To the Editor—Carbapenem-resistant Enterobacteriaceae (CRE) are a leading cause of nosocomial infections. In the CRE group, the Klebsiella pneumoniae carbapenemase (KPC) producers stand out among the others and have been associated with serious infections and high mortality rates, mainly in intensive care units.Reference Nordmann, Naas and Poirel 1 Apart from that, antimicrobial resistance among these isolates has increased worldwide, therefore limiting the therapeutic alternatives against KPC.Reference Carmeli, Akova and Cornaglia 2
Early detection of colonized or infected patients is crucial for the rapid management of patients and to establish infection control practices in order to avoid further dissemination and to curb the rise of antimicrobial resistance.Reference Carmeli, Akova and Cornaglia 2
We conducted a prospective survey from July 1, 2013, through November 30, 2015, to assess the impact of CRE type involved on the clinical outcomes and the emergence of antimicrobial resistance among CRE urinary or bloodstream isolates in a cohort of critically ill patients from an adult intensive care unit of a tertiary hospital in Porto Alegre, Southern Brazil.
Patients were included at the time of their first urine culture in which CRE were recovered. Isolates with reduced susceptibility to carbapenems (meropenem, imipenem, and/or ertapenem) were identified by MicroScan Walkaway automated system (Beckman Coulter) and confirmed by Etest (AB Biodisk). The presence of carbapenemase was detected by phenotypic testing and by gene detection using a polymerase chain reaction procedure, as previously described.Reference Perez, Rodrigues and Dias 3
The primary outcomes (or clinical outcomes) were determined by result of a subsequent urine culture (negative or recurrent/subsequent bacteriuria) and/or blood culture with the same CRE within 90 days and mortality at 30 days. Development of antimicrobial resistance (which was the microbiologic outcome in this study) was evaluated comparing results from the first CRE isolate with those obtained in a subsequent sample (urine or blood) for amikacin, gentamicin, polymyxin B, tigecycline, and fosfomycin.
During the study period, a total of 109 patients were included. In 85 patients, KPC-2-producers (mostly Klebsiella pneumoniae [Kp]) were recovered whereas, in the remaining 24, a culture with carbapenemase nonproducers was obtained. Of the 85 patients with KPC-2-Kp bacteriuria, 19 died during the 30-day period, 27 had a negative urine culture or were discharged, 14 had bacteriuria with a microorganism other than KPC-2-producers, and 25 had a recurrent KPC-2-Kp bacteriuria. Moreover, 15 patients, including 5 patients who also had a recurrent urinary isolate, had an episode of bacteremia due to KPC-2-Kp and the 30-day mortality for these patients was 47% (Table 1). Regarding carbapenemase nonproducers, no patients were bacteremic, and only 4 of them had recurrent bacteriuria.
TABLE 1 Microbiologic Characteristics and Clinical Outcomes After CRE Bacteriuria
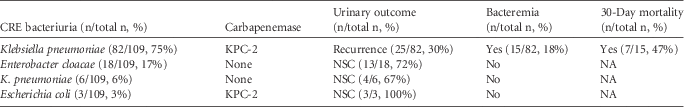
NOTE. CRE, carbapenem-resistant Enterobacteriaceae; NA, not applied; NSC, negative subsequent culture.
In 35 patients, a KPC-2-Kp isolate was recovered in a subsequent bacteriuria/bacteremia case and a minor increase in resistance was observed for polymyxin B (34% vs 43%), gentamicin (57% vs 69%), amikacin and tigecycline (14% vs 26%). For fosfomycin, used more often nowadays as therapy to treat urinary tract infections due to KPC producers, a significant increase in resistance was detected (11% vs 34%; OR, 4.04 [95% CI, 1.1–14.2], P=0.03), driven by prior fosfomycin use, as previously described.Reference Perez 4 On the other hand, no increase of antimicrobial resistance was observed among isolates of carbapenemase nonproducers.
In this study, the urine specimen was used as a starting point for surveillance for KPC-2-Kp isolated during hospitalization because KPC-2-Kp was found most commonly in urine culture. Criteria for asymptomatic bacteriuria, as reported by the Centers for Disease Control and Prevention/ National Healthcare Safety Network, 5 are primarily designed for surveillance purposes, and only patients with KPC-2-Kp bacteriuria who do not meet these criteria are thought to have urinary tract infection and receive treatment.
The results presented here show that the outcomes of CRE bacteriuria/bacteremia are influenced by both the choice of antimicrobial treatment and the CRE isolate type. The selective pressure imposed by antibiotic usage has been strongly associated with the emergence of resistance, as observed in this study and in previous reports regarding polymyxins,Reference Perez and Dias 6 tigecycline,Reference Kumar 7 and fosfomycin,Reference Perez 4 which are considered “reappraised” therapeutic options to treat multidrug-resistant microorganisms.
Unequivocally, endemic KPC-2-Kp has become quite more competitive than multidrug-resistant noncarbapenemase isolates that proved to be self-limited, with neither bacteremia case nor development of resistance observed in this study (Table 1); KPC-2-Kp is probably favored by the presence of a more robust resistance mechanism, such as the production of carbapenemase, although bla KPC-2 gene has not been associated with virulence by itself.Reference van Duin, Cober and Richter 8
In conclusion, KPC-2-Kp isolates presented with recurrent/subsequent bacteriuria as the main urinary outcome and as such developed cases of bacteremia with a high 30-day mortality rate being observed. Increase in resistance rates was observed for all agents evaluated, possibly driven by previous use similar to prior observations for KPC-2-Kp recovered from surveillance rectal swab samples.Reference Perez, Rodrigues and Dias 9 These findings and the poor outcomes for KPC-2-Kp infection underscore the urgent need for better surveillance and stewardship programs to combat these antibiotic stains.
ACKNOWLEDGMENTS
Financial support. Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil.
Potential conflicts of interest. The author reports no conflicts of interest relevant to this article.



