associations were found. However, between-study heterogeneity was not apparent in cohort studies. In males, dietary vitamin C intake was significantly associated with hip fracture (overall RR=0·47; 95 % CI 0·28, 0·79, P 0·004). In females, a non-significant association was seen (overall RR=0·90; 95 % CI 0·71, 1·15, P 0·394). In both females and males, 29 % decreased incidence of hip fracture was seen by greater dietary vitamin C intake (overall RR=0·71; 95 % CI 0·56, 0·92, P 0·009). The association between dietary vitamin C intake and hip fracture was significant in participants aged 70 years or older (overall RR=0·72; 95 % CI 0·57, 0·92, P 0·009). No publication bias was found (Begg’s test P=0·74 and Egger’s test P=0·83).
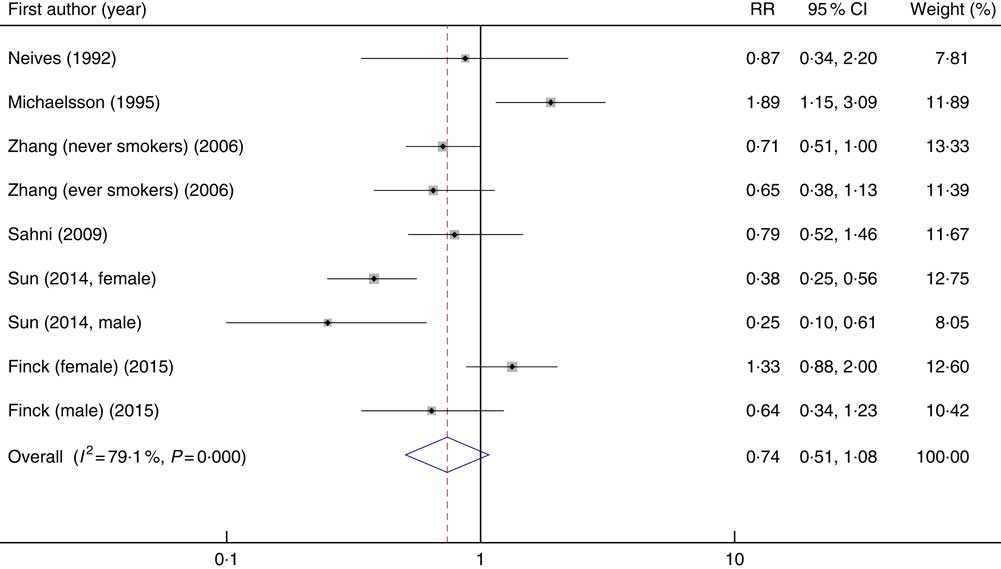
Fig. 3 Forest plot of the association between dietary vitamin C intake and the risk of hip fracture. RR, relative risk.
Table 4 Subgroup analysis of dietary vitamin C intake and risk of hip fracture (Relative risks (RR) and 95 % confidence intervals)
Vitamin C intake and risk of osteoporosis
Out of four included studies in the systematic review, one study was excluded from the meta-analysis( Reference Zhang, Zhang and Shi 61 ). This study reported the risk of osteoporosis for one unit vitamin C consumption. Three studies considered the risk of osteoporosis in categorised of vitamin C intake which were included in the meta-analysis( Reference Kim and Lee 21 , Reference Park, Heo and Park 26 , Reference Sugiura, Nakamura and Ogawa 43 ). These studies included 3378 individuals with 1301 cases of osteoporosis. In the meta-analysis of effect sizes obtained from three included studies, we found that higher vitamin C intake was inversely associated with the risk of osteoporosis (overall RR=0·67; 95 % CI 0·47, 0·94, P: 0·022; I 2=0 %; P heterogeneity=0·999) (Fig. 4). No publication bias was found (Begg’s test P=0·60 and Egger’s test P=0·95).
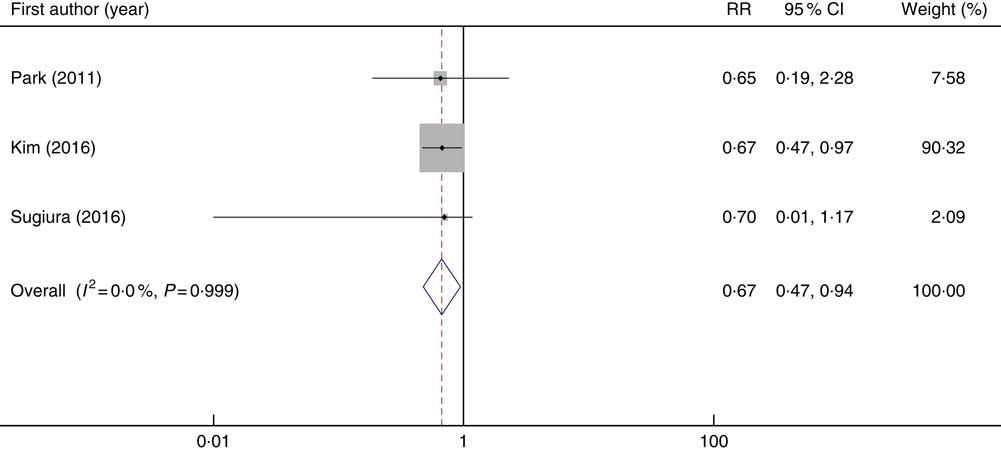
Fig. 4 Forest plot of the association between dietary vitamin C intake and the risk of osteoporosis. RR, relative risk.
Discussion
In this meta-analysis, we found that greater dietary intake of vitamin C was positively associated with a higher BMD at the femoral neck and lumbar spine. In addition, greater dietary vitamin C intake was significantly associated with reduced risk of hip fracture and osteoporosis. This is the first meta-analysis that examined the association of dietary vitamin C intake with risk of fracture, osteoporosis and BMD.
Osteoporosis is a chronic condition that affects a large number of elderly people( Reference Liu, Liu and Liu 62 ). The incidence of fracture has increased recently in parallel to ageing throughout the world( Reference Melton, Kearns and Atkinson 63 ). Among several factors that might influence the risk of fractures and osteoporosis, dietary intakes are of great importance. Vitamin C is essential for collagen synthesis and osteoblastogenesis. Ascorbic acid specifically stimulated type I and III collagen synthesis( Reference Carinci, Pezzetti and Spina 64 – Reference Barnes 67 ). In addition, vitamin C deficiency stimulates osteoclastogenesis( Reference Hie and Tsukamoto 68 ). During osteoclastogenesis, ascorbic acid acts as an oxidant, first stimulating osteoclast formation, later limiting osteoclast life span. This action results in adaptation of osteoclastogenesis( Reference Hie and Tsukamoto 68 , Reference Le Nihouannen, Barralet and Fong 69 ). Although vitamin C indeed has a major effect on collagen synthesis, the main and most crucial effect is on osteoblast differentiation. Vitamin C can prevent the loss of osteoblast differentiation markers (Osterix, osteocalcin, runt-related transcription 2, bone morphogenetic protein 2) and attenuate bone loss, as well as stimulate bone formation( Reference Aghajanian, Hall and Wongworawat 70 ). Vitamin C actually is a marker of dietary fruit and vegetable intake, healthy dietary patterns and total antioxidant intake. Greater intake of fruit, vegetables and antioxidants, and healthier dietary pattern, was associated with bone health( Reference Ward, Prentic and Kuh 71 , Reference Sahni, Mangano and McLean 72 ).
We found that greater intakes of dietary vitamin C were associated with higher BMD at femoral neck and lumbar spine. Previous studies have reached the same findings( Reference Kim, Cho and Park 23 – Reference Prynne, Mishra and O’Connell 27 , Reference Hall and Greendale 29 , Reference Macdonald, New and Golden 34 , Reference Morton, Barrett-Connor and Schneider 52 , Reference Yang and Kim 60 ). However, some investigations indicated no association between dietary vitamin C intake and BMD’s correlation coefficient at the femoral neck and lumbar spine( Reference Chan, Woo and Leung 38 , Reference Wang, Villa and Marcus 58 , Reference Wolf, Cauley and Pettinger 59 ). Such findings might be explained by the validity of the FFQ used in different studies and lack of controlling for several confounders. However, it must be noted that these correlations are considered as weak correlations.
In terms of hip fracture, we found a significant inverse association between greater dietary vitamin C intake and the risk of hip fracture in both males and females. Although these findings were in agreement with previous publications( Reference Sun, Li and Xie 19 , Reference Sahni, Hannan and Gagnon 20 ), other studies have found a non-significant association between dietary vitamin C intake and the risk of hip fracture( Reference Finck, Hart and Lentjes 22 , Reference Holbrook, Barrett-Connor and Wingard 39 , Reference Key, Appleby and Spencer 40 , Reference Simon and Hudes 42 , Reference White, Atchison and Gornbein 44 , Reference Martínez-Ramírez, Pérez and Delgado-Martínez 46 – Reference Zhang, Munger and West 49 ). These findings might be explained by different selection bias, different study designs and sample sizes, as well as lack of controlling for potential confounders and the validity of the FFQ used in different studies.
Another finding of our study was that dietary intakes of vitamin C were inversely associated with the risk of osteoporosis. Our finding was in agreement with that of the study by Kim & Lee( Reference Kim and Lee 21 ). Although other publications in this regard indicated non-significant or direct associations( Reference Park, Heo and Park 26 , Reference Sugiura, Nakamura and Ogawa 43 , Reference Zhang, Zhang and Shi 61 ), such findings might be explained by the lack of controlling for potential confounders, low sample sizes and low incidence of osteoporosis during follow-up.
Although the present study is the first meta-analysis that examined the association of dietary vitamin C intake and BMD, fracture or osteoporosis, it has some limitations that should be considered. Searching was limited to published articles. Although there was no evidence of publication bias, lack of considering unpublished studies might have influenced the findings. Some studies reported the β-correlation coefficient and we did not include them in the meta-analysis. Owing to the cross-sectional design of most studies that reported mean BMD, our findings in this regard cannot indicate causality. Differences in study design lead to differences in their reliability. We could not consider this point because of the lack of enough studies in this regard. Although significant heterogeneity was seen, due to lack of enough studies, we could not perform subgroup analysis.
In conclusion, we found that greater dietary vitamin C intake was associated with higher BMD at the femoral neck and lumbar spine. In addition, reduced risk of hip fracture and osteoporosis were associated with greater dietary vitamin C intakes. However, some questions still need to be answered to determine causality. Further prospective cohort studies with long duration of follow-up, valid instruments for measurement of dietary vitamin C, BMD, fracture and osteoporosis are warranted to support the relation between dietary vitamin C and BMD, risk of fracture and osteoporosis.
Acknowledgements
This study was supported by the Tehran University of Medical Sciences, Tehran, Iran.
The authors’ contributions were as follows: H. M., S. S.-b. and K. D. designed the research; H. M. conducted the research and performed statistical analysis; H. M. and S. S.-b. wrote the paper; and S. S.-b. had responsibility for final content. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.










