INTRODUCTION
Encephalitis is a complex syndrome of multiple aetiologies and pathogeneses. The clinical diagnosis of encephalitis is complicated as the symptoms and signs are similar to many other serious neurological diseases. Infection is the commonest cause of acute encephalitis where an aetiological diagnosis is made; however, in most cases the cause remains undetected [Reference Granerod and Crowcroft1]. When an infection is detected in a patient with encephalitis, another complexity presents itself: with what certainty can we say the infectious agent is actually causing encephalitis? In 1890 Robert Koch described a series of conditions that must be met to establish a microorganism as the cause of a disease and additional criteria were added later by Rivers for virus infections; however, not all microbes associated with encephalitis can be shown to fulfil these criteria [Reference Rivers2].
Ascribing a specific pathogen as the cause of the encephalitis is not straightforward. The definitive or ‘gold standard’ way to establish an infectious aetiology is to identify the pathogen in diseased brain tissue. Whilst brain biopsy has 99% sensitivity and 100% specificity for herpes simplex encephalitis (HSE) its use is limited in the living as only a small amount of tissue is obtained for investigation, limited areas of the brain can be sampled, and the procedure may be associated with surgical complications [Reference Burtis and Ashwood3]. Therefore a number of problems face both the diagnostician and researcher. First, in most cases it is not possible to study directly the diseased tissue. In addition, the frequency or strength of the link between the putative aetiological agent and the neurological syndrome varies among organisms.
Encephalitis is often described as a rare complication of common human infections. Thus, the detection of organisms outside the central nervous system (CNS) occurs with frequency, particularly for viruses that establish lifelong latency in their hosts such as lymphotropic and neurotropic herpesviruses. Other potentially neurotropic organisms are associated with asymptomatic carriage in non-sterile sites, such as the nasopharynx. Hence, detection of an organism in a non-sterile site or extraneural tissue confers less diagnostic weight than detection of an organism within brain tissue. Analysis of cerebrospinal fluid (CSF) is the key diagnostic intervention in patients with suspected CNS infections; however, being more representative of pathological processes affecting the meninges as opposed to the parenchyma of the brain it is only a surrogate for brain tissue. Furthermore, the methods of microbe detection have different diagnostic weight. For instance, isolation or culture of an organism indicates the presence of viable microbes, which cannot necessarily be implied when specific microbial sequences are detected by polymerase chain reaction (PCR). Therefore there exists a hierarchy of sample locations for attributing causality (see Fig. 1).
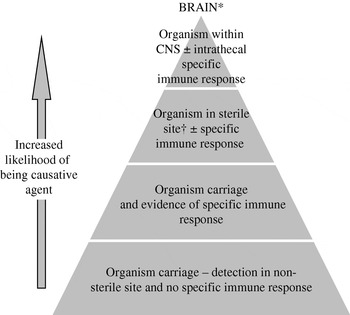
Fig. 1. Hierarchy of diagnostic tests for defining causal relationship between a microbe and the syndrome encephalitis. * This hierarchy is not relevant for all bacteria and viruses, e.g. rabies virus. † Normally sterile site=blood, CSF, joint, pleural, or pericardial fluid.
As Rivers classically demonstrated detection of a microbe is not sufficient to attribute causality but this association can be strengthened by demonstrating a microbe-specific immune response [Reference Rivers2]. Serological tests may show primary or recent infection or activity, which confers more aetiological weight; particularly when the detected organism is one that establishes latency. Neurological complications are more frequent at the time of primary infection with viruses such as Epstein–Barr virus (EBV) or human herpesviruses-6 and -7 (HHV-6/-7) in the immunocompetent [Reference Ward4]. Furthermore, through study of CSF and blood samples paired in time, the microbe-specific immune response can be demonstrated to occur locally within the intrathecal space. When found this implies a very strong association between organism and neurological syndrome. Thus without brain biopsy the hierarchical zenith for diagnosis of infectious encephalitis would be both the demonstration of an organism and its specific immune response within the intrathecal space. Critically, however, infection is a dynamic process and therefore at different time points during infectious encephalitis the probability of detecting either the causal microbe or the microbe-specific immune response varies.
Approximately one third of acute encephalitides are thought to have an immune-mediated pathogenesis, with acute disseminated encephalomyelitis (ADEM), being the most commonly identified subtype [Reference Johnson5]. The clinical manifestations of ADEM, often referred to as ‘post-infectious’ encephalitis, and acute infectious encephalitis may be identical and difficult to distinguish on clinical grounds alone [Reference Sejvar6]. There are at present no universally standardized diagnostic criteria for ADEM. Diagnosis is complicated and numerous controversies exist, for instance how to make the distinction from a first presentation of multiple sclerosis [Reference Young, Weinshenker and Lucchinetti7]. Other more recently described causes of immune-mediated encephalitis include voltage-gated potassium channel antibodies and antibodies against the N-methyl-d-aspartate (NMDA) receptor [Reference Dalmau8–Reference Vincent and Bien10].
This paper is the outcome of an initiative to streamline aetiological case definitions for acute encephalitis. Aetiological case definitions for encephalitis have recently emerged from the USA, but are otherwise lacking in the published literature [Reference Sejvar6, Reference Tunkel11–Reference Glaser13]. We present what is to our knowledge the first set of specific case definitions and laboratory criteria by organism for infectious causes of encephalitis and extend these to include immune-mediated causes. The more uniform case definitions are between regions, the more easily epidemiological studies of encephalitis can be compared.
METHODS
Discussions began on aetiological case definitions for acute encephalitis following implementation of the Health Protection Agency (HPA) Prospective Aetiological Study of Encephalitis in the UK (http://www.hpa.org.uk/webw/HPAweb&HPAwebStandard/HPAweb_C/1195733813070?p=1191942149529) [Reference Granerod14]. This study has involved national UK experts of encephalitis based both at the HPA and across the National Health Service. A diagnosis of encephalitis was made either clinically or pathologically (or both) as defined below. The aetiological case definitions apply to all cases with a clinical or histopathological diagnosis of encephalitis.
Encephalitis was defined as follows:
(1) Clinical definition: Any person, of any age, admitted to hospital with encephalopathy (altered level of consciousness persisting for >24 h and including lethargy, irritability or a change in personality and behaviour) and ⩾2 of the following:
• fever or history of fever (⩾38°C);
• seizures and/or focal neurological findings (with evidences of brain parenchyma involvement);
• CSF pleocytosis (>4 WBC/μl);
• electroencephalogram findings compatible with encephalitis;
• abnormal results of neuroimaging in keeping with encephalitis.
(2) Pathological definition: The presence of non-pyogenic inflammatory infiltrates, commonly in the form of T lymphocytes and microglia, within the brain. This may also involve the meninges (meningoencephalitis) and spinal cord (myelitis). In polioencephalitis/poliomyelitis the inflammation is predominantly localized to grey matter, in leucoencephalitis to white matter, and in panencephalitis inflammation is present in both grey and white matter.
The causes were divided into those whose pathogenesis was due to a direct effect of the organism, and those with an immune-mediated pathogenesis. A combination of expert opinion and literature-based methods was used. The HPA Study Steering Group, all with extensive experience in encephalitis, provided multidisciplinary expertise in virology, microbiology, epidemiology, neurology, neuroimmunology, and infectious diseases. Individual advice on specific rarer causes of CNS infection, including amoebae, Brucella, Borrelia, Leptospira and Toxoplasma species, was obtained from UK reference laboratories whilst advice on antibody-associated encephalitis and histopathology was obtained from other laboratories with specific expertise. Guidance on diagnostic criteria for ADEM was sought from paediatric neurologists with expertise in this field. This expert opinion was supplemented by literature searches. Relevant articles on aetiology and diagnosis of encephalitis were retrieved from Pubmed by comprehensive albeit non-systematic searching. For ‘aetiology’ for example, specific criteria applicable to a particular agent were used to find relevant publications, e.g. ‘granular cell neuronopathy’ and ‘fulminant JC encephalopathy’ were entered to find papers specific to JC virus. The first meeting of the Steering Group to discuss these definitions was held in London in July 2007, with the first draft of the definitions circulated soon afterwards. Following a teleconference in November 2007 a subsequent draft was disseminated to the group. Specific questions and issues were addressed at a meeting held in January 2008 with an updated version circulated in November 2008, and a final review shortly after. Due to the complexities and caveats discussed above, we adopted a probabilistic approach for causal inference based on strength of association. Causes of encephalitis were defined as confirmed, probable, possible, and excluded (see Fig. 2). The category possible is not just used to capture diagnoses which are suggested but not confirmed by the available test results, but also to flag that a certain diagnosis has not been ruled out. The case definitions are primarily intended for use in clinical epidemiological studies of encephalitis. However, the definitions also have implications for clinicians, public health professionals and laboratory staff.

Fig. 2. Flowchart for defining aetiologies in acute encephalitis.
RESULTS
Aetiological case definitions for acute encephalitis caused by an acute infectious agent are displayed in Table 1. Case definitions for encephalitis which is predominantly immune-mediated are displayed in Table 2. These case definitions apply to all cases with a clinical picture of encephalitis or histopathological evidence of encephalitis (see Fig. 2).
Table 1. Acute encephalitis caused by an infectious agent

ADEM, Acute disseminated encephalomyelitis; ADV, adenovirus; AAFB, alcohol and acid-fast bacilli; ASOT, antistreptolysin O titre; CD, case definition; CF, complement fixation; CMV, cytomegalovirus; CNS, central nervous system; CSF, cerebrospinal fluid; EBNA, Epstein–Barr virus nuclear antigen; EBV, Epstein–Barr virus; EIA, enzyme immunoassay; ELISA, enzyme-linked immunosorbent assay; HGE, human granulocytic ehrlichiosis; HHV-6, human herpesvirus-6; HHV-7, human herpesvirus-7; HI, haemagglutination inhibition; HIV, human immunodeficiency virus; HME, human monocytic ehrlichiosis; HSV, herpes simplex virus; IFA, immunofluorescence assay; IHA, indirect haemagglutination; JEV, Japanese encephalitis virus; LA, latex agglutination; LCMV, lymphocytic choriomeningitis virus; MA, microagglutination; MAC-ELISA, IgM antibody capture ELISA; ME, meningoencephalitis; MRI, magnetic resonance imaging; MTB, Mycobacterium tuberculosis; PAM, primary amoebic meningoencephalitis; PCR, polymerase chain reaction; PML, progressive multifocal leukoencephalopathy; RPR, rapid plasma reagin; RSV, respiratory syncytial virus; SLEV, St Louis encephalitis virus; TBEV, tick-borne encephalitis virus; TPHA, treponema pallidum haemagglutination test; TPPA, treponema pallidum particle agglutination test; VCA, viral capsid antigen; VDRL, venereal disease research laboratory; VZV, varicella-zoster virus; WNV, West Nile virus.
* Patients may meet more than one possible aetiological case definition but only one probable aetiological case definition.
† Intrathecal antibody production implies local synthesis of microbe-specific antibody within the CNS. Microbe-specific intrathecal antibody production can be identified by two methodologies: either by calculation of an antibody index or through antigen-specific immunoblotting of CSF and serum antibody following isoelectric focusing [Reference Monteyne36].
‡ Based on data from very few cases.
§ Does not include post-infectious encephalitis due to streptococci.
¶ Presents more often as meningoencephalitis.
Table 2. Encephalitis which is predominantly immune-mediated

ADEM, Acute disseminated encephalomyelitis; CNS, central nervous system; GAD, glutamic acid decarboxylase; MRI, magnetic resonance imaging; MS, multiple sclerosis; NMDA, N-methyl-d-aspartate.
* Patients may meet more than one possible aetiological case definition but only one probable aetiological case definition.
DISCUSSION
Defining the causal relationship between a microbe and the syndrome encephalitis is complex. It is thought that over 100 different infectious agents may cause encephalitis, often as one of the rarer manifestations of infection. The gold-standard investigation to identify a causative agent in encephalitis is the examination of diseased brain tissue; however, in most cases this is not clinically justified. We present the UK perspective on aetiological case definitions for acute encephalitis. We also extend them to include immune-mediated causes, which often cannot be distinguished from acute infectious encephalitis on clinical grounds alone.
These case definitions are primarily the product of consultation with an array of experts with extensive experience in encephalitis; they have additionally been supplemented by a search of published literature. The complexities of defining aetiologies in encephalitis cannot be addressed from a single discipline but rather require a multidisciplinary effort like the one used for these purposes. Different approaches incorporate different perceptions and allow for establishment of a more precise set of definitions. Validation of these definitions in future studies of encephalitis will be beneficial, as will their application in paediatrics where the spectrum of causative agents and disease may be different.
Each organism should be considered separately as some are well-established causes of encephalitis whereas others have only recently been linked to the disease. Aetiological confirmation for the latter may require both nucleic acid detection in CSF and serological tests such as IgM and IgG antibody detection, whereas in the former only one of these may suffice. Nucleic acid detection by PCR may also be difficult to interpret as a positive result may be obtained in the absence of specific neurological signs, for example Treponema in syphilis. Other dimensions, including test type and sample type, need to be considered when defining the role of an agent in encephalitis. We emphasize the importance of detecting a specific intrathecal compartment effect or detection of the organism or nucleic acid within the CSF as this confers more aetiological weight. This is evident throughout our definitions and in many cases guides a confirmed vs. a probable categorization.
Inevitably difficulties were faced in producing these definitions. Differentiating between probable and possible cases is difficult, especially for viruses that establish latency within the host and are often common human infections that only occur sporadically as encephalitis. Negative post-mortem histology is not documented in the exclusion criteria for any cause. It is difficult to exclude diagnoses based on negative neuropathology unless the patient dies in the acute stage without treatment, and in some forms of encephalitis the low number of autopsy cases examined to date makes even this difficult without making assumptions. Documenting post-mortem criteria for organism confirmation is also difficult for some causes, for example HHV-6 and EBV, where histopathological evidence is based on data from only few cases. We propose a definition for the diagnosis of the syndrome ADEM although the cause or causes are still poorly defined. Perhaps in the future a similar set of definitions can be devised specifically for ADEM. Our case definitions have focused on the most common causes of encephalitis, as well as newly described antibody-mediated causes [Reference Dalmau8, Reference Vincent9]. Some immune-mediated forms of encephalitis are not included; however, those we do not discuss are of subacute onset and some are very contentious in terms of their existence, for example Hashimoto's encephalopathy/encephalitis.
Published studies have used different criteria to link potential aetiological agents to encephalitis. A recent French study used clinical data, imaging findings and biological test results to classify patients as having confirmed, probable or possible causes of encephalitis [Reference Mailles and Stahl15]. The California Encephalitis Project defined the association between an identified agent and the encephalitis case ‘as confirmed, probable or possible, based on the type of specimen in which the potential aetiological organism was detected, the strength of the previously established associations between the agent and encephalitis, and the clinical and epidemiological characteristics of the disease’ [Reference Glaser13]. Parallels to our case definitions include the consideration of type of specimen, strength of previously established association, clinical characteristics, and emphasis on CNS to establish aetiology. In contrast, epidemiological profiles did not feature much in our case definitions. Only case definitions for certain agents, for example amoebae, Borrelia burgdorferi and rabies, included epidemiological criteria; exposures are very well defined for these organisms. Although our main emphasis was laboratory criteria, we integrate imaging and histopathology into our case definitions. For example, ‘MRI suggestive/not suggestive of ADEM’ is used to differentiate acute infectious and post-infectious encephalitis associated with group A streptococci and M. pneumoniae, as the pathogenic mechanisms for these organisms remain unclear. A characteristic MRI was also used to define HIV and JC cases as sufficient evidence is available on which to base these diagnostic criteria [Reference Yousry16, Reference McArthur, Brew and Nath17]. Recent US clinical practice guidelines for the management of encephalitis emphasize the importance of neuroimaging in encephalitis and imply its potential use in distinguishing aetiologies [Reference Tunkel11]. Our definitions incorporate the most up-to-date research such as the need to exclude chromosomal integration before confirming HHV-6 as the definitive cause [Reference Ward18]. As a third of all acute encephalitides are thought be immune-mediated and often the distinction from acute infectious encephalitis cannot be made on clinical grounds alone, it is crucial to include non-infectious causes in any case definitions for acute encephalitis.
Yet other studies in North America have based their criteria solely on the type of specimen in which the organism was detected [Reference Kolski19, Reference Nicolosi20]. Other studies have not made the criteria they used explicit. In one study, prior to the routine use of molecular diagnostics, causative agents were assigned by isolation of the organism from the nasopharynx [Reference Rantala and Uhari21]. The fact that so many case definitions are used, and that they are of such varying quality, makes comparison between existing studies difficult.
This paper is an important addition to the limited literature available on case definitions of encephalitis. Encephalitis is of global public health concern and as a result numerous studies are either underway or being planned. Achieving consensus for these aetiological case definitions will facilitate comparison between studies and ultimately result in a better understanding of the causes of this devastating neurological condition. They provide a framework for regular review and updating as the knowledge base increases both clinically and through improvements in diagnostic methods, while the importance of new and emerging pathogens as causes of encephalitis can be assessed against the principles laid out here.
ACKNOWLEDGEMENTS
The authors are grateful to the following for their expert advice on specific case definitions: Dr Richard Pebody (HPA Immunisation Department, London), Dr Susan O'Connell (HPA Lyme Borreliosis Unit, Southampton), Professor Peter Chiodini (HPA Parasitology Reference Laboratory, London), Dr Edward Guy (HPA Toxoplasma Reference Laboratory, Swansea), Dr Alison Johnson (HPA Leptospira Reference Unit, Hereford), Dr Richard Cooke (HPA Brucella Reference Unit, Liverpool), Dr Sarosh Irani (Immunology Neurosciences Group, John Radcliffe Hospital, Oxford), Professor Angela Vincent (Immunology Neurosciences Group, John Radcliffe Hospital, Oxford), Professor John Watson (HPA Respiratory Diseases Department, London), Professor Bruce Brew (Expert in HIV Neurology, University of New South Wales, Sydney, Australia), Dr Geoff Keir (Neuroimmunology and CSF Laboratory, National Hospital for Neurology, London), Dr Cheryl Hemingway (Paediatric Neurologist, Great Ormond Street Hospital for Children, London), Dr Jean-Pierre Lin (Paediatric Neurologist, Evelina Children's Hospital, London), Dr Ming Lim (Paediatric Neurologist, Evelina Children's Hospital, London), Dr Ray Borrow (Manchester Medical Microbiology Partnership, HPA Regional Laboratory), Dr Ed Kaczmarski (Manchester Medical Microbiology Partnership, HPA Regional Laboratory).
K.N.W. acknowledges funding from the University College London Hospitals/University College London Comprehensive Biomedical Research Centre (CBRC) of the National Institute for Health Research. N.W.S.D. is funded by the Peel Medical Research Trust.
DECLARATION OF INTEREST
None.






