Introduction
Measles and rubella infections in Iran decreased due to the mass campaign of measles and rubella vaccination in 2003 [Reference Esteghamati1] but still, there are suspected cases. National Measles Laboratory (NML) in Tehran University of Medical Sciences receives samples for about 6000 suspected cases of measles and rubella in 1 year. Fortunately, the Measles/Rubella incidence rate is less than expected. It is worth noting that some other viral infections show similar signs with measles and rubella such as some arboviruses [Reference Gourinat2]. Over the past decade, a considerable global emergence/re-emergence of arboviral diseases occurred [Reference Dash3]. Arboviruses cause some of the most important emerging infectious diseases which are associated with fever and rash in tropical and subtropical regions [Reference Afzal4, Reference Gubler5]. Of these viruses, chikungunya virus (CHIKV) belonging to the alphavirus genus in the Togaviridae family[Reference Powers and Logue6] has re-emerged in Africa, the Indian Ocean islands, South and Southeast Asia, with an emergence seen in the Americas, the Pacific and Europe due to the transatlantic and transpacific migration of patients [Reference Burt7]. Another arbovirus is dengue virus (DENV), an RNA virus that belongs to the Flaviviridae family with four closely related but antigenically different DENV serotypes 1–4 [Reference Rasheed, Butlin and Boots8]. It is responsible for about 400 million estimated infections annually resulting in asymptomatic or mild dengue fever to dengue haemorrhagic fever [Reference Murray, Quam and Wilder-Smith9]. Currently, DENV infection is a major health threat and the most important human arbovirus disease in both the tropics and subtropical regions [Reference Khan10, Reference Wilder-Smith11]. The CHIKV and DENV transmission between humans occurs through different Aedes species including Aedes aegypti and Aedes albopictus mosquitoes which are distributed throughout the world [Reference Afzal4, Reference Kraemer12]. Signs of disease in CHIKV and DENV infections are associated with arthralgia, fever, rash and also other manifestations such as muscle pain, headache, nausea and fatigue [Reference Afzal4]. In Iran, the epidemiology of DENV and CHIKV remains poorly understood. Iran is a country located in West Asia. Iran is bordered to the east by Afghanistan and Pakistan and to the west by Turkey and Iraq, countries where epidemics and endemics of arboviruses including CHIKV and DENV have occurred [Reference Amarasinghe and Letson13, Reference Humphrey14].
Therefore, Iran is strategically placed at a remarkable risk zone for these arboviral diseases. In addition, the introduction of exotic vectors into Iran is possible [Reference Doosti15]. Active entomological surveillance of Ae. albopictus and Ae. aegypti was carried out in spring, summer and winter, 2008–2014 in eight provinces [Reference Doosti15] following the first dengue case report in Iran in 2008 [Reference Chinikar16]. Ae. albopictus larvae were collected in the Sistan & Baluchestan province bordering Pakistan in 2009 [Reference Doosti15]. In 2013, Ae. albopictus adult mosquitoes were also detected in a coastal location neighbouring Chabahar in the same province [Reference Doosti15]. Ae. albopictus is regarded as a conspicuously menacing and versatile species that inhabits both temperate and tropical climate regions [Reference Nejati17, Reference Roiz18]. Since this vector has been detected in different parts of Sistan & Baluchestan, there is a possibility of dengue fever and chikungunya fever in southeast Iran. At present, there are just a few studies from Iran on dengue fever in blood donors [Reference Aghaie19] and on patients who tested negative for Crimean-Congo Haemorrhagic Fever in Sistan & Baluchestan province [Reference Chinikar20] and there is no report from Iran on dengue fever in patients with rash and fever showing non-specific febrile illness. Between 2017 and 2018, an investigation was cariied out in the Sistan and Baluchistan Province on alleged CHIK patients. Laboratory results from qRT-PCR or ELISA tests performed revealed a quarter (25.1%) of the samples tested positive [Reference Pouriayevali21]. In addition, another study in 2017 depicted the southernmost regions as areas at a high risk of Ae. albopictus establishment [Reference Nejati17]. Hence, this study was implemented with the aim of assessing the serology of chikungunya and dengue fever in suspected patients.
Methods
Serum specimens
NML in the School of Public Health, Tehran University of Medical Sciences, every year, receives about 6000 samples from suspected cases of measles and rubella, and laboratory diagnosis was made using IgM capture enzyme-linked immunosorbent assay (ELISA) for measuring IgM anti-measles and IgM anti-rubella antibody.
Serum samples were collected randomly from eight provinces (Table 1) from December 2016 to November 2017. The patients had fever and rash, but the sera were negative for measles and rubella IgM. We have studied the areas where active entomological surveillance of Ae. albopictus and Ae. aegypti was carried out in spring, summer and winter, 2008–2014 in those regions.
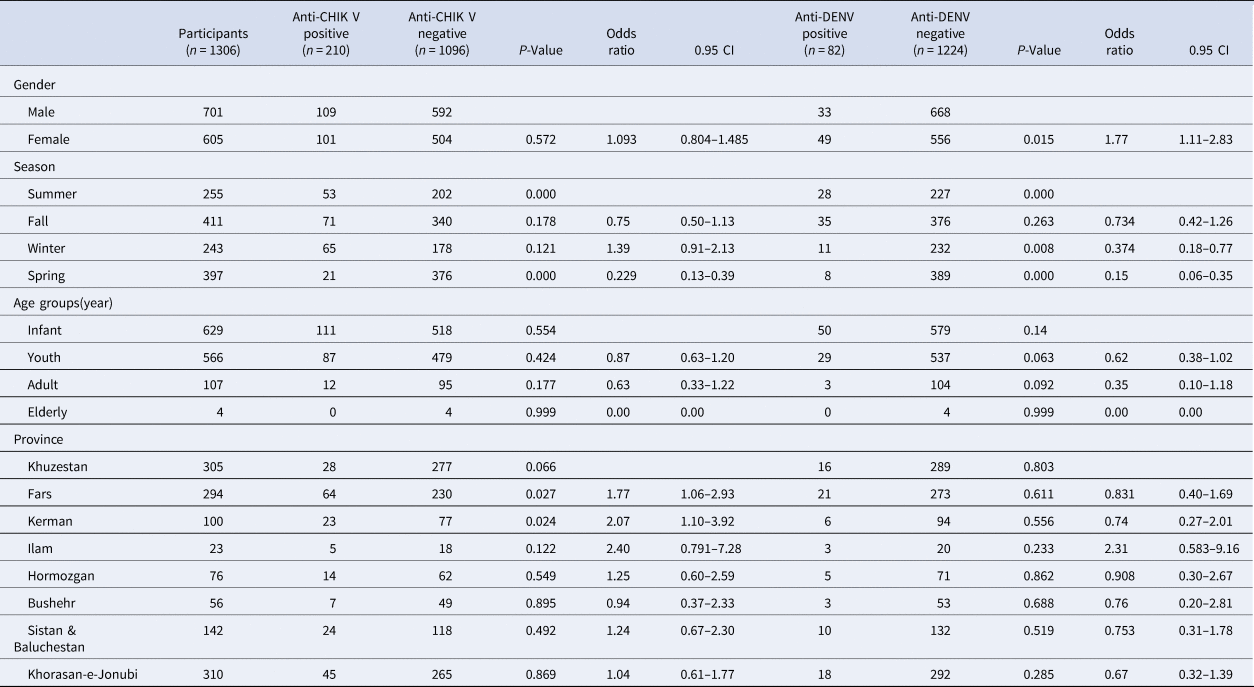
Table 1. Anti-CHIKV and anti-dengue seropositivity rates in studied samples
Iran is a country with an area of 1,648,000 km2 located in the Middle East with a diverse climate [Reference Askarian22]. Among the studied provinces during 2017, Ilam and Sistan & Baluchestan had the highest (398.8 mmHg) and the lowest (28.7 mmHg) average annual rainfall respectively [23]. Khuzestan had the highest (50.4 °C) maximum temperature and Ilam had the lowest (−8.9 °C) minimum temperature [24].
Enzyme-linked immunosorbent assay
The presence of DENV IgM and CHIKV IgM were examined by a commercial Semiquantitative IgM ELISA kit according to the manufacturer's instructions (EUROIMMUN Anti-CHIKV/IgM) and (EUROIMMUN Anti-DENV/IgM; PerkinElmer, Lübeck, Germany).
Statistical analyses
Statistical analyses were performed using the SPSS software, version 16. Fisher's exact test was performed for the evaluation of qualitative variables including sex, province and season. In order to explain the relationship between dengue and chikungunya infection and independent variables, logistic regression was performed. P-Value <0.05 was considered significant.
Results
In this study, serum samples from 1306 patients from eight provinces were tested.
The overall CHIKV and DENV IgM seropositivity was 16.07% (210/1306) and 6.27% (82/1306) respectively. A summary of the characteristics of the study of all the patients was presented (Table 1).
The rate of chikungunya seropositivity among patients from the eight provinces ranged from 9.18% in Khuzestan (28/305) to 23% in Ilam (23/100) (Fig. 1). The rate of dengue seropositivity among patients from the eight provinces ranged from 5.24% in Khuzestan (16/305) to 13.04% in Ilam (3/23) (Fig. 2). The highest rate of chikungunya seropositivity was found in Kerman, Fars and Ilam, and the highest rate of dengue seropositivity was found in Ilam, Fars and Khorasan-e-Jonubi. Logistic regression analysis showed a significant increase in the CHIKV IgM antibody seropositivity rate in Kerman (OR = 2.07, 95% CI: 1.10–3.92; P = 0.024) and Fars (OR = 1.77, 95% CI: 1.06–2.93; P = 0.027).

Fig. 1. Map showing the CHIKV Seropositivity rate in study areas in Iran, 2017.

Fig. 2. Map showing the DENV Seropositivity rate in study areas in Iran, 2017.
The patients were divided into four age groups comprising less than or equal to 1 year (infants), 2–18 years (youths), 19–64 years (adults) and more than 64 years (elderly). The frequency distribution of the various age groups encompassing groups 1 to 4 stated above was 48.2% (629/1306) for infants, 43.3% (566/1306) for youths, 8.2% (107/1306) for adults and 0.3% (4/1306) for elderly respectively. The mean (±s.d.) age of the studied cases was 6.05 (±9.536). The highest anti-CHIKV seropositivity rate was observed in infants (17.6%). Also, most of the dengue positive cases belonged to infants (7.9%). Although the CHIKV seropositivity rate was higher in infants, CHIKV seropositivity was not statistically significant based on the age group (P = 0.3). Besides, the observed difference between anti-dengue and age in this study was not significant (P = 0.09).
In total, 109/701 (15.54%) in the male group and 101/605 (16.69%) in the female group were positive for anti-IgM CHIKV antibodies and 33/701 (4.07%) in the male group and 49/605 (8.09%) in the female group were positive for anti-IgM DENV antibodies.
The highest dengue seropositivity rate was seen in the female group in youths (logistic regression, OR 1.77, 95% CI 1.11–2.83; P = 0.015).
In terms of season, there was a statistically significant difference in the anti-DENV and anti-CHIKV IgM antibody seropositivity rate between the patients grouped based on the season (χ 2 test, P < 0.01), and the infection rate in summer was higher than in other seasons (logistic regression, P < 0.01).
Discussion
The seropositive cases in our study reveal possible CHIKV and DENV infection in Iran.
As mentioned before, among the studied provinces, Kerman and Fars had a significant increase in the CHIKV seropositivity rate in comparison with other provinces. Kerman and Fars are located in tropical regions, so these differences can be explained in part by the increasing transmission risk at high temperatures [Reference Vairo25]. Another possible explanation for this is that Kerman and Fars provinces are located in southern Iran. Kerman is neighbouring Sistan and Baluchestan, where the presence of Ae.. Albopictus and cases of dengue fever and chikungunya fever have been reported. So, it could be suggested that CHIKV has possibly been imported through travellers to this neighbouring endemic country.
The majority of reported dengue cases in previous studies in Iran were from patients who had travelled to endemic countries especially in southeastern Asia. Among them, only one case, unlike previous ones, had been bitten by an infected vector inside Iran [Reference Ghasemzadeh, Dalaki and Safari26].
However, some patients had no travel history to dengue-endemic countries [Reference Aghaie19, Reference Chinikar20].Besides, revelations from the only current study done in the Sistan and Baluchistan Province since three decades ago (since 1971), indicated that all the CHIKV+ RNA patients had lately traveled to Pakistan according to their records and five of the specimens had analogy with the newly identified cases of the 2016-2017 Pakistani epidemic native to the satellite ECSA genotype of the Indian Ocean descent [Reference Pouriayevali21]. Unfortunately, in our study, the travel history of patients is not available.
As a result, the virus may have been spread by patients who have travelled to areas where one or two major vectors, Ae. aegypti and Ae. albopictus have established. Moreover, an autochthonous silent or paucisymptomatic circulation cannot be excluded and may have found its origin in border countries where vectors are established and autochthonous transmission have been described.
Since 2005, Ae. aegypti and/or Ae. albopictus have been found in Afghanistan, Algeria, Lebanon, Oman, Palestine, Syria, Turkey and Iran [Reference Humphrey27]. Although outbreaks of suspected dengue occurred in Pakistan, Yemen, Saudi Arabia, Sudan and Madagascar in 2005–2006, there is no report of autochthonous transmission of DENV from these countries so far [Reference Humphrey27]. Despite the fact that Ae. aegypti is regarded as the primary vector traditionally, some recent epidemics were caused by Ae. albopictus, which suggests that Ae. aegypti is becoming superseded by Ae. albopictus due to virus mutation [Reference Chia, Ng and Chu28]. Ae. albopictus has been distributed widely in Madagascar, Southern Europe and North and South America, Africa, the Indian Ocean and South East Asia via increased international travel and vehicle component such as water-containing tyres imported from elsewhere [Reference Chia, Ng and Chu28]. For example, vehicles travelling from Turkey, Bulgaria and Syria may introduce Ae. albopictus in Iran [Reference Doosti15]. A number of factors may have promoted the distribution of Aedes-borne viruses such as urbanisation [29], heavy rainfall [Reference Malik30], war and economic disturbance in Iraq, Syria and Yemen [Reference Amarasinghe and Letson13], migration [Reference Amarasinghe and Letson13] and heavy trade in the Red Sea region [Reference Malik30], that may pose risk for spread of DENV from Pakistan to Iran or Afghanistan [Reference Aghaie19]. With this regard, a significant difference in the high CHIKV and dengue seropositivity rate in summer was observed (P < 0.01) in our study. On the other hand, vector mosquitoes are mainly active in the summer season [Reference Vairo25]. Based on this finding, we could hypothesise that the vector active season has played an important role in facilitating the autochthonous transmission of imported viruses in Iran. Considering the fact that the presence of mosquito vectors, as well as the infectivity of these vectors, have not yet been studied in Iran since 2014, it is possible that these regions could be the foci of these vectors if the climate conditions were suitable.
Although our study shows no significant difference in the different age groups, however, the prevalence suggests that dengue fever often occurs in children, despite the fact that the older age group has been witnessing an increase in dengue fever infection in recent years [Reference Chinikar20].
Even though in our study, the presence of the CHIKV IgM antibody is not associated with gender, on the other hand, the anti-dengue IgM antibody is higher in females than in males, which it is not in line with previous reports that exhibited an excess of male patients in Iran [Reference Chinikar20], Malaysia [Reference Shekhar and Huat31] and Singapore [Reference Yew32]. In line with our study, other researchers have reported a greater proportion of female dengue cases or a slightly differing result of equal proportions of male and female patients in South America [Reference Kaplan33–Reference García-Rivera and Rigau-Pérez36]. The higher dengue fever in males has been attributed to displacements resulting from more business travels executed by males than females in endemic areas [Reference Chinikar20], while an explanation for the greater frequency of female dengue cases stems from the indoor habits of mosquitoes. Considering the fact that women spend more time at home [Reference Guzman37], they are more susceptible to arboviruses.
Given that chikungunya and dengue fever epidemic is engendered by either Ae. aegypti and/or Ae. albopictus, in a population with low herd or community immunity [Reference Chia, Ng and Chu28], it is not surprising that Iran may be at high risk for the occurrence of chikungunya and dengue fever epidemic. At this point, it is worth mentioning that the present study is not without its limitations. Firstly, the cross-reactivity between these arboviral infections particularly that of Flaviviruses, with other viral infections such as West Nile Virus, which had been previously reported in Iran was not taken into consideration [Reference Ziyaeyan38]. Another limitation can be explained by the fact that polyclonal stimulation of the immune system or antibody persistence may have had an impact on the specificity of the positive results of the ELISA test. Hence, diagnostic reports should be accompanied by neutralisation assays including the detection or isolation of the virus from patients in order to confirm the presence of these viruses in Iran. The main strength of this study is the large sample size.
Conclusion
The results from this study suggest that there has been an exposure to DENGV and CHIKV by a local population in Iran. This is an important issue for future research studies and further population-based work is required in order to investigate and assess the degree of exposure of the population to the presence of dengue and CHIKV in Iran.
Acknowledgement
The Research reported in this publication was supported by the Elite Researcher Grant Committee under award number [958911] from the National Institute for Medical Research Development (NIMAD), Tehran, Iran
Conflict of interest
None.





