INTRODUCTION
Acute rotavirus gastroenteritis (ARGE) is characterized by a more severe clinical course and a higher number of potential complications than other types of infectious acute gastroenteritis (AGE), and is observed predominantly in children aged <2 years [Reference Huppertz, Salman and Giaquinto1, Reference Gimenez-Sánchez2]. Mild fever occurs in 65·5%, severe dehydration in 49·9%, and metabolic acidosis in 79·2% of cases [Reference Forster3], with the dehydration rate being 32% higher for rotavirus [Reference Gimenez-Sánchez2]. Vomiting, diarrhoea, or severe febrile ARGE can lead to moderate to severe dehydration requiring acute rehydration in hospital [Reference Forster3]. In the developed world, complications from ARGE requiring hospitalization occur in >10% of children, with hypertonic dehydration (5·3%) and seizures (1·7%) being the most frequent reasons for hospital admission [Reference Rinder4]. Aggravating factors affect the severity of the condition, with rates of hospitalization differing between countries and regions in Europe [Reference Forster3].
The isolated influence of perinatal factors, as well as the demographic (maternal and family issues) and clinical aspects of this type of hospitalization have all been reported [Reference Huppertz, Salman and Giaquinto1, Reference Wilking5–Reference Dennehy8]. To date, the known characteristics of rotavirus that lead to a predisposition to hospitalization include: congenital malformations, low birth weight (LBW), male gender, maternal smoking, single mother, daycare for children aged <24 months, siblings aged <1 year at home, maternal age <25 years, low level of maternal education (<secondary school), and lack of breastfeeding [Reference Dennehy8, Reference Payne9]. Furthermore, 41·2% of ARGE cases have been associated with acute respiratory tract infections (RTIs) [Reference Yang and Fang10], with respiratory signs appearing in 60·7% of ARGE cases [Reference Zvizdić11]. However, the role of common respiratory viruses in enteric infections has yet to be elucidated [Reference Paloniemi, Lappalainen and Vesikari12, Reference Paloniemi13]. Human coronaviruses were detected in 6·4% of children with AGE and in 7·1% of those with symptoms of both AGE and RTI [Reference Paloniemi, Lappalainen and Vesikari12]. Additionally, 83% of ARGE cases were found to be comorbid with human bocaviruses in children aged <4 years [Reference Paloniemi13, Reference Zeng14]. Rotavirus hospitalizations are also more prevalent in children with dystrophy, iron-deficiency anaemia, intestinal parasitosis, rhinopharyngitis, or pneumococcal meningitis. In fact, symptoms of upper RTI, acute pharyngitis, and maculopapular eruption accompany ARGE in 13·3%, 10% and 16·6% of cases, respectively [Reference Mihalache15]. However, until now we have found no studies evaluating the confluence of comorbidities, clinical course, and epidemiology of ARGE cases requiring hospitalization within the same cohort.
The aim of this study is to explore the interrelationship of clinical and epidemiological factors associated with community-acquired rotavirus requiring hospitalization in Spain.
METHODS
Design and setting
This retrospective cohort study was performed as part of a larger project carried out in Castile-La Mancha (CLM), an autonomous region in central Spain, in order to describe the burden borne by hospitals due to community-acquired rotavirus both before and after a 3-year period in which rotavirus vaccines were made available in Spain. Data were obtained for all AGE-associated hospitalizations in CLM from 1 January 2003 to 31 December 2009. Two data sources were used: the Minimum Basic Data Set (MBDS) of the CLM Health Service and the Statistics on Health Establishments with an Inpatient Regimen, both of which represent a population coverage of 99·37%.
Definition of the variable of interest
A total of 23 006 hospital discharges with any records that included a primary or secondary diagnosis of AGE were retrieved from the MBDS. According to the Ninth Revision of the International Classification of Diseases, Clinical Modification (ICD-9-CM), AGE cases are classified as either bacterial or parasitic (001–005, 006–007, 008·0–008·5), viral (008·6–008·8), ‘of undetermined cause’ (009·0–009·3), ‘unspecified non-infectious AGE’ (558·9), or ‘diarrhoea’ (787·91).
Community-acquired rotaviruses were defined as having the ICD-9-CM code 008·61 either in the primary diagnosis field of the MBDS or in one of the secondary diagnosis fields (SDFs) together with a principal diagnosis code of ‘undetermined aetiology’, ‘cause-unspecified’, or ‘diarrhoea’.
Other possible cases of community-acquired AGE were identified from records that had the ICD-9-CM bacterial, parasitic, or viral AGE codes, as specified above, in the primary diagnosis field, as well as those records that contained these codes in a SDF together with a principal diagnosis of ‘undetermined aetiology’, ‘cause-unspecified’, or ‘diarrhoea’. Concomitant AGE cases were defined as having any of the bacterial, parasitic, or viral AGE codes in the SDF. The algorithm explaining the final count has been described elsewhere [Reference Redondo, Cano and Simón16].
Definition of the independent (exposure) variables
ICD-9-CM codes in the SDFs of whatever AGE cases identified were revised, from the first to the thirteenth, to assess the features of all AGE hospitalizations:
Complications
• Dehydration: 276·5 (low volume), 276·50 (unspecified volume decrease), 276·51 (dehydration), 276·52 (hypovolaemia), 276·0 (hypernatraemic), 276·1 (hyponatraemic).
• Acidosis: 276·2 (unspecified, respiratory, lactic or metabolic); not diabetic.
• Hypoglycaemia: 251·2 (unspecified).
• Seizures: 779·0 (newborn seizures), 780·31 (unspecified simple febrile seizures), 780·32 (complex febrile seizures), and 780·39 (others: unspecified attacks, recurrent seizures or syncope; seizures not otherwise specified).
• Intravenous (IV) rehydration: 39·96 in any of the first to eighth ‘surgical and/or obstetric procedures’ fields of the MBDS.
Comorbidities
• Perinatal problems (PNPs): V13·7 (perinatal problems), V21·3 (state of LBW: V21·30–V21·35), and 760–779 (diseases in the perinatal period).
• Disorders of pondostatural development (DPSD): 783·0 (anorexia), 783·4 (lack of expected normal physiological development), 783·40 (unspecified lack of normal physiological development), 783·41 (inadequate weight gain), 783·43 (short stature), 263·9 (unspecified protein-calorie malnutrition), 783·2 (abnormal weight loss), 783·21 (weight loss), and 783·22 (underweight).
• Malabsorption syndrome: 579·8 (specified intestinal absorption defect), 579·0 (celiac disease), and 579·9 (unspecified intestinal malabsorption).
• Gastroesophageal reflux disease (GERD): 530·81.
• Atopic dermatitis and related conditions: 691·0 (diaper rash), 691·8 [atopic, flexural, or intrinsic (allergic) eczema; atopic or diffuse (Brocq) neurodermatitis; Besnier's prurigo].
• Iron-deficiency anaemia: 280·9.
• RTI. Upper RTI: 460 (nasopharyngitis/common cold), 462 (pharyngitis), 463 (tonsillitis), 464·0 (laryngitis: 464·00 and 464·01, without/with obstruction), 464·1 (tracheitis: 464·10 and 464·11), 464 (unobstructed laryngitis), 465·8 (upper RTI of multiple locations), 465·9 (upper RTI of unspecified location). Otitis: 381·00 [unspecified non-suppurative otitis media (OM)], 381·01 (serous OM), 381·4 (non-suppurative OM, not classified as acute or chronic), 382·00 and 382·01 (suppurative OM without/with ruptured eardrum), 382·4 (unspecified suppurative OM), 382·9 (unspecified OM). Lower acute RTI: 466·0 (bronchitis), 466·11 (respiratory syncytial virus bronchiolitis), 466·19 (bronchiolitis due to other agents), 480·9 (unspecified viral pneumonia), 481 (pneumococcal pneumonia), 485 and 486 (unspecified bronchopneumonia).
• Urinary tract infection (UTI): 599·0.
• Concomitant gastrointestinal infection: Salmonella (003·0, 003·1, 003·8, 003·9), Pseudomonas (008·42), Campylobacter (008·43), Clostridium difficile (008·45), adenovirus enteritis (008·62), other viral enteritis (toroviruses) (008·69).
• Oral candidiasis: 112·0.
Epidemiological features
• Sex and age groups (<7 and 7–11 months; 1, 2, 3, 4, 5–9, 10–14 and ⩾ 15 years).
• Hospitalization period: 2003–2005 (prior to vaccine availability) vs. 2007–2009 (3-year period when Rotarix (GlaxoSmithKline, Spain) and RotaTeq (Sanofi Pasteur MSD, Spain) vaccines became commercially available simultaneously in Spain).
• Season of hospitalization: December–February, March–May, June–August, September–November.
Statistical analysis
First, we calculated both the number of cases and the percentages of the codes most frequently noted in the hospital discharge information for cause-specific infectious (known) AGE, as well as for AGE of unknown aetiology.
The frequency of all independent variables or features was described both in the group of patients hospitalized for ARGE, as well as in the group of those hospitalized for other AGE. A simple (bivariate) logistic regression analysis was performed to compare the occurrence of each feature in these groups. The odds ratio (OR) and 95% confidence interval (CI) showed the effect size of each independent variable in the ARGE group relative to that in the AGE group. A χ 2 test was used to explore the association between dehydration and other characteristics of the ARGE cases identified.
To analyse the associations between community-acquired ARGE requiring hospitalization and the epidemiological and clinical factors described above (both complications and comorbidities), we constructed a multivariable, logistic, step-wise regression model by introducing the variables that were found to be significant in the bivariate analysis at each step in the block for their entry or removal.
All analyses were performed with Stata v. 11 statistical software (StataCorp LP, USA); the level of significance (P) was assigned to values <0·05.
Ethics
The Ethical Review Board of The Service of Information and Sanitary Statistics of the Counselling of Health and Social Welfare in CLM approved this study prior to establishing a Firm Commitment Requirement of a Confidentiality Agreement, as set forth by Royal Decree 994/1999, which regulates the security measures for automated files containing personal data.
RESULTS
We identified 17 415 AGE cases from 22 998 registries, of which 1657 were identified as ARGE (99·7% were emergency admissions). The median number of rotavirus admissions per year was 203 [interquartile range (IQR) 190–263], accounting for 10% of discharges with a classification of AGE throughout the study period (27% in children aged <5 years) (Table 1). The second and third most common cause-specific AGE in children aged <5 years were Salmonella and Campylobacter, which made up 7% and 3% of all AGE cases, respectively, in this age group (Table 1). Half of all rotavirus hospitalizations occurred in children aged <2 years, with a median age of 1 year (IQR 0·59–1·63); 56% were male (Table 2). In contrast, 57% of admissions for other AGE occurred in children aged ⩾10 years, and only 14% appeared in children aged <1 year (median age 32·3, IQR 2·4–73·2) (Table 2).
Table 1. Number of discharges of general AGE cases, by age group (source: MBDS, CLM, Spain, 2003–2009)

AGE, Acute rotavirus gastroenteritis; MBDS, Minimum Basic Data Set; CLM, Castile-La Mancha; Cj, Campylobacter jejuni.
* Diagnoses of localized Salmonella infection (no AGE) (003·21–003·24) were excluded.
† Rest of AGE cases defined by a specific code in the primary diagnosis field of MBDS, together with AGE cases coded as AGE ‘of undetermined aetiology’ (009·0–009·3), ‘Unspecified non-infectious AGE’ (558·9), or ‘Diarrhoea’ (787·91), with a specifically coded AGE diagnosis in any secondary field of the MBDS.
‡ Unknown AGE, AGE coded as AGE ‘of undetermined aetiology’ (009·0–009·3), ‘Unspecified non-infectious AGE’ (558·9), or ‘Diarrhoea’ (787·91), without a specifically coded AGE diagnosis in any secondary field of the MBDS.
Table 2. Description and bivariate analysis of characteristics of patients hospitalized for rotavirus (source: MBDS, CLM, Spain, 2003–2009)
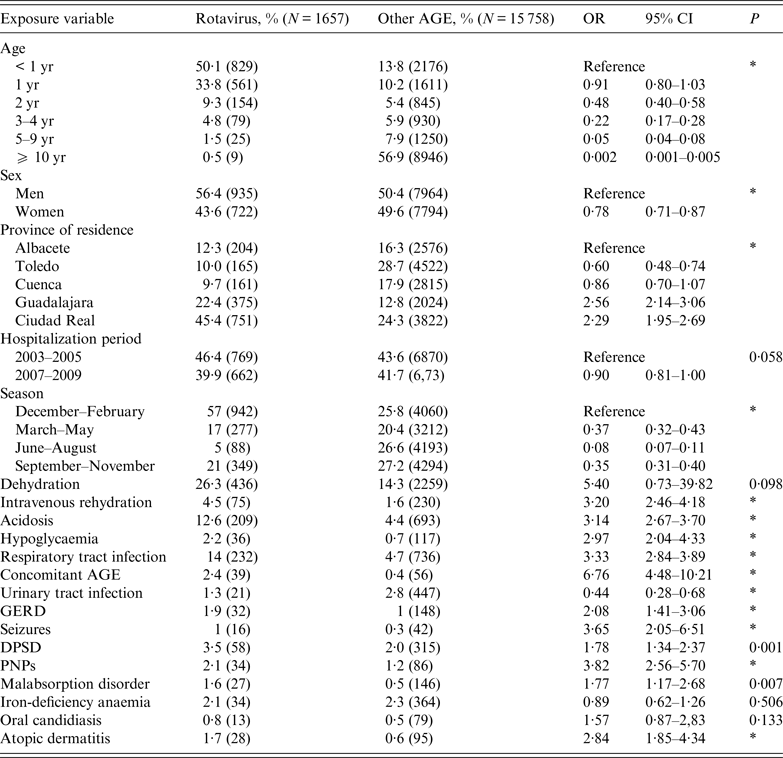
MBDS, Minimum Basic Data Set; CLM, Castile-La Mancha; AGE, acute gastroenteritis; OR, Odds ratio; CI, confidence Interval; GERD, gastroesophageal reflux disease; DPSD, disorders of pondostatural development; PNPs, perinatal problems.
* P < 0·0001.
Eighteen percent of patients with ARGE had one co-infection (P = 0·027); only 0·1% presented with two co-infections. Of all rotaviruses complicated with dehydration, 14% had one or more co-infections. Frequent co-infecting rotavirus agents included adenovirus (70%), followed by Campylobacter (19%) and Salmonella (8%). Almost 68% of rotavirus hospitalizations occurred in Ciudad Real and Guadalajara. With respect to other AGE cases, dehydration, acidosis, and hypoglycaemia complications were 12%, 8%, and 2% more frequent, respectively; the need for IV infusion was 3% greater. Moreover, the occurrence of concomitant RTIs and AGE was 9% and 2% higher, respectively. Rotaviruses were also significantly more commonly associated with GERD, seizures, or PNPs, among others (Table 2).
Dehydration was four times more frequent in ARGE patients aged <5 years (OR 4·36, 95% CI 1·20–12·96, P = 0·024). Twenty-one percent (48/232) of children with ARGE and a RTI (P = 0·035) and 9% (3/32) of those with ARGE accompanied by GERD (P = 0·028) were dehydrated. However, dehydration was not a significant complication in children with ARGE and a malabsorption disorder (P = 0·695), nor in the case of ARGE with a concomitant AGE (P = 0·053). Twenty-seven percent (118/436) of rotavirus dehydrations presented with acidosis (P < 0·0001). Dehydration was associated with acidosis when ARGE and RTI were simultaneously present (P < 0·0001), but not in the case of ARGE and a concomitant AGE (P = 0·578), nor in children with ARGE who also presented with malabsorption syndrome (P = 0·512) or GERD (P = 0·320). Neither patients with ARGE and PNPs nor ARGE and DPSD showed a greater risk of dehydration (P = 0·708 and P = 0·258, respectively).
In the multivariate analysis, the characteristics that were found to be independently associated with rotavirus hospitalizations were age, province, season of hospitalization, acidosis (all P < 0·0001), dehydration (P = 0·0003), IV rehydration (P = 0·0020), RTI (P = 0·0033), and concomitant AGE (P = 0·0355) (Table 3). At age 2 years, the risk of rotavirus hospitalization decreased to less than half and between 3 and 4 years, to a quarter. In summer, admissions were reduced by 92% (95% CI 0·05–0·14). Compared to other AGE cases, dehydration was 12 times more likely in ARGE cases (95% CI 1·52–40·38) (Table 3), while acidosis (95% CI 1·24–1·83), the need for IV infusion (95% CI 1·29–2·35), and the presence of a concomitant AGE (95% CI 1·03–2·25) or RTI (95% CI 1·09–1·98) were almost double.
Table 3. Multivariate analysis of those characteristics associated with rotavirus hospitalizations in the bivariate analysis (source: MBDS, CLM, Spain, 2003–2009)

MBDS, Minimum Basic Data Set; CLM, Castile-La Mancha; OR, odds ratio; CI, confidence interval; AGE, acute gastroenteritis.
* P < 0·0001.
In the 3 years that Rotarix and RotaTeq were commercially available in Spain, the frequency of both ARGE and other non-specific codified viral enteritis occurring in children aged <5 years decreased by 14·5% and 18·9%, respectively, in regard to the pre-vaccine period. Additional information on the prevalence of the AGE most commonly coded in hospital discharge records, classified by hospitalization period and age group, is provided in Supplementary Table S1. A more detailed analysis of the decrease in ARGE incidence rates throughout the vaccination period is explained elsewhere [Reference Redondo, Cano and Simón16]. Despite that analysis revealing a significant decline in the incidence rate of ARGE in 2007–2009 compared to 2003–2005 (incidence rate ratio 0·86, 95% CI 0·78–0·96), in the present study no significant differences were found between the percentage of children with ARGE accompanied with PNPs, PDPE, or dehydration in 2007–2009 compared to 2003–2005 (P = 0·260, 0·414 and 0·069, respectively).
DISCUSSION
ARGE cases carry an increased risk of dehydration and metabolic acidosis, a greater need for IV rehydration, and higher comorbidity rates with RTIs and other AGE. While dehydration with acidosis occurred in a third of cases with a simultaneous RTI, both together were hardly observed in children with a concomitant malabsorption syndrome, and in no cases did they present with a concomitant infectious AGE.
Our results agreed with the need for IV rehydration that other authors have previously noted [Reference Wildi-Runge17]. In Europe, the severity of infection accounts for 53% of cases compared to 31% for other cases of AGE [Reference Forster3]. Although there is no threshold of severity to indicate the need for hospitalization, rotavirus infections requiring admission are those assessed by clinicians as severe or at risk of becoming so. Consequently, the severity of an AGE correlates significantly with the incidence of hospitalization [Reference Freedman, Eltorky and Gorelick18].
The proportion of enteropathogenic concomitant AGE in ARGE cases is reported to be 11%, with Campylobacter spp. being the most frequent [Reference Lan19]. We estimated a lower frequency of concomitant AGE cases, with adenovirus as the most common cause of all co-infections; moreover, the proportion of concomitant Salmonella observed in our study was higher than that reported in the literature [Reference Lan19]. However, we found no significant increase in gastrointestinal complications in these cases, in contrast to what could reasonably be expected [Reference Valentini20], probably due to a problem of underdiagnosis in the MBDS.
Functional gastroesophageal reflux is common during early infancy [Reference Singendonk21]. Since vomiting both characterizes ARGE and constitutes a side-effect of the rotavirus vaccine [22], we hypothesized that GERD could be contributing to the general severity of the infection and, in particular, to dehydration in the youngest infants. However, although 97% of GERD cases occurred in children aged <2 years, the entity was not significantly associated with ARGE in the multivariable analysis. As expected, complications of intussusception or necrotizing enterocolitis were not codified [Reference Calle23, Reference Redondo-González24].
Prematurity, LBW, and congenital pathologies are all associated with increased risk for ARGE hospitalization [Reference Bruijning-Verhagen25], but we could not specifically evaluate the role of premature birth because it was not coded in the MBDS. Instead, we computed the percentage of ARGE cases that were attributable to PNPs and DPSD and their implications. In fact, the percentage of premature birth and LBW in rotavirus hospitalizations calculated from the MBDS in Spain between 1999 and 2009 was as low as 0·08–0·84% [26]. Hence, we presume relevant miss-codification from the MBDS when it is used as data source of risk factors for rotavirus.
Because of its neurotropism, rotavirus is the most important agent in benign seizures caused by an AGE [Reference Lloyd27, Reference Ueda28], with seizures occurring in 3% of ARGE cases with no pre-existing neurological conditions [Reference Reimerink29]. In CLM, seizures were coded less often and only 1·3% of ARGE cases with seizures were complicated with dehydration. It is well known that early rotavirus infection in the first 2 years of life is associated with a higher risk for recurrent wheezing, although no further associations have been found with other atopic manifestations [Reference Reimerink29]. In this sense, we did not find atopic dermatitis to be significantly more frequent in ARGE cases.
One limitation of this study is that many aspects of clinical histories are either recorded unevenly or not included in coded diagnoses. Although fever is present in up to 79% of ARGE cases [Reference Gimenez-Sánchez2], we could not analyse it as a factor because its encoding rate was <0·5%. Similarly, we could not assess vomiting or LBW individually as a perinatal risk factor. Breastfeeding was likewise not coded in the MBDS. Under-coding of terms may explain the lack of statistical significance of certain characteristics evaluated in our study. Indeed, an important disagreement between the clinical data contained in the MBDS and the main diagnosis has been reported [Reference Calle23]. Regarding the ability of the MBDS to specifically identify ARGE cases, it has only been described for nosocomial infections [Reference Redondo-González24].
To improve the detection of community-acquired rotaviruses from the MBDS, we examined the secondary diagnosis codes 008·61 accompanying undefined AGE as the principal diagnosis. Dehydration and hypovolaemic shock were not considered as they require multiple differential diagnoses with other entities. Nevertheless, their lack of inclusion as principal diagnostic codes accompanying the secondary code 008·61 may have led to a certain amount of misclassification [Reference Lindenauer30]. As only 0·3% of ARGE cases corresponded to elective admissions, the risk of bias due to nosocomial ARGE that might have been labelled as community-acquired should be minimal. Finally, some misclassifications are assumed to be inherent in the MBDS because of the nature of the coding process.
The main strength of our research is that most factors studied here had not been previously analysed either singly or in a multi-causal manner. We evaluated their interactions to correct for possible confounding that may have led to over- or under-estimation, or even misdirection when they had been analysed separately in previous studies. In this sense, PNPs, DPSD, GERD, seizures, and atopic conditions were all shown to be unassociated with rotavirus in the multivariate analysis. This was probably due to the confounding effect of age since these factors were coded most often in the youngest patients (70% in children aged <7 months).
A model based on administrative data led us to explore the joint influence of intertwined factors associated with the severity of ARGE. Age group and concomitant RTIs appear to contribute to the need both for IV rehydration and metabolite replacement to counteract metabolic acidosis. Although several of these conclusions only reinforce and validate what is already known, the SDFs included in the MBDS have proven to be an efficient tool for elucidating the clinical and epidemiological behaviour of infectious AGE cases, resembling the information obtained by chart review. The finding that adenovirus is the leading cause of concurrent AGE should be explored further because of its possible role in acute respiratory and gastrointestinal manifestations of ARGE.
Recent studies have demonstrated that risk-based immunization programmes are not optimum in terms of coverage for people at risk, proposing additional strategies to reduce the remaining morbidity [Reference Cromer31]. Likewise, modern dynamic models of rotavirus transmission have assessed that universal immunization programmes would be the preferred option to bring additional herd immunity benefits for the prevention of severe ARGE cases [Reference Atkins32, Reference Shim33]. In this sense, our study provides further suggestive evidence for policy-makers seeking a possible future universal vaccination.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268816000881.
DECLARATION OF INTEREST
None.





