Introduction
Severe fever with thrombocytopenia syndrome (SFTS) is an emerging infectious disease that is caused by SFTS virus (SFTSV) [Reference Yu1]. From 2010 to 2016, more than 5000 confirmed SFTS cases have been reported in at least 23 provinces of mainland China, and the case-fatality rate of SFTS infection was 5.3% [Reference Zhu2, Reference Zhan3]. SFTS cases have also been reported in South Korea, Japan and Vietnam, and a similar disease has occurred in the USA [Reference Kim4–Reference McMullan7]. Due to the heavy burden, lack of vaccines, effective therapies and high-fatality rates, the disease has become an important health issue.
Some SFTS patients had been bitten by ticks before the onset of illness [Reference Deng8]. Animals might be a reservoir host in the life cycle of SFTSV in nature [Reference Huang9]. SFTSV has been isolated successfully from some ticks [Reference Luo10]. Until now, the natural transmission mode of SFTSV among humans, hosts and vectors has remained unclear.
In this study, we systemically reviewed three aspects of SFTS: SFTS cases and asymptomatic infections (human level), seroprevalence and SFTSV infection rates in animals (animal level), and SFTSV positivity rate in ticks and vertical transmission among ticks (tick level). Thereafter, the incidence rate of SFTS in the healthy population, the positive ratio of anti-SFTSV antibodies in animals and the infection rate of SFTSV in ticks were calculated to study the transmission mode of SFTSV. This mode of transmission was beneficial for blocking the transmission route and reducing the incidence of SFTS.
Materials and methods
This systematic review followed the guidelines provided in the Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The PRISMA checklist is included in the Supporting Information.
Search strategy
An electronic search of the Chinese National Knowledge Infrastructure databases and the Wan Fang Data, PubMed, Web of Science and Embase databases was performed for all eligible papers (published from 1 January 2011 until 1 December 2019; English and Chinese publications) using a range of search strings ('severe fever with thrombocytopenia syndrome’ or ‘SFTS’ or ‘fever, thrombocytopenia and leukopenia syndrome’ or ‘FTLS’ or ‘severe fever with thrombocytopenia syndrome virus’ or ‘SFTSV’ or ‘fever, thrombocytopenia and leukopenia syndrome virus’ or ‘FTLSV’ or ‘Huaiyangshanvirus’ or ‘HYSV’ or ‘New bunyavirus’ or ‘NBV’ or ‘Dabie mountain virus’ or ‘DBMV’ or ‘Dabie bandavirus’ or ‘Huaiyangshan banyangvirus’ or ‘BHAV’). Additional studies obtained from the references of the original articles were also included.
Eligibility criteria
The article had been accepted for publication with full text available and should meet one of the following conditions: (1) SFTS patients must be confirmed and baseline information could be extracted, (2) people who had an asymptomatic infection were confirmed by SFTSV antibodies (IgM and IgG), (3) animals were confirmed by SFTSV antibodies or RNA and (4) transmission medium was confirmed by SFTSV RNA. Exclusion criteria included abstracts, conferences, letters, reviews, duplicated publications, and overlapping data sets.
SFTS patients mentioned in the selected studies were confirmed as meeting one or more of the following criteria: (1) the virus was isolated from the patient's samples, (2) SFTSV RNA was detected in the patient's serum and (3) a fourfold or greater increase in antibody titres was detected between paired patient serum samples collected from the acute and convalescent phases of infection.
Data extraction and quality assessment
For this meta-analysis, the following information was extracted from every eligible article: first author, year of publication, country, province; year of admitted patients, confirmed cases, death number, test method, and patients' age (SFTS patients); investigation time, sample size and the number of asymptomatic infected people with SFTSV (asymptomatic infections); sampling time and sample size of infected animals; sampling time of transmission medium and testing result.
The included studies were assessed using Study Quality Assessment Tools provided by the National Institute of Health [11], which consisted of good, fair and poor. Based on the quality assessment for studies, we evaluated the articles' quality.
Statistical analysis
The statistical analyses were performed using SPSS 13.0 software (SPSS, Inc., Chicago, IL), R software (version 4.0.0), and STATA version 12.0 (STATA Corporation, College Station, Texas, USA) [Reference Luo12, Reference Wang13]. Means and s.d. were calculated to describe continuous variables with normal distribution, medians and ranges or interquartiles were calculated to describe the abnormal distribution. Each study calculated the event rates and proportions with confidence limits by the R software package [Reference Luo12]. The χ 2 test was used for comparison between groups, Cochran Q and I2 statistics were used to assess the heterogeneity among the studies [Reference Higgins14]. A Cochran Q test with a P-value of <0.05 was considered to be statistically significant. An I2 value of more than 75% indicated high heterogeneity, and then a random-effect model was used. Otherwise, a fixed-effect model was performed. In some circles, researchers tended to start with the fixed-effect model, and then switched to the random-effects model if there was a compelling reason to do so [Reference Michael15]. Because of the high heterogeneity, a random effects model was more suitable when combining results from the studies. A leave-one-out sensitivity analysis was carried out to assess the impact of each study on the overall pooled estimate. Publication bias was appraised using Begg's test or Egger's test [Reference Egger16, Reference Begg17]. A P-value of <0.05 was considered statistically significant.
Results
Literature search
A total of 4273 articles were retrieved through the database searches. A total of 2704 articles were excluded because they were duplicates and 1362 irrelevant studies were removed. Then, a total of 207 articles were evaluated for eligibility. A total of 115 studies were excluded for the following reasons: lack of some indicators, failure to extract data and overlapping data. For the same province, we selected documents with a large span of years and a large number of cases. After close scrutiny, 92 studies were included (Fig. 1).
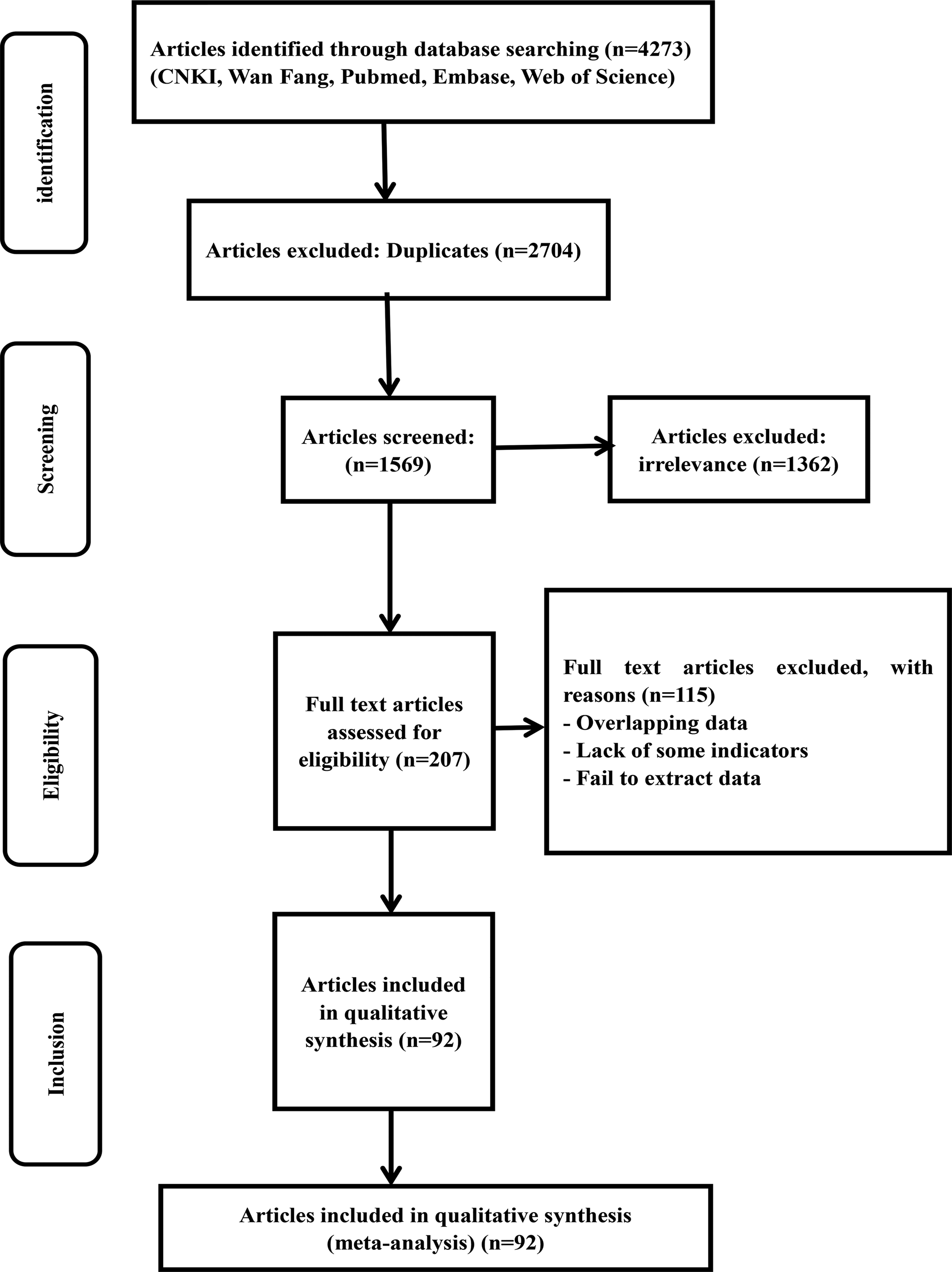
Fig. 1. Flow chart of the study selection process in this meta-analysis.
Study characteristics and quality assessment
The basic characteristics of the included studies and the quality assessment of the results are shown in Tables 1–5. These studies were published between 2011 and 2019 and carried out in three countries with different geographical locations. Sixty-nine studies were performed in China, 16 studies were conducted in South Korea and seven studies were conducted in Japan. The quality of the assessment result, based on the Study Quality Assessment Tools, is shown in 15 studies that were of high quality, 10 studies were of poor quality and 76 studies were of moderate quality.
Table 1. Basic characteristics of SFTS patients
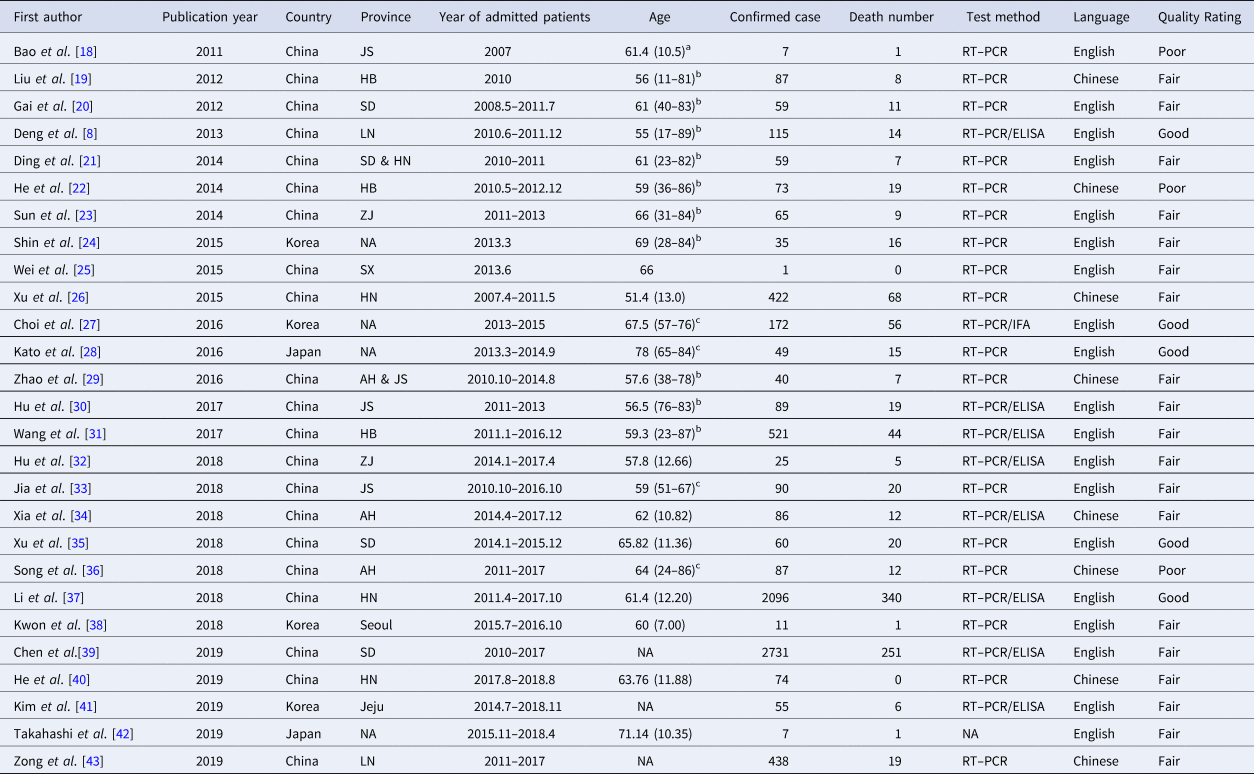
Abbreviations: NA, not available; JS, Jiangsu; HB, Hubei; SD, Shandong; LN, Liaoning; HN, Henan; ZJ, Zhejiang; SX, Shaanxi; AH, Anhui; RT–PCR, reverse transcription–polymerase chain reaction; IFA, immunofluorescence assay; ELISA, enzyme-linked immunosorbent assay.
a Values the mean (s.d.).
b Values are listed as median (ranges).
c Values are listed as median (interquartiles).
Table 2. Basic characteristics of person-to-person transmission

Abbreviations: NA, not available; RT–PCR, reverse transcription–polymerase chain reaction; IFA, immunofluorescence assay; ELISA, enzyme-linked immunosorbent assay; HCW: health care worker.
Table 3. Characteristics of asymptomatic infected persons
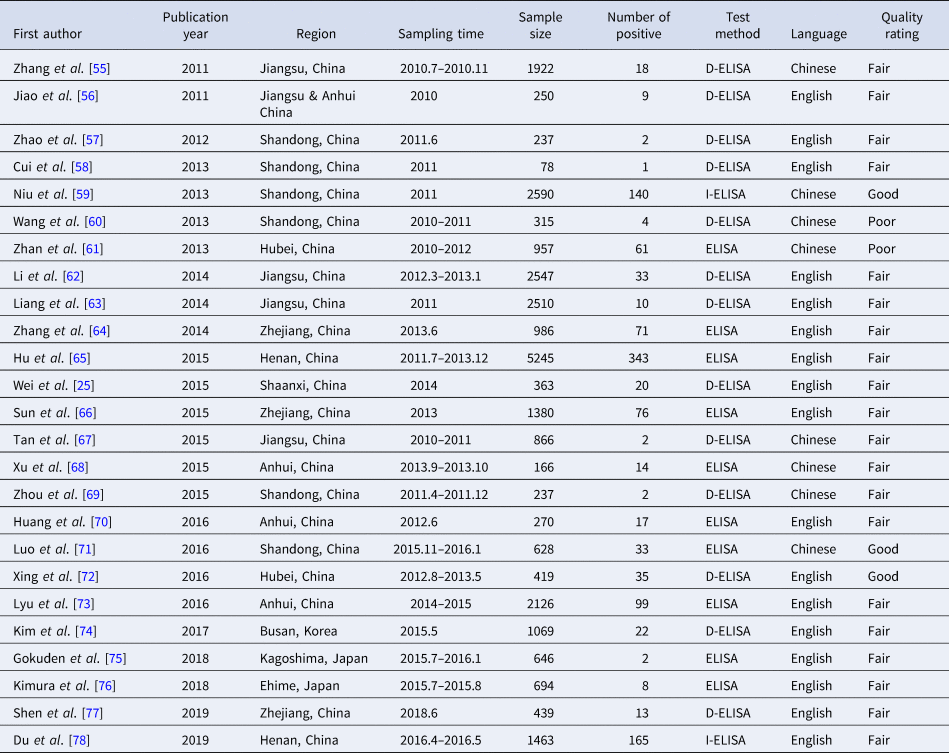
Abbreviations: ELISA, enzyme-linked immunosorbent assay; D-ELISA, double-antigen sandwich enzyme-linked immunosorbent assay; I-ELISA, indirect enzyme-linked immunosorbent assay.
Table 4. SFTSV seroprevalence in animals
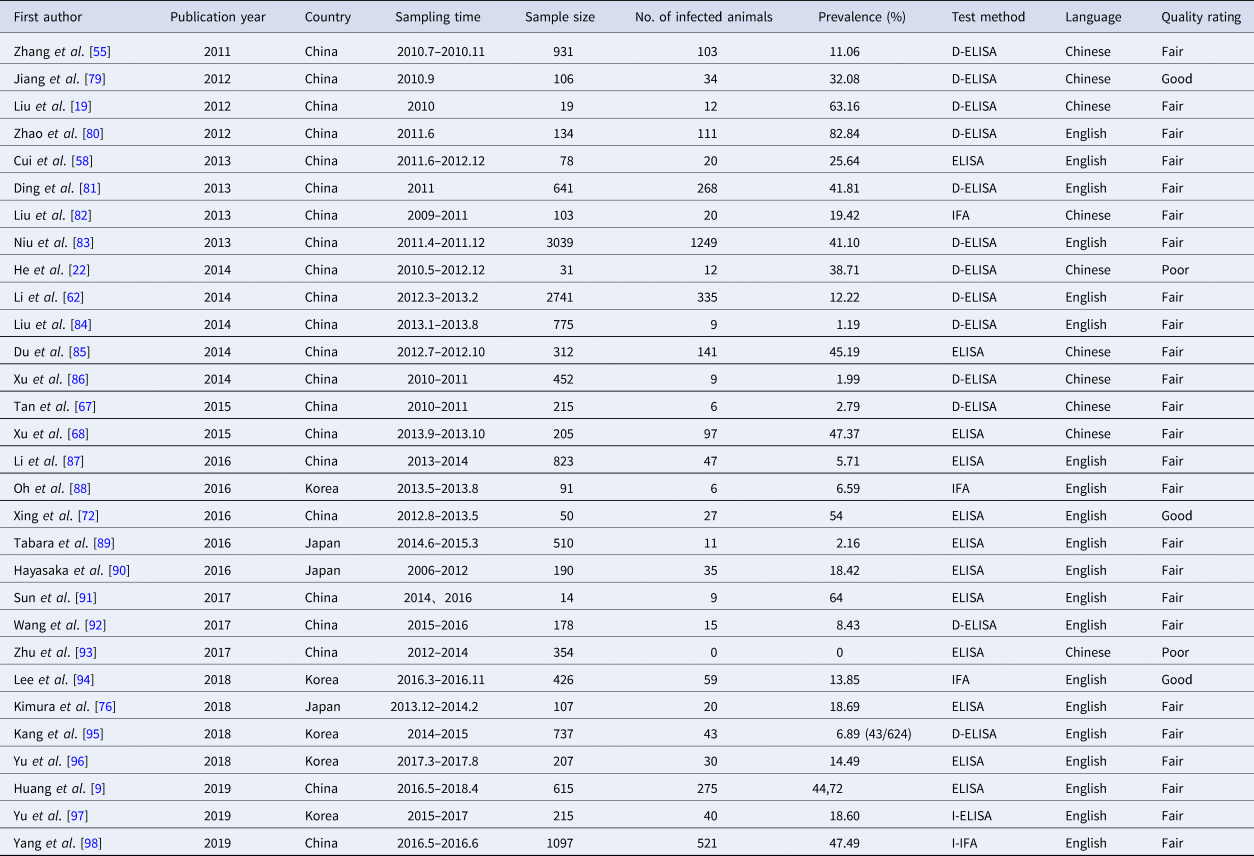
Abbreviations: ELISA, enzyme-linked immunosorbent assay; D-ELISA, double-antigen sandwich enzyme-linked immunosorbent assay; I-ELISA, indirect enzyme-linked immunosorbent assay; IFA, immunofluorescence assay; I-IFA, indirect immunofluorescence assay.
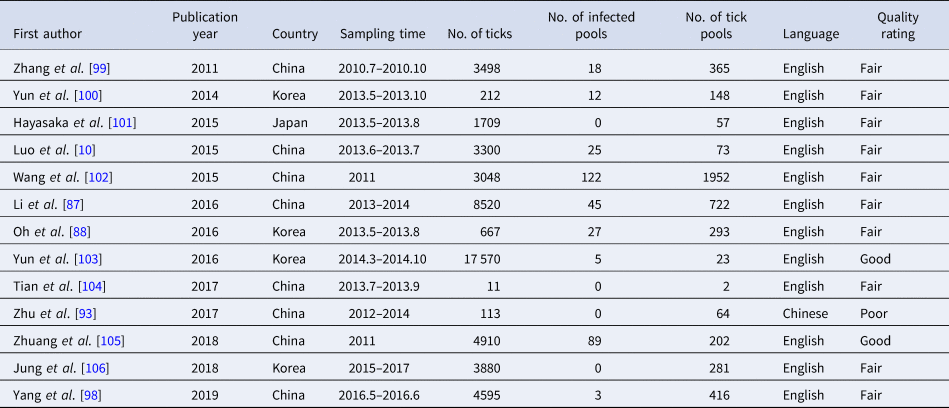
Table 5. SFTSV tick infections rates and vertical transmission characteristics
SFTS cases and asymptomatic infections
Epidemiology of SFTS patients
As shown in Table 1, 27 studies were included in the meta-analysis. A total of 7554 confirmed cases were collected from these studies. The geographical distribution was mainly in China (Zhejiang, Liaoning, Henan, Shandong, Jiangsu, Anhui, Hubei province), Japan and South Korea (Fig. 2A). SFTS showed strong seasonality, the cases were mainly reported from April to October and peaked between May and July, and cases during those three months accounted for 54.39% (3792/6972) of all cases (Fig. 2B). Of the 7409 cases, 47.52% (3521/7409) were male and 52.48% (3888/7409) were female. The median age of the patients was 61 years old (range: 11–89). The case-fatality rate was 0.15 (95% CI 0.11, 0.18) and the vast majority of the cases were farmers (82.89%, 4307/5196), including agricultural and forest workers living in rural areas (Fig. 3A). A total of 296 of 1384 SFTS patients indicated that they had been bitten by ticks, the biting rate was 0.21 (95% CI 0.16, 0.26), and the result showed statistically significant heterogeneity (I 2 = 77.0%, P < 0.001) (Fig. 3B).
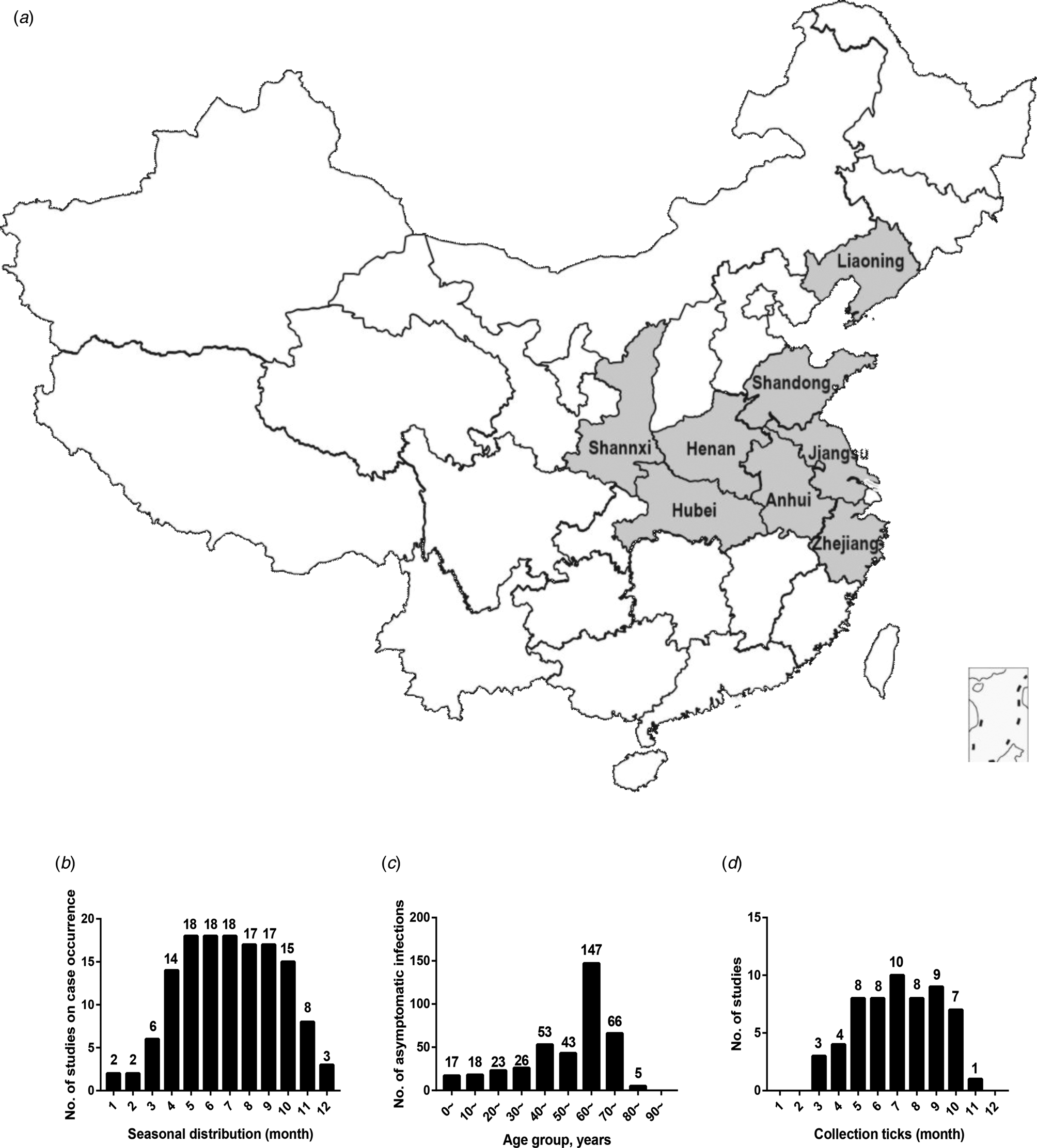
Fig. 2. (a) Geographic distribution of SFTS in mainland China. (b) Seasonal distribution of published studies on case occurrence. (c) Age distribution of asymptomatic infections. (d) The relationships between collected ticks and number of published studies. The horizontal ordinate represented the month and the ordinate represented the number of studies that meet the requirements (b and d). The horizontal ordinate represented the age group and the ordinate represents the number of asymptomatic infections (c).
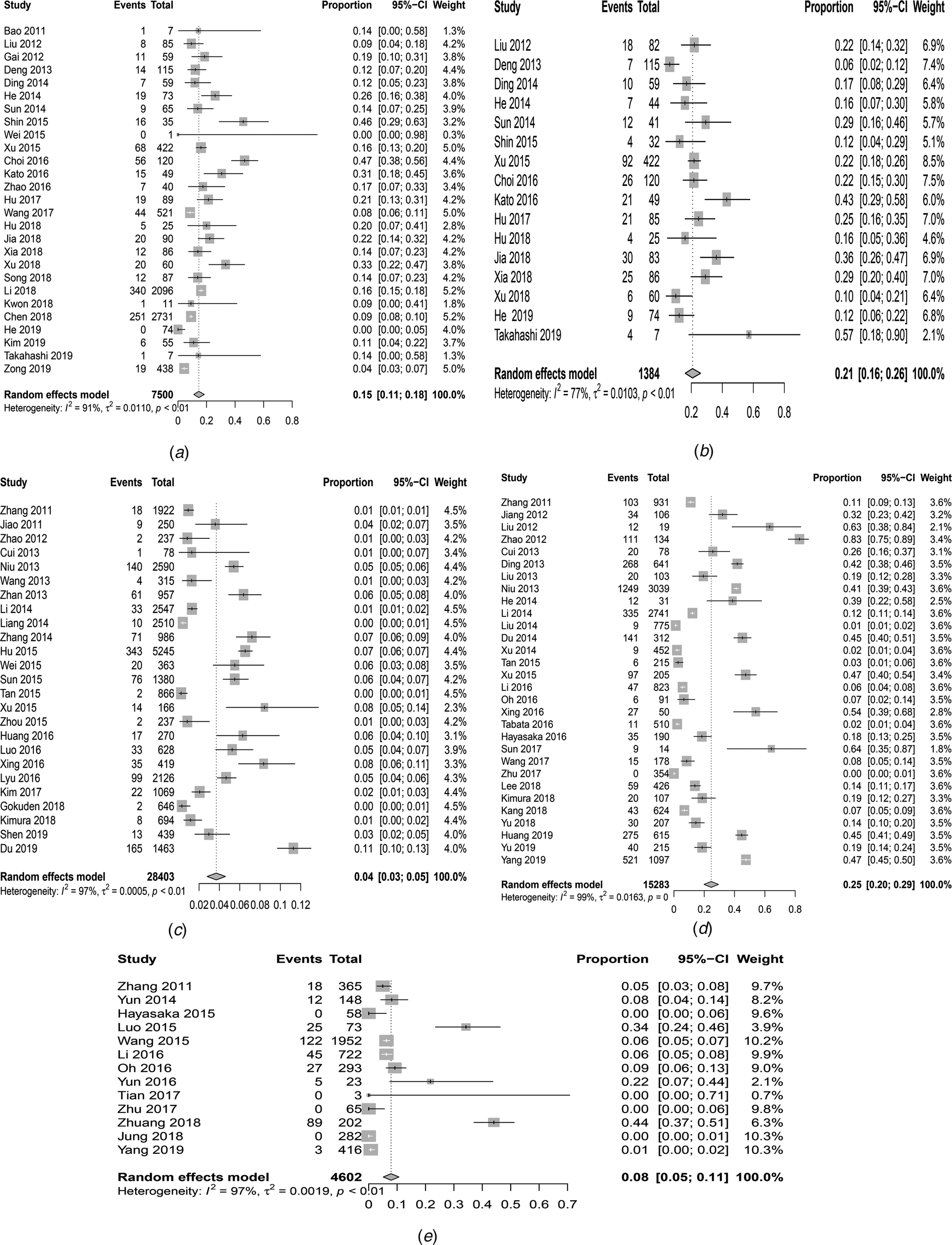
Fig. 3. Forest plots of the meta-analysis on a panel of prevalence. (a) The pooled case-fatality rate of SFTS. (b) The pooled biting rate by ticks. (c) The overall seroprevalence of SFTSV among the healthy population. (d) The overall seroprevalence of total antibodies against SFTSV in animals. (e) Infection rate of SFTSV in ticks.
Person-to-person transmission
Person-to-person transmission of SFTSV was mostly reported in hospitals from China, Japan or South Korea. According to epidemiological investigations and laboratory analyses, 12 studies reported that SFTSV could be transmitted from person to person by contact with blood or bloody respiratory secretions, especially in inadequately protected people (Table 2). There were 84 secondary patients, but only three tertiary patients were described or found in articles. All 27 studies with 7554 confirmed patients tried to find the index cases but did not describe them in these articles.
Asymptomatic infections
The 28 403 blood samples in 25 studies from 2011 to 2019 were collected from healthy people. These were a random sample of healthy people from some populations in each study. Through collecting serum of healthy people and testing their IgM and IgG, we could calculate the overall pooled seroprevalence of SFTSV antibodies among the healthy population. Based on the data extractability, 24 075 healthy people, including 11 647 males and 12 428 females (male to female ratio: 0.94:1), were extracted from 17 studies. Among healthy people (24 075), 5059 had clear occupational descriptions in these studies and 3999 (79.05%) of them were farmers (Table 3).
A total of 931 healthy people were tested positive for SFTSV antibodies, 449 (48.23%) cases were male and 482 (51.77%) cases were female, there was no significant difference between the male and female groups (t = −0.202, P = 0.84). The participants were grouped by decades and the results showed that a large number of asymptomatic infections were 60–70 years (Fig. 2C). In addition, the overall pooled seroprevalence of SFTSV antibodies among the healthy population in the random-effect model was 0.04 (95% CI 0.03, 0.05) (Fig. 3C).
Seroprevalence and SFTSV infection rates in animals
The seroprevalence of SFTSV in animals
We analysed 30 studies to determine the overall seroprevalence of SFTSV in animals (Table 4). These included studies that did not describe the sampling method but just described that animal serums were collected at the survey site (there were SFTS cases nearby) for laboratory testing of IgM and IgG. The overall seroprevalence of SFTSV in animals was 0.25 (95% CI 0.20, 0.29) and is displayed as a forest plot in Figure 3D. The goats and sheep were 0.49 (95% CI 0.34, 0.65) and 0.42 (95% CI 0.31, 0.57) in cattle, 0.16 (95% CI 0.10, 0.22) in chickens, 0.26 (95% CI 0.17, 0.35) in dogs and 0.04 (95% CI 0.01, 0.07) in pigs. The χ 2 test was used for comparison of the seroprevalence in different animals and there was a statistically significant difference in the positivity rates of these animals (χ 2 = 1204.92, P < 0.001). The seroprevalences of SFTSV in goats and cattle were higher than those in animals.
The positivity rates of SFTSV RNA in animals
In our included studies, RT–PCR was used to detect SFTSV RNA in these animals. Fourteen articles reported that the infection rates of SFTSV RNA in animals ranged from 0.23 to 26.31%. The positivity rates of SFTSV RNA detected in sheep and goats were 0.03 (95% CI 0.01, 0.04), 0.04 (95% CI 0.01, 0.08) in cattle, 0.02 in chickens, 0.03 in dogs, 0.02 in pigs, 0.02 (95% CI 0.01, 0.04) in shrews, 0.02 in yellow weasels, 0.03 in hares, 0.01 (95% CI 0.00, 0.02) in deer and 0.02 (95% CI 0.01, 0.03) in rodents. There were statistically significant differences in the positivity rates of different animals (χ 2 = 31.97, P < 0.001), and the positivity rate of SFTSV RNA in cattle was higher than that in goats (χ 2 = 4.49, P = 0.03). Twenty-three strains of the virus were isolated from animal specimens in eight articles, including cattle, dogs, rodents, shrews, water deer, wild boars, goats, sheep, yellow weasels, and hedgehogs.
SFTSV positivity rate in ticks and vertical transmission
The infection rate of SFTSV in ticks
A total of 4598 ticks were included in 13 studies. These included studies that did not describe the sampling method but just described that ticks were collected at the survey site (there were SFTS cases nearby) for laboratory testing of SFTSV RNA. SFTSV RNA was detected in 346 ticks via laboratory tests and we found that there were not positive numbers in four articles (Table 5). The infection rate of SFTSV in ticks was 0.08 (95% CI 0.05, 0.11) and the result had significant heterogeneity (I 2 = 97.0%, P < 0.001) (Fig. 3E). There were 65 strains of SFTSV isolated from ticks. The seasonal change in tick numbers was consistent with SFTS case reports (Fig. 2D).
The positivity rate of SFTSV in vertical transmission
In vertical transmission, the experiment of ticks in the laboratory and the detection of naturally infected oviposited ticks were reported in previously published articles. Luo et al. reported that ticks fed on SFTSV-infected mice could acquire the virus and transovarially transmit it to other developmental stages of ticks. Wang et al. reported that 2 of 22 egg masses oviposited by blood-fed Haemaphysalis longicornis females were positive for SFTSV [Reference Wang102]. Three studies containing 168 ticks were analysed. A total of 117 ticks (eggs, larvae, nymphs or adult ticks) were infected through female tick oviposits. The positivity rate of SFTSV was 0.55 (95% CI: 0.12, 0.97) and the heterogeneity was also statistically significant (I 2 = 97.3%, P < 0.001). For the other transmission media, SFTSV has been detected by RT–PCR in gamasid mites, chigger mites and gadflies. The gamasid mites and chigger mites in the nine groups were all positive for SFTSV genomic nucleic acids. A total of 38 gadflies were divided into 16 groups and the results showed that three groups of gadflies were positive by RT–PCR. The role of other bloodsucking insects such as vectors and reservoirs for SFTSV needs to be further investigated [Reference Wang107, Reference Liu108].
Virus isolation and phylogenetic analysis
Eight articles reported that virus isolation was attempted on all viral RNA-positive serum samples, and phylogenetic analysis of the S segment of these SFTSV isolates was performed. For further analysis, the S segment of 445 SFTSV complete sequences obtained from GenBank was analysed, and we found that most of the viral isolates from animals and ticks were genetically close to the SFTS patient-derived isolates, and there was a clear boundary in these isolates in the three countries (Fig. 4). Niu et al. also showed that all sequences of the isolates from domesticated animals, SFTS patients and H. longicornis ticks shared more than 95% identity, which demonstrated a close evolutionary relationship among those SFTSV isolates from domesticated animals, ticks and SFTS patients by pairwise distance analysis [Reference Niu83].
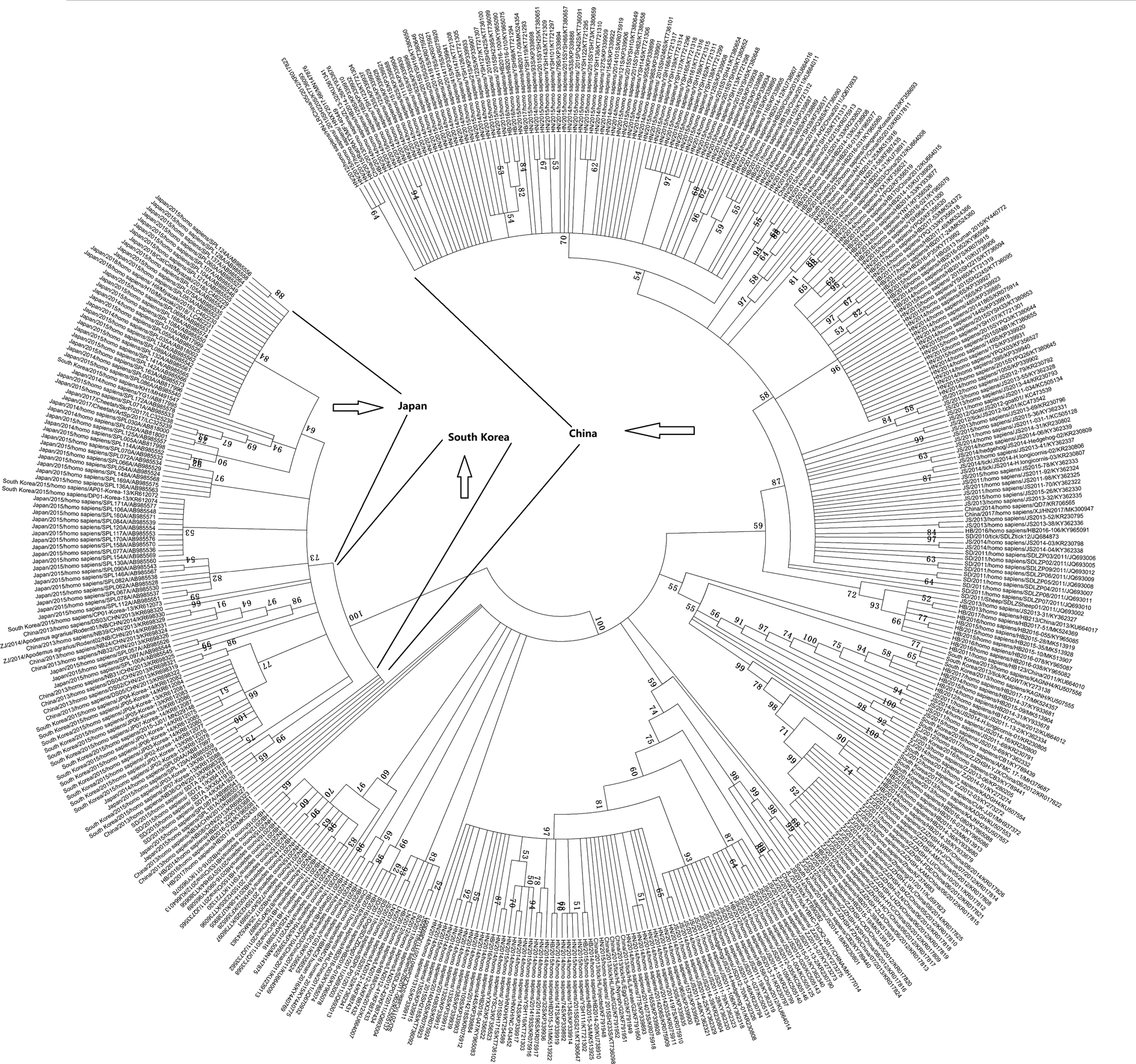
Fig. 4. Phylogenetic analysis of the S segment of 445 SFTSV complete sequences obtained from GenBank.
Subgroup analysis
To explore the potential sources of high heterogeneity in the meta-analysis, we performed a subgroup analysis by country. The pooled case-fatality rate of SFTS in China was 0.13 (95% CI 0.10, 0.17) (I 2 = 91.0%, P < 0.01), 0.29 in Japan (95% CI 0.18, 0.42) (I 2 = 00.0%, P = 0.39), and 0.26 in South Korea (95% CI 0.11, 0.50) (I 2 = 86.0%, P < 0.01). The more details with other prevalences are presented in Supplementary Table S1.
Sensitivity analysis and publication bias
The sensitivity analysis was performed, which indicated little change in the data (Supplementary Table S2). The incidence rate of SFTS in the sensitivity analysis was stable and had no significant effect on the merger rate.
Egger's test and Begg's test were conducted to evaluate publication bias. The results showed that Egger's test t value was 2.76 (P = 0.011), and Begg's test z value was 1.77 (P = 0.076) in the case-fatality rate of SFTS. Egger's test t value was 4.44 (P = 0.000) and Begg's test z value was 2.15 (P = 0.032) in the overall seroprevalence of SFTSV among the healthy population. Egger's test t value was 3.52 (P = 0.002) and Begg's test z value was 2.27 (P = 0.023) in the seroprevalence of SFTSV in animals. Egger's test t value was 3.23 (P = 0.008) and Begg's test z value was 0.92 (P = 0.360) in the infection rate of SFTSV in ticks.
Discussion
This systematic review and meta-analysis were performed to study the transmission mode of SFTSV. The epidemiology of SFTS cases has the following characteristics: (1) most patients were older (60–70 years), (2) May to July was the peak of the SFTS cases in these epidemic areas and (3) most of the reported cases were farmers living or working in wooded and hilly areas, where ticks were commonly found. The epidemic areas of SFTS were mainly in the central and eastern China, mostly in mountainous and hilly rural areas, while there was no case in the western region, which might be due to the topography of mountains and plateaus. Infection and death cases were mainly found in central China, where H. longicornis ticks were spread [Reference Zhan3]. For the person-to-person transmission of SFTSV, we discovered the index and secondary patients but only three tertiary patients were described or found. The index cases died soon after becoming infected, suggesting that their transmission rate might be low. The asymptomatic infection rate was calculated and was high among the healthy population. This result was similar to a previously reported study [Reference Li109]. These observations indicated that the rate of asymptomatic infection increased with the SFTS epidemic situation.
Animal hosts and vectors of SFTSV are still unclear, but some case-control studies have reported that raising animals is a risk factor for human SFTS and working and living with domesticated animals, especially those showing high levels of SFTSV antibodies, increases SFTS incidence rates [Reference Ding110, Reference Chen111]. It is possible that SFTSV could be transmitted directly from animals (other than ticks) to humans through contact with blood and/or other body fluids. However, there are few articles at present, and we could not perform further research. The seroprevalence of SFTSV in animals was conducted in previous studies. Du and Chen et al. investigated whether SFTSV has a wide spectrum of animal hosts, including domestic and wild animals. The prevalence of SFTSV is high among specific animal species [Reference Du78, Reference Chen111]. In our study, we searched and analysed the publications, and the results showed that the overall seroprevalence of SFTSV in animals was 25%. Previous studies reported that the sequences of SFTSV isolated from animals were highly homologous to SFTSV from human cases [Reference Huang9, Reference Niu83, Reference Oh88]. The included study also showed that the virus was isolated from animals, such as cattle, goats and hedgehogs. A natural infection study also showed that goats were infected by ticks in the SFTS-endemic region. The goats were viraemic over a very short period (<24 h) after the viral infection and soon occupied by a timely mounting antibody response that effectively controlled the infection. The whole cohort did not show any specific clinical signs of illness and all survived infection [Reference Jiao112].
Ticks, especially H. longicornis, are suspected to be potential vectors and have a broad animal host range in nature [Reference Liu113]. The positivity rate of SFTSV indicated that SFTSV could be carried by ticks and transmitted vertically through female tick oviposits. Luo et al. fed H. longicornis ticks to SFTSV-infected mice, and the results indicated that ticks could acquire SFTSV from infected mice. The team also fed SFTSV-infected ticks on Kunming albino mice, and the results showed that ticks transmitted SFTSV to mice through feeding. These results from a laboratory study confirmed that ticks could serve as a vector and reservoir of SFTSV and were consistent with those of epidemiological investigations [Reference Luo10]. A previous study reported that the prevalence of SFTSV infection among ticks collected from vegetation was lower than that among ticks collected from animals. Ticks were vectors of SFTSV, similar to other insect-borne diseases, and SFTSV could not spread among ticks except for vertical transmission, so the SFTSV infection rate of vegetated ticks was fairly low [Reference Wang102]. Although the SFTSV of mites and gadflies was detected, we did not have much evidence that they were the main routes of transmission.
The case-fatality rate of SFTS has a wide range among endemic areas, the reason might be that SFTS cases were first found in China, the case-fatality was high at first and then gradually decreased. Later, Japan and South Korea successively reported cases, which led to a large range. The overall seroprevalence of SFTSV among the healthy population was almost the same in the three countries. The seroprevalence of SFTSV in animals has varied widely among the three countries, the reason for this discrepancy might include different geographical locations. In this study, we found SFTSV seroprevalence was high in China and was relatively low in Japan. However, this result should be interpreted with caution because of the limited number of studies and sample size in Japan and South Korea, which might lead to a lack of representativeness. Because only one study regarding Japan was included, we could not perform further analysis in the infection rate of SFTSV in ticks.
Our study had several strengths. The poor, moderate and high-quality studies were pooled for a relatively large sample size. Through the analysis of previous studies, we summarised the transmission mode of SFTSV, which had guiding significance for cutting off the transmission channels. Nevertheless, this meta-analysis also had some limitations. First, significant heterogeneity brought into question the suitability of performing this meta-analysis. Second, publication bias might distort the estimates of rates, so the results should be interpreted with caution.
Conclusion
According to the current study, the transmission patterns of SFTSVs can be summarised as shown in Figure 5. Ticks can serve as transmitting vectors of SFTSV and act as reservoir hosts as well. Animals can be infected with SFTSV by tick bites and as a reservoir host, but most animals are latent, and the infected animals might introduce SFTSV into areas where ticks were present. SFTSV circulates continuously between animals and ticks in nature. Humans are infected by SFTSV-infected ticks and might be infected by direct contact with infected blood or body fluids of patients.
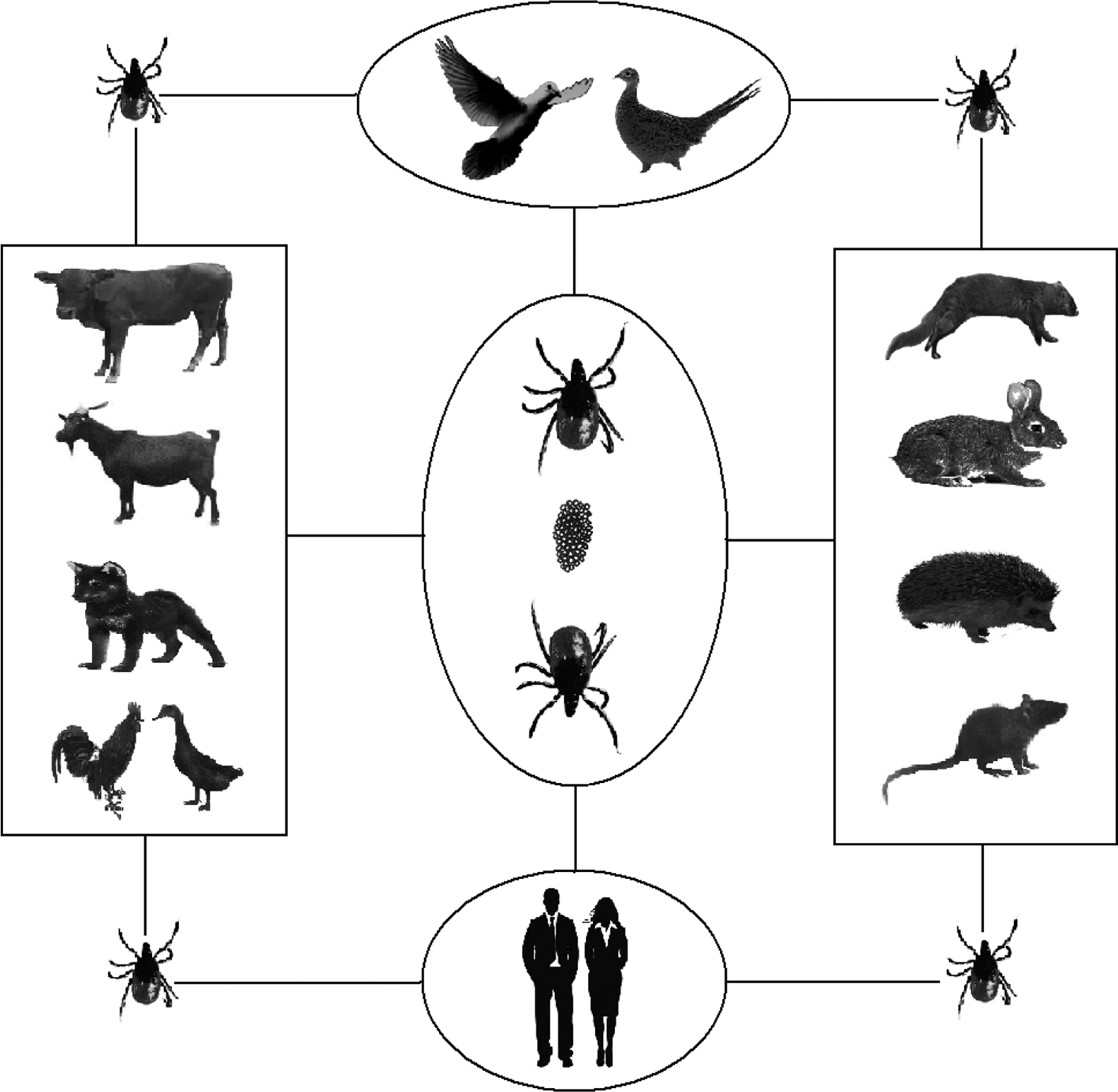
Fig. 5. Transmission models of SFTSV among ticks, animals and humans.
Supplementary material
Additional file 1: Table S1: Subgroup analysis by country in the meta-analysis.
Additional file 2: Table S2: Sensitivity analysis in this review. The supplementary material for this article can be found at https://doi.org/10.1017/S0950268820002290.
Acknowledgements
None.
Author contributions
All authors made a substantial contribution to the study. XYH and ZQH designed and conceived the study. KH and WSG supervised and edited the manuscript. BHW and YL conducted an electronic search, extracted data and analysed it. XYH and ZQH wrote the initial manuscript. KH and WSG revised manuscript. YL, KH and WSG critically analysed the manuscript. All authors gave final approval for the manuscript to be submitted for publication.
Financial support
This work was supported by the National Natural Science Foundation of China (81573204 and 81773500) and Henan Medical Science and Technology Program (2018010029 and 2018020510).
Conflict of interest
The authors declare no conflict of interest.
Data availability statement
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.













