Introduction
Suicidal behavior is a major public health problem causing approximately 800 000 deaths worldwide each year (WHO, 2017). Major psychiatric disorders and psychiatric/cognitive traits are risk factors for suicidal behavior (Bachmann, Reference Bachmann2018), best conceptualized in a stress diathesis model (Mann & Rizk, Reference Mann and Rizk2020). The stressor can be internal, as in a major psychiatric disorder, or external, as in psychosocial stressful life events. The diathesis includes a variety of traits related to mood regulation, decision-making, learning and memory, and social cognitions. Family, adoption, and twin studies indicate moderate heritability of suicidal behavior, with an estimated h 2 of 30–50% (Bondy, Buettner, & Zill, Reference Bondy, Buettner and Zill2006; Oquendo et al., Reference Oquendo, Sullivan, Sudol, Baca-Garcia, Stanley, Sublette and Mann2014; Statham et al., Reference Statham, Heath, Madden, Bucholz, Bierut, Dinwiddie and Martin1998). This heritability is related in part to major psychiatric disorders, and in part to associated traits that comprise the diathesis for suicidal behavior (Mann & Rizk, Reference Mann and Rizk2020; van Heeringen & Mann, Reference van Heeringen and Mann2014).
Most genome-wide association studies (GWASs) of suicidal behavior have included few or no suicide decedent cases, the most extreme or severe phenotype (Erlangsen et al., Reference Erlangsen, Appadurai, Wang, Turecki, Mors, Werge and Agerbo2020; Galfalvy et al., Reference Galfalvy, Haghighi, Hodgkinson, Goldman, Oquendo, Burke and Mann2015; Mullins et al., Reference Mullins, Bigdeli, Børglum, Coleman, Demontis, Mehta and Lewis2019; Ruderfer et al., Reference Ruderfer, Walsh, Aguirre, Tanigawa, Ribeiro, Franklin and Rivas2020; Strawbridge et al., Reference Strawbridge, Ward, Ferguson, Graham, Shaw, Cullen and Smith2019; Willour et al., Reference Willour, Seifuddin, Mahon, Jancic, Pirooznia, Steele and Potash2012). Consequently, few studies have examined the genetic architecture of suicide death (Docherty et al., Reference Docherty, Shabalin, DiBlasi, Monson, Mullins, Adkins and Coon2020; Otsuka et al., Reference Otsuka, Akiyama, Shirakawa, Okazaki, Momozawa, Kamatani and Hishimoto2019; Strawbridge et al., Reference Strawbridge, Ward, Ferguson, Graham, Shaw, Cullen and Smith2019). Given the clinical, demographic, and biologic differences between non-fatal suicide attempt and suicide death (DiBlasi, Kang, & Docherty, Reference DiBlasi, Kang and Docherty2021; Mann & Rizk, Reference Mann and Rizk2020), the two phenotypes may involve both similar and different qualitative and quantitative genetic architecture. Recent GWASs (Docherty et al., Reference Docherty, Shabalin, DiBlasi, Monson, Mullins, Adkins and Coon2020; Mullins et al., Reference Mullins, Bigdeli, Børglum, Coleman, Demontis, Mehta and Lewis2019; Otsuka et al., Reference Otsuka, Akiyama, Shirakawa, Okazaki, Momozawa, Kamatani and Hishimoto2019; Ruderfer et al., Reference Ruderfer, Walsh, Aguirre, Tanigawa, Ribeiro, Franklin and Rivas2020) reported higher single nucleotide polymorphism (SNP)-based heritability for suicide death than for non-fatal suicide attempt. However, to date, there is no GWAS comparing the shared polygenic effects with psychiatric disorders or traits between suicide attempt and suicide death in a same study. Another limitation of most studies is the lack of information about the components of suicide-related endophenotypic psychopathology such as depression/hostility/impulsivity/aggression severity.
In the present study, we utilized individual-level genome-wide variant data and deep clinical phenotyping including severity scores for depression/hostility/impulsivity/aggression obtained by the same research team in both non-fatal suicide attempt and suicide death. We tested whether polygenic risk scores (PRSs) from large publicly-available GWASs of suicide-related psychiatric disorders and diathesis-related traits were associated with non-fatal suicide attempt and suicide death, and compared the results from the two groups. Furthermore, we hypothesized that polygenic effects of certain psychiatric traits may affect specific clinical endophenotypic components of suicidal behavior such as depression from the stress domain and hostility/impulsivity/aggression traits from the diathesis domain. For this purpose, we tested whether the above PRSs correlated with the severity of depression/hostility/impulsivity/aggression in our study sample.
Materials and methods
Subjects
This study was approved by the relevant Institutional Review Boards at Columbia University/New York State Psychiatric Institute, McGill University and University of Munich. The demographic/clinical characteristics of the dataset are shown in Table 1 and a part of details were previously published (Galfalvy et al., Reference Galfalvy, Haghighi, Hodgkinson, Goldman, Oquendo, Burke and Mann2015).
Table 1. Characteristics of subjects in our suicidal behavior dataset

M, male; F, female; SA, non-fatal suicide attempters; SD, suicide decedents; C, controls.
a This column shows the statistical information on difference for sex and age distributions among groups. **p < 0.01 by Fisher's exact test for sex comparison and Mann–Whitney U test for age comparison, respectively.
b Mean score ± standard deviation. The details for each score and the sample overlap in availability of each score are shown in online Supplementary Table S1 and Fig. S1.
c Quantitative depression severity scores were calculated by combining Z scores from HDRS and ADS obtained separately for each site.
Our GWAS sample was limited to individuals of European origin and included 577 cases with suicidal behavior (260 non-fatal suicide attempters and 317 suicide decedents) and 874 unmatched non-psychiatric controls, recruited from three sites (New York, USA; Montreal, Canada, Munich, Germany) between 1991 and 2011. Non-fatal suicide attempters were ascertained from psychiatric clinics of the three sites (87 samples from New York, 97 from Montreal and 76 from Munich). Suicide decedents were obtained from the Medical Examiner's Office of the two sites (121 samples from New York and 196 from Montreal). The definition of a suicide attempt was a self-injurious act involving at least partial intent to end one's life. Postmortem cases (suicide decedents) and controls (sudden death but not suicide) were clinically characterized by psychological autopsies, and suicide status was determined using the Columbia Classification Algorithm for Suicide Assessment (Posner, Oquendo, Gould, Stanley, & Davies, Reference Posner, Oquendo, Gould, Stanley and Davies2007). As previously described (Galfalvy et al., Reference Galfalvy, Haghighi, Hodgkinson, Goldman, Oquendo, Burke and Mann2015), the psychiatric diagnoses were determined in most subjects by psychiatrists or clinical psychologists and evaluated using the SCID-1 or SCID-NP version, and controls were free of axis I diagnoses, cluster B personality disorders, substance use disorders and a lifetime history of suicide attempts. For a subset of non-fatal suicide attempters, acute depression severity scores were obtained close to the time of blood sampling using one of two different instruments (each site had used only one instrument): the Hamilton Depression Rating Scale (Hamilton, Reference Hamilton1960) or the Allgemeine Depressions-Skala (ADS) (Meyer & Hautzinger, Reference Meyer and Hautzinger2001). Z-scores for these two scales were calculated separately for each site and combined into a quantitative depression severity score for the live subjects. In addition, trait hostility was assessed by the Buss–Durkee Hostility score (BDHS) (Buss & Durkee, Reference Buss and Durkee1957) in a subset of suicide attempters, trait impulsivity by the Barratt Impulsivity Scale (BIS-10) (Barratt, Reference Barratt1965) in a subset of suicide attempters and controls, and trait aggression by the Brown–Goodwin Aggression History score (BGAH) (Brown, Goodwin, Ballenger, Goyer, & Major, Reference Brown, Goodwin, Ballenger, Goyer and Major1979) in a subset of suicide attempters, suicide decedents, and controls (Table 1). The demographics for the subjects who completed questionnaires and those who did not and the details for each score is shown in online Supplementary Table S1 and the sample overlap in the availability of each score is shown in online Supplementary Fig. S1. All study subjects or the families of decedents gave written informed consent to participate in this study.
Genotype and QC
Details of assay and data processing are reported in our previous paper (Galfalvy et al., Reference Galfalvy, Haghighi, Hodgkinson, Goldman, Oquendo, Burke and Mann2015). Briefly, cases and controls were genotyped concurrently using the genotyping platform HumanOmni1-Quad BeadChip. Quality control was performed using PLINK 1.9 (Purcell et al., Reference Purcell, Neale, Todd-Brown, Thomas, Ferreira, Bender and Sham2007); SNPs with a call rate <0.95, minor allele frequency <0.01, and p < 1.0 × 10−6 for the Hardy–Weinberg Equilibrium in controls were excluded. Then, we performed a principal component (PC) analysis and compared the results to HapMap Phase 3 population cluster (Price et al., Reference Price, Patterson, Plenge, Weinblatt, Shadick and Reich2006; Purcell et al., Reference Purcell, Neale, Todd-Brown, Thomas, Ferreira, Bender and Sham2007), confirming all subjects were from the CEU (Northern European ancestry from Utah) (online Supplementary Fig. S2).
Generation of polygenic risk scores of various psychiatric disorders and traits
To generate PRSs of various psychiatric disorders and traits based on publicly available summary data from GWASs (online Supplementary Table S2) using PRSice-2 (Choi & O'Reilly, Reference Choi and O'Reilly2019), we selected GWASs indexing psychiatric disorders and traits reported to carry increased risk of suicidal behavior. We excluded GWASs with insufficient sample sizes (case N < 5000 for case–control GWAS or total N < 10 000 for continuous trait GWAS) to avoid the use of underpowered PRSs. We selected 22 summary data sets for psychiatric disorders [major depressive disorder (MDD), bipolar disorder (BD), schizophrenia (SCZ), anxiety disorders, post-traumatic stress disorder (PTSD), anorexia nervosa, attention-deficit hyperactivity disorder (ADHD), autism spectrum disorder (ASD) and Alzheimer's disease], insomnia, substance use phenotypes (alcohol dependence, lifetime cannabis use, and cigarettes per day), personality traits [neuroticism, extraversion, aggression, anti-social behavior, risk tolerance, and sensitivity to environmental stress and adversity (SESA)] and cognitive abilities [educational attainment, cognitive performance, and intelligence quotient (IQ)]. We selected GWASs based on only participants of European ancestry except for PTSD (Duncan et al., Reference Duncan, Ratanatharathorn, Aiello, Almli, Amstadter, Ashley-Koch and Koenen2018), and because the PTSD sample size of only European cases was small (N = 2424), we included PTSD data from other ethnic groups. There was no overlap between the samples in the GWAS summary statistics and our own cohort. The threshold (PT) for selecting ‘risk’ SNPs was sequentially set at 1 × 10−4, 0.001, 0.01, 0.05, 0.1, 0.2, 0.3, 0.4, and 0.5, excluding SNPs in the major histocompatibility complex (MHC) region. We then performed LD clumping to select the eligible SNPs for PRS (clump-kb: 250, clump-p: 1.0, clump-r2: 0.1).
Polygenic risk score analysis targeting suicidal behavior
First, we used our GWAS of suicidal behavior (577 suicidal behavior cases v. 874 controls) as the target set. The variance in the target datasets explained by the discovery set-based PRS was estimated based on Nagelkerke's R 2 from a logistic regression model with sex, age, and 10 PCs as covariates and converted to R 2 on the liability scale (Lee, Goddard, Wray, & Visscher, Reference Lee, Goddard, Wray and Visscher2012), which accounts for the prevalence rate in the general population (0.02 for suicidal behavior) (Nock et al., Reference Nock, Borges, Bromet, Alonso, Angermeyer, Beautrais and Williams2008) and the proportion of cases in our suicidal GWAS. The significance level for the PRS analysis was set at 2.5 × 10−4 [the PRSs of 22 disorders and traits were analyzed meaning 9 PT = 0.05/(22 × 9)]. We also designated associations with 2.5 × 10−4 ⩽ p < 0.05 as ‘nominal’. Second, in sensitivity analyses, we conducted additional PRS analyses dividing our GWAS suicidal behavior cases into non-fatal suicide attempt (N = 260) and suicide death (N = 317) as target sets, respectively. Then, we compared the shared contributions of polygenic effects across 22 disorders/traits between non-fatal suicide attempt and suicide death by using Spearman's rank correlation test and Wilcoxon signed-rank test. We also considered the directional effects of these PRSs between non-fatal suicide attempt and suicide death by using two-sided exact binomial test. Third, to control the polygenic effect of comorbid MDD, we conducted a subgroup analysis to test the association between MDD-PRS and suicide death status in suicide decedents without diagnosed MDD (N = 113) and also excluded suicide decedents with unknown psychiatric history (N = 90) because about half may have had ‘possible MDD’. Fourth, to test the differences in polygenic architecture between non-fatal suicide attempt and suicide death, we conducted PRS analysis setting the 22 psychiatric disorders/traits derived from large publicly available GWASs as the discovery set and the genotyped dataset for direct comparison of ‘non-fatal suicide attempters v. suicide decedents’ as the target set. For calculation of statistical power of our PRS analysis, we set the current sample size, α = 2.5 × 10−4 [0.05/(22 × 9)] and genetic correlation between suicidal behavior and each psychiatric trait = 0.1–0.5 estimated based on the previous papers (Mullins et al., Reference Mullins, Bigdeli, Børglum, Coleman, Demontis, Mehta and Lewis2019, Reference Mullins, Kang, Campos, Coleman, Edwards, Galfalvy and Ruderfer2021). To have statistical power >0.8 in our sample size, the estimated minimum detectable effect sizes of each psychiatric trait on the comparison of ‘suicidal behavior v. controls’, ‘non-fatal suicide attempt/suicide death v. controls’ and ‘non-fatal suicide attempt v. suicide death’, were OR = 1.3–1.4, 1.4–1.6, and 1.5–1.7, respectively.
Polygenic risk score analysis for clinical scores in suicidal behavior cases and non-psychiatric controls
To investigate the relationship between the suicide-related clinical severity scores in our suicidal cases and non-psychiatric controls and PRSs for the 22 psychiatric disorders and traits listed above, we calculated PRSs for each trait using the best PT in each subject as above. Then, linear regression was run with the PRS score as predictor and the depression/hostility/impulsivity/aggression severity score as response where applicable, with sex, age, and 10 PCs as covariates. The regression R 2 was used to estimate the proportion of variance in each clinical measure that is explained by each PRS score. The significance level for each regression analysis was set at 0.0023 (the PRS of 22 traits were analyzed = 0.05/22).
Results
Using 22 GWAS summary statistics of various psychiatric disorders and traits as the discovery dataset and our own genotyped dataset of suicidal behavior [766 810 SNPs; λ genomic control (GC) = 1.05 (online Supplementary Fig. S3)] as the target dataset, we found that PRSs for 9/22 psychiatric disorders and traits were statistically associated (p < 2.5 × 10−4) with the risk of suicidal behavior as follows: (1) major depressive disorder: maximum OR = 1.51, maximum variance explained R 2 = 2.26%; (2) BD: OR = 1.49, R 2 = 1.80%; (3) SCZ: OR = 1.51, R 2 = 2.04%; (4) ADHD: OR = 1.25, R 2 = 0.68%; (5) alcohol dependence: OR = 1.26, R 2 = 0.69%; (6) SESA: OR = 1.26, R 2 = 0.75%; (7) educational attainment: OR = 0.80, R 2 = 0.70%; (8) cognitive performance: OR = 0.82, R 2 = 0.61%; and (9) IQ: OR = 0.81, R 2 = 0.62% (Fig. 1, online Supplementary Table S3 and Fig. S4). In addition, PRSs for another 6/22 psychiatric disorders and traits showed nominal associations (2.5 × 10−4 ⩽ p < 0.05) with the risk of suicidal behavior as follows: (1) PTSD: OR = 1.23, R 2 = 0.59%; (2) anorexia nervosa: OR = 1.15, R 2 = 0.28%; (3) insomnia: OR = 1.12, R 2 = 0.18%; (4) lifetime cannabis use: OR = 1.14, R 2 = 0.25%; (5) antisocial behavior: OR = 1.20, R 2 = 0.47%; and (6) risk tolerance: OR = 1.16, R 2 = 0.29%. The PRSs for anxiety disorders, ASD, Alzheimer's disease, cigarettes per day, neuroticism, aggression and extraversion did not show a detectable association with suicidal behavior (p ⩾ 0.05) (Fig. 1 and online Supplementary Table S3).

Fig. 1. Odds ratios of suicidal behavior based on polygenic risk scores for psychiatric disorders and traits. We used each GWAS summary statistics available (all statistics, except that for PTSD, were obtained using the European dataset) as the discovery set and genotyped case–control GWAS data of suicidal behavior including both non-fatal suicide attempt and suicide death as the target set. Each odds ratio (dot) and 95% confidence interval (error bar) is shown at the PT threshold attaining the lowest p value, adjusted with age, sex and 10 PCs. The significance level for the PRS analysis was set at 2.5 × 10−4. Full results are shown in online Supplementary Table S3.
As an exploratory or sensitivity analysis to compare the findings for non-fatal suicide attempt and suicide death, we performed the same PRS analyses after subdividing our suicidal behavior cohort into non-fatal suicide attempters and suicide decedents. As shown in Fig. 2a, all 22 psychiatric disorders and traits showed the same direction (p for binomial tests = 4.8 × 10−7) of the polygenic effects between non-fatal suicide attempters and suicide decedents. The polygenic effects of the 22 traits were significantly correlated between non-fatal suicide attempters and suicide decedents (Spearman's ρ = 0.85, p = 9.4 × 10−7) and there was no statistical difference (p for Wilcoxon signed-rank test = 0.24) in these effects between the two groups. The 95% CIs of the ORs of all 22 psychiatric disorders and traits overlapped between non-fatal suicide attempt and suicide death (Fig. 2b and online Supplementary Table S4).
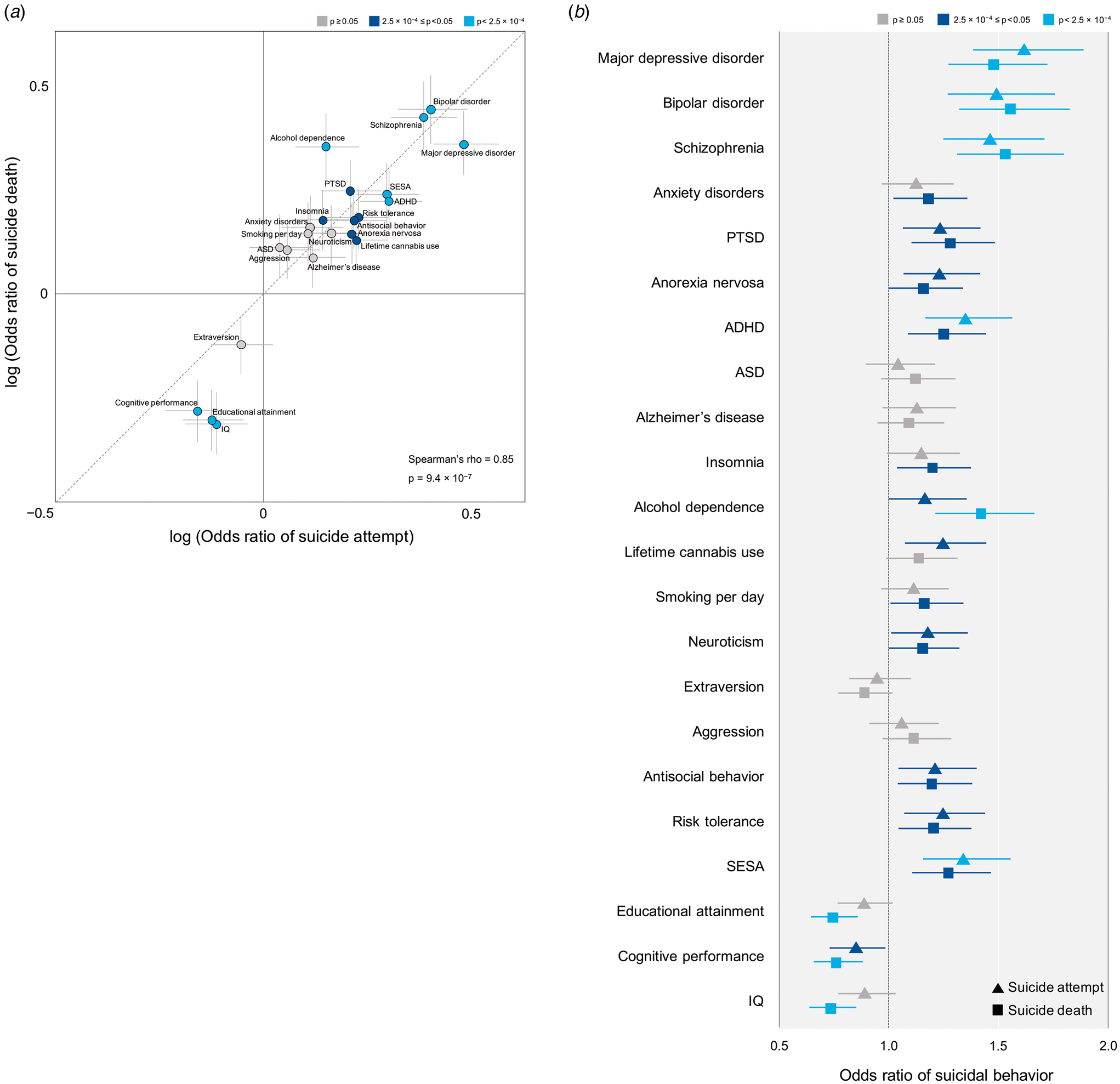
Fig. 2. Comparison of odds ratio by polygenic risk scores of psychiatric disorders and traits between suicide attempt and suicide death. We used each GWAS summary statistics available (all are European dataset except PTSD) as the discovery set and genotyped case–control GWAS data of non-fatal suicide attempt or suicide death as the target set. (a) Log (odds ratio) (dot) and standard error (grey lines) at the PT threshold attaining the lowest p value, adjusted with age, sex, and 10 PCs. The color of each dot refers to p value obtained from PRS analysis of suicidal behavior. Statistics obtained from Spearman's rank correlation test are presented. (b) Odds ratio (suicide attempt as triangle and suicide death as square) and 95% confidence interval (error bar), adjusted with age, sex, and 10 PCs. The color of each dot refers to p value obtained from PRS analysis of suicide attempt and suicide death separately. Full results are shown in online Supplementary Table S4.
We conducted a subgroup PRS analysis to test the association between MDD-PRS and suicide death by excluding suicide decedents with diagnosed/possible MDD and controls. We found similar associations between MDD-PRS and suicide death without MDD [p = 8.3 × 10−4, OR (95% CI) = 1.44 (1.16–1.78)], compared with the analysis using the whole sample of suicide decedents (online Supplementary Table S5 and Fig. S5).
In order to test the differences in polygenic architecture between non-fatal suicide attempt and suicide death, we also investigated the polygenic effects of the 22 traits in our genotyped dataset for direct comparison for ‘non-fatal suicide attempters v. suicide decedents’. None of the 22 psychiatric PRSs had significant differences of polygenic architecture between non-fatal suicide attempt and suicide death (p > 2.5 × 10−4) (online Supplementary Table S6).
In order to test whether the PRSs for 22 traits are associated with suicide-related clinical scores, we conducted regression analyses using our suicidal behavior cases and controls, respectively. Among the depression/hostility/impulsivity/aggression severity score, aggression was the only trait measured in all three groups (non-fatal suicide attempters, suicide decedents, and controls). Significant positive correlation after adjustment for multiple testing (R 2 = 0.11, p < 0.0023) was observed between MDD-PRS and trait aggression in our 407 suicidal behavior cases comprised of 230 non-fatal suicide attempters and 178 suicide decedents (Table 2 and online Supplementary Table S7). No statistically significant association was found in the control group (online Supplementary Table S8).
Table 2. Significant associations between polygenic risk scores for psychiatric traits and suicide-related clinical scores in suicidal behavior cases

Suicide behavior cases included both 230 non-fatal suicide attempters and 178 suicide decedents. Statistical values were from linear regression model adjusted with sex, age, and 10 PCs as covariates. The significance level was set at 0.0023. Full results are shown in online Supplementary Table S7.
Discussion
We found that the PRSs for MDD, BD, SCZ, ADHD, alcohol dependence, SESA, educational attainment, cognitive performance, and IQ from large publicly available GWASs were significantly associated with suicidal behavior (p < 2.5 × 10−4). In addition, the PRSs for PTSD, anorexia nervosa, insomnia, lifetime cannabis use, antisocial behavior, and risk tolerance showed nominal associations with suicidal behavior (2.5 × 10−4 ⩽p < 0.05). Sensitivity analyses showed that all 22 psychiatric disorders/traits had comparable polygenic effects in both non-fatal suicide attempters and suicide decedents. Excluding MDD suicides did not reduce the strength of the association of the MDD PRS with suicide death. We also found that MDD-PRS correlated with the severity of trait aggression in our suicidal behavior cases.
Polygenic association between psychiatric disorders/traits and suicidal behavior including both non-fatal suicide attempt and suicide death
The largest polygenic variance in suicidal behavior was explained by the PRS scores for major psychiatric disorders (MDD, BD, and SCZ). Other significant associations (ADHD, alcohol dependence, high SESA, lower educational attainment, lower cognitive performance, and lower IQ) and nominal significant associations observed with PTSD, anorexia nervosa, ADHD, insomnia, lifetime cannabis use, antisocial behavior, and risk tolerance, all explained less than half the variance of the major associations. Our results are consistent with prior clinical studies where these psychiatric disorders and traits were identified as clinical risk factors for suicidal behavior (Bachmann, Reference Bachmann2018; Balazs & Kereszteny, Reference Balazs and Kereszteny2017; Hansson Bittár, Falkstedt, & Sörberg Wallin, Reference Hansson Bittár, Falkstedt and Sörberg Wallin2020; Mann, Waternaux, Haas, & Malone, Reference Mann, Waternaux, Haas and Malone1999; Phillips & Hempstead, Reference Phillips and Hempstead2017; Stanley et al., Reference Stanley, Boffa, Rogers, Hom, Albanese, Chu and Joiner2018). Some European ancestry GWASs reported shared polygenic effects between suicidal phenotypes and the disorders/traits that we found to be significant (MDD, BD, SCZ, ADHD, alcohol drink per day, lower educational attainment, and lower IQ) or nominally (PTSD) (Coombes et al., Reference Coombes, Markota, Mann, Colby, Stahl, Talati and Biernacka2020; Docherty et al., Reference Docherty, Shabalin, DiBlasi, Monson, Mullins, Adkins and Coon2020; Levey et al., Reference Levey, Polimanti, Cheng, Zhou, Nuñez, Jain and Gelernter2019; Lim et al., Reference Lim, Rijsdijk, Hagenaars, Socrates, Choi, Coleman and Pingault2020; Mullins et al., Reference Mullins, Bigdeli, Børglum, Coleman, Demontis, Mehta and Lewis2019; Strawbridge et al., Reference Strawbridge, Ward, Ferguson, Graham, Shaw, Cullen and Smith2019). On the other hand, to our knowledge, we identified the first evidence for shared polygenic effects on suicidal behavior with the GWASs for high SESA and lower cognitive performance, which fits with our stress diathesis model that includes cognitive deficits and abnormal stress responsivity as part of the diathesis for suicidal behavior (Mann & Rizk, Reference Mann and Rizk2020). Validation of our PRS results using other statistical methods such as genetic-correlation analysis (van Rheenen, Peyrot, Schork, Lee, & Wray, Reference van Rheenen, Peyrot, Schork, Lee and Wray2019) was prevented by the small sample size of our suicidal cohort in this study. However, our results are in line with the largest GWAS of suicidal behavior, which found genetic correlations with various diathesis-related psychiatric disorders/traits such as MDD, BD, SCZ, PTSD, anorexia nervosa, ADHD, alcohol dependence, risk taking, sensitivity, and intelligence (Mullins et al., Reference Mullins, Kang, Campos, Coleman, Edwards, Galfalvy and Ruderfer2021).
Comparison of polygenic effects for psychiatric disorders/traits between non-fatal suicide attempt and suicide death
In a sensitivity analysis, we subdivided our suicidal behavior group into non-fatal suicide attempters and suicide decedents, and found comparable polygenic effects for all of the psychiatric disorders and traits in both subgroups. This finding is consistent with a reported strong PRS association between suicide attempt and suicide death (Mullins et al., Reference Mullins, Kang, Campos, Coleman, Edwards, Galfalvy and Ruderfer2021). While different genetic architecture between suicide attempt and suicide death may be indicated by the clinical, demographic, and biological differences previously reported (DiBlasi et al., Reference DiBlasi, Kang and Docherty2021; Mann & Rizk, Reference Mann and Rizk2020), our study indicates there are also common polygenic elements that support the premise of prior genetic studies that used mixed suicidal cohorts (suicide attempters and suicide decedents), the feasibility of meta-GWAS using mixed suicidal phenotypes, and those of other multi-omics studies of suicidal behavior that target the full spectrum of lethality and intent. To test the similarity of polygenic architecture between non-fatal suicide attempt and suicide death, we first employed a non-psychiatric healthy population as the control cohort for both the non-fatal suicide attempter and suicide decedent cohorts. This was because of prior results indicating a biological diathesis for suicidal behavior, some of which are shared between non-fatal and fatal suicidal behavior (Edwards et al., Reference Edwards, Ohlsson, Mościcki, Crump, Sundquist, Lichtenstein and Sundquist2021). That indicates that polygenic architecture of suicidal behavior (including both non-fatal suicide attempt and suicide death) may be distinct from that of a non-suicidal healthy population. Therefore, we considered that a non-suicidal healthy population as control is better initial comparison population and also allows comparison of the polygenic architecture of non-fatal suicide attempt and that of suicide death.
In addition, we tested whether the 22 psychiatric PRSs derived from large publicly available GWAS summary statistics could statistically explain the difference in polygenic architecture between non-fatal suicide attempt and suicide death by using direct comparisons of the genotyped dataset for non-fatal suicide attempters v. suicide decedents. While none of the PRSs had significant association (p > 2.5 × 10−4), the sample size of this direct comparison was even smaller than our main PRS analysis with non-psychiatric controls. This null finding should be interpreted with caution in light of low statistical power for this direct comparison to detect difference.
Association analysis between PRSs of psychiatric disorders/traits and suicide-related clinical scores
In terms of the association of the PRSs and suicide-related clinical scores, we found that the trait aggression score was positively correlated with the PRS for MDD only in suicidal behavior cases, not in the control group. Trait aggression has been associated with the use of a violent method (Dumais et al., Reference Dumais, Lesage, Lalovic, Seguin, Tousignant, Chawky and Turecki2005), lethality of attempt (Placidi et al., Reference Placidi, Oquendo, Malone, Huang, Ellis and Mann2001), and with earlier-onset MDD and higher rates of familial MDD (Brent et al., Reference Brent, Oquendo, Birmaher, Greenhill, Kolko, Stanley and Mann2002). In this study, we showed that the strength of the relationship between MDD-PRS and suicide was comparable to that seen when we excluded all suicides with definite or possible MDD. Recent Mendelian randomization analysis (Lim et al., Reference Lim, Rijsdijk, Hagenaars, Socrates, Choi, Coleman and Pingault2020) of the UK Biobank data also indicated that the genetic liability for MDD was associated with self-harm independently of diagnosis. While this finding suggests that MDD might be genetically associated with trait aggression in the diathesis of suicidal behavior (partially independent of comorbid MDD), and not with trait aggression in the general population, there remains the possibility that our finding between trait aggression and MDD-PRS in suicidal cases might be confounded by other clinical factors (e.g. the severity of comorbid MDD). Of note, sample size for the clinical phenotype variables regarding depression/hostility/impulsivity/aggression severity scores in our cohort varied from 109 to 417, which limited our ability to compare the effect sizes and significance levels across clinical phenotypes. In addition, given the limited statistical power after adjustment for multiple testing for some of these tests, no conclusions can be drawn from the null findings in the association analyses between suicide-related clinical scores and each psychiatric PRS.
Limitations
Our findings should be interpreted in the context of several limitations. First, our suicidal behavior cohort was small for a GWAS dataset, and power analyses showed that most of the actual effect sizes in our PRS analysis were below those detectable with 80% power. This limited the power to detect differences between the polygenic architectures of non-fatal suicide attempt and suicide death (especially comparison analyses between non-fatal suicide attempters v. suicide decedents). Therefore, we carefully note again that the similarity in the polygenic architecture between non-fatal suicide attempt and suicide death presented in this study is not proof of no significant differences between non-fatal suicide attempt and suicide death. Second, discovery datasets for PRS analysis were obtained from discovery GWAS summary statistics for psychiatric disorders/traits with different sample size (N = 20 223–766 345), which means the confidence of the assessment of the PRSs differed across the disorders and traits, making it hazardous to compare the magnitudes of shared polygenic effects with suicidal behavior. Third, associations between suicidal behavior and various psychiatric PRSs might be exaggerated due to the genetic effects of comorbid MDD or non-MDD psychiatric disorders. In this study, we indicated similar correlations between MDD-PRS and suicide death after excluding suicide decedents with diagnosed/possible MDD. This suggests that our PRS findings are due mostly to the polygenic correlates of ‘suicide death’ itself. Nevertheless, a GWAS to examine the genetic effects of trait risk factors for suicidal behavior independent of psychiatric diagnoses requires a large psychiatric non-suicidal behavior control group. Fourth, given that polygenic architecture of the 22 psychiatric disorders and traits tested here overlap each other, our multiple testing correction might be quite conservative, potentially resulting in some false-negative findings. We should also note that the similarity in the polygenic architecture between non-fatal suicide attempt and suicide death might be exaggerated due to such genetic overlap between the 22 psychiatric traits themselves. Fifth, our sample characteristics had significant differences in sex and age distributions among groups as shown in Table 1, and, in addition, there were some significant differences in age in our control sample between those with and without impulsivity/aggression severity scores (online Supplementary Table S1). While we controlled for sex and age effect in all analyses here, we cannot exclude the possibility that a selection bias reflected in these significant differences may affect our findings. The sex distribution of our cohort had a natural epidemiological imbalance (females were nearly two times more frequent in non-fatal suicide attempters, males were nearly three times more frequent in suicide deaths, while the proportions were approximately equal in controls). Simply controlling for sex-related effects may obscure important genetic relationships that explain why males are three times more common in suicide decedents and females are twice as common in non-fatal suicide attempter groups. Similarly, previous studies have associated different characteristics with suicidal behavior in different age groups; for instance, more impulsive/aggressive personality traits and stronger familial transmission in the young, while higher comorbidity rate of depression and physical diseases were associated with increasing age of suicidal behavior (Fässberg et al., Reference Fässberg, Cheung, Canetto, Erlangsen, Lapierre, Lindner and Waern2016; McGirr et al., Reference McGirr, Renaud, Bureau, Seguin, Lesage and Turecki2008; Melhem et al., Reference Melhem, Brent, Ziegler, Iyengar, Kolko, Oquendo and Mann2007; Otsuka et al., Reference Otsuka, Akiyama, Shirakawa, Okazaki, Momozawa, Kamatani and Hishimoto2019). Therefore, sex- and age-stratified analysis with larger sample sizes can more precisely determine whether the genetic effects underlying each association studied differ by sex and age. Such analyses with larger sample size are important because the sex- and age-differences between non-fatal suicide attempters and suicide decedents are related potentially to sex- and age-dependent differences in genetic effects and in gene–gene interactions (Edwards et al., Reference Edwards, Ohlsson, Mościcki, Crump, Sundquist, Lichtenstein and Sundquist2021). Sixth, we removed the MHC region from our PRS analysis because estimation of PRSs in these regions is made difficult due to high polymorphic diversity in the MHC region (Choi, Mak, & O'Reilly, Reference Choi, Mak and O'Reilly2020). Therefore, we cannot exclude the possibility that exclusion of MHC may have an impact on the current PRS findings because disease associations with the MHC region variants have been reported in psychiatric genetics (especially in SCZ) (Tamouza, Krishnamoorthy, & Leboyer, Reference Tamouza, Krishnamoorthy and Leboyer2021).
Conclusions
Our PRS analysis suggests shared polygenic effects between various psychiatric disorders and traits and suicidal behavior. While we found comparable polygenic effects for major psychiatric disorders/traits between non-fatal suicide attempters and suicide decedents, the generalizability of our results may be limited by low statistical power (due to small sample size) to detect differences between non-fatal suicide attempt and suicide death. Further studies using larger sample sizes are needed to build a more detailed quantitative genetic architecture of suicidal behavior and to clarify the difference in genetic architecture between non-fatal suicide attempt and suicide death.
Financial support
This work was supported in part by SENSHIN Medical Research Foundation, JSPS KAKENHI Grant No. JP17H04249/20KK0194 and NIMH Grant No. MH082041. JJM received royalties from the Research Foundation of Mental Hygiene for commercial use of the C-SSRS. The other authors declare no conflict of interest.
Ethical standards
Contributing studies provided individual-level genotype data or summary statistics consistent with their institutional review board-approved protocols. All procedures complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291721004700.







