Introduction
Locally advanced breast cancer (LABC) accounts for 10–20% in the West, while in India, it represents 30–40% of all cases. Reference Nair, Shet and Parmar1 LABC is a heterogeneous group characterised by large primary tumour (>5 cm), with/without skin or chest wall involvement and/or presence of matted axillary lymph nodes (ALN) and/or involvement of internal mammary nodes (IMN) or supraclavicular fossa (SCF) nodes without distant metastases. Thus, LABC includes all stage III patients and some with stage IIB disease (T3N0). Reference Giuliano, Edge and Hortobagyi2
A clinical presentation of LABC merits adjuvant radiotherapy regardless of type of surgery done or use of neoadjuvant or adjuvant chemotherapy used. Adjuvant radiotherapy has been proven to be effective for tumour control as well as improving overall survival. The benefit from adjuvant radiotherapy is proportionally larger for LABC compared to early breast cancer. 3,4
With regard to target volume for adjuvant radiotherapy, some controversy exists on the prophylactic inclusion of the internal mammary chain (IMC) in the radiation targets. A recent meta-analysis of 3 randomised trials: EORTC 22922–10925, MA.20, and French showed that comprehensive regional nodal irradiation significantly improves progression-free, distant metastasis-free and overall survival in stage I–III breast cancer. Reference Budach, Bölke, Kammers, Gerber, Nestle-Krämling and Matuschek5 The main challenge lies in the identification of the sub-group that will benefit the most from prophylactic IMC irradiation as the inclusion of IMC makes planning as well as delivery complex. Reference Wadasadawala and Bajpai6
Delivering IMC irradiation with conventional techniques like partially wide tangents and photon-electron combination leads to significant dose inhomogeneity as well as excessive exposure of heart and lung. Over the few decades, there are significant technological advancements in radiotherapy. Intensity-modulated radiotherapy (IMRT) has emerged as a promising option, which can also be utilised to deliver simultaneous integrated boost (SIB) to the lumpectomy cavity in case of breast conservation. The SIB technique improves dose conformity at the tumour bed, reduces spillage in the adjacent breast as well as to the organs at risk (OAR) compared to a sequential boost. Reference Hijal, Fournier-Bidoz and Castro-Pena7–Reference van der Laan, Dolsma, Maduro, Korevaar, Hollander and Langendijk10 The IMPORT HIGH trial currently in follow-up is a randomized trial testing the benefit of concomitant boost (SIB) at two doses levels: 48 Gray (Gy) or 53 Gy in 15 fractions(F) versus standard radiotherapy 40 Gy/15F to whole breast +16 Gy/8F sequential boost. Reference Coles, Griffin and Kirby11 It intends to study whether high dose using IMRT can reduce radiation-induced side effects while maintaining cure rates. The initial 3-year result of late adverse effects are similar in the standard and low dose level test arm but significantly higher in the 53 Gy arm.
Helical tomotherapy (HT) is a unique platform that permits daily 3D image verification coupled with IMRT technique. It is akin to a helical computed tomography (CT) scanner delivering radiation in a helical rotational manner using a modulated fan beam. Reference Mackie, Holmes and Swerdloff12
Its advantages include online correction of set-up errors due to daily imaging and delivery of continuous radiation in cranio-caudal direction suppressing junction problems from multiple fields while treating larger complex volumes, that too with excellent dose conformality. Reference Cendales, Schiappacasse, Schnitman, García and Marsiglia13
Few series have reported outcome of patients with initially positive IMN receiving optimal systemic therapy, surgery, and loco-regional radiotherapy including IMN. Reference Kim, Chang and Choi14,Reference Kim, Jeong and Shin15 These studies report heterogenous use of radiotherapy dose, fractionation as well as techniques. In the current study, we intend to report the outcomes of HT for IMN irradiation in clinically positive nodes diagnosed radiologically and includes dosimetric evaluation, early toxicity as well as short-term clinical outcome.
Methods and Materials
Study design and patients
All consecutive patients with LABC who received adjuvant breast/chest wall irradiation along with SCF and IMN using HT technique at our institute between November 2014 and May 2019 were identified. Electronic medical records of 65 patients were reviewed for clinico-pathological details, and treatment history including the radiotherapy treatment planning parameters and acute toxicities. The clinical, treatment and pathological details have been reported in Tables 1, 2, and 3, respectively. The diagnosis of IMN involvement was made on the cross-sectional imaging done for staging. No cytological or histological confirmation of the IMN involvement was attempted. None of the patient received prophylactic IMN irradiation. This study was approved by the institutional ethics committee, and waiver of consent was granted.
Table 1. Clinical characteristics

a One patient had occult primary.
* 2 patients had contralateral axillary nodal metastases, 2 patients had solitary liver metastases and 4 patients had sternal metastases.
cT: clinical tumour stage; cN: clinical nodal stage; cM: clinical metastatic stage; ER: estrogen receptor; PR: progesterone receptor; CT: computed tomography; PET: positron emission tomography.
Table 2. Treatment characteristics

* One patient had occult primary hence did not undergo primary surgery.
BCS: breast conserving surgery; MRM: modified radical mastectomy; AI: aromatase inhibitor; TAM: Tamoxifen; OS: Ovarian suppression.
Table 3. Pathological characteristics

IBC: invasive breast carcinoma; Tis: tumour insitu; pT: Pathological tumour stage; pN: pathological nodal stage.
The median age was 45 years (range 23–68). All patients treated with curative intent were included. Eight patients had oligometastatic disease: 4 had disease in the sternum out of which one patient also had ipsilateral 5th rib metastases and one also had parasternal disease. Two patients had solitary nodule in liver which were treated with radiofrequency ablation (RFA). Two patients with contra-lateral axillary metastases underwent bilateral axillary dissection.
Sixty-one patients received neo-adjuvant chemotherapy (NACT), while 4 patients underwent upfront surgery followed by chemotherapy as they were operated outside. All patients received anthracyline and taxane-based chemotherapy. Thirty-four patients had partial response and 23 patients had complete response, 2 had stable disease whereas 2 had progression (response assessed clinic-radiologically). All 36 hormone-receptor-positive patients received appropriate hormonal therapy as per institutional protocol. Nineteen of the 22 patients who were HER 2neu positive received trastuzumab in neo-adjuvant or adjuvant setting of which only 12 patients received maintenance therapy due to affordability issues.
Immobilisation and volume delineation
All patients were immobilised in supine position in a customised vacuum bag with both arms abducted over head. Planning images without contrast with 3–5 mm slice thickness were acquired from the level of mandible to mid-abdomen on CT simulator (GE DISCOVERY IQ). Images were exported to the tomotherapy treatment planning system (Accuray Inc., Sunnyvale, CA, version 5.1.0). Clinical reference using wire placement on patient’s body during CT acquisition aided in the delineation of clinical target volume (CTV) for the breast and/or chest wall. The target volumes were contoured according to the ESTRO guidelines. Reference Offersen, Boersma and Kirkove16 In all patients, primary (breast/chest wall) and regional nodes comprising of SCF and IMC were included in the radiotherapy targets, while sternum was included in four patients with sternal metastases. Axilla was not irradiated as full axillary clearance was performed in all patients as per institutional protocol.
A uniform 5 mm margin to the CTV was given for generating the planning target volume (PTV) for the primary as well as nodes and tumour bed (TB). The PTV primary was cropped from the skin by 5 mm. The CTV was restricted to the pectoral muscles posteriorly but included only in T4 disease especially post mastectomy. For IMN, one intercostal space below the involved space was included in the target volume. Additional targets like the sternum was treated in 4 patients, whereas 2 patients had bilateral IMN irradiation. Departmental guidelines for TB delineation were followed taking into account the seroma, base clips, post-operative changes and wired surgical scar. Thoracic OARs: individual lung, total lung, heart, left anterior descending artery (LAD), contra-lateral(C/L) breast, oesophagus, spinal cord and thyroid were contoured. Brachial plexus dosimetry was not included as none of the patients had axillary radiation and also dose prescribed was below the tolerance limit of brachial plexus.
Helical tomotherapy planning
A jaw width of 5·02 cm was used for planning with pitch of 0·287 and maximum modulation factor used was 3. The dose prescribed to the primary and nodal targets was 50 Gy in 25 fractions, and all cases of breast conserving surgery (BCS) received a SIB to the PTV_Boost. SIB was given in 16 patients out of which 12 received 61 Gy and 4 received 58 Gy and one received sequential boost. One patient with occult primary did not receive boost. The planning objectives were defined to achieve coverage of 95% of the target volume with 95% of the prescribed dose (it was difficult to achieve 98% dose coverage). However, in cases with unfavourable anatomy, a coverage up to 90% was considered acceptable (Figure 1). Target objectives were chosen according to priority – PTV IMN followed by PTV SCF then PTV CW/BR. For OARs, the planning objectives were set as V20 Gy < 35% for total lung, whereas V25 Gy < 10% for heart as per QUANTEC guidelines. Reference Kong, Ritter and Quint17 OARs according to priority were set LAD > Heart high dose (high dose region defined as area within 2·5 margin from PTV; whereas low dose is area obtained after subtracting OAR from high dose region) > Lung HD > C/L Breast > Heart LD > Lung LD > C/L Lung > Oesophagus > Spine > Body.

Figure 1. Coverage of <95% of target volume.
Standard criteria for target volumes (volumes receiving 95, 90 and 107%, mean dose) and OARs (mean, maximum, volume receiving 5 Gy (V5 Gy), V10 Gy, V20 Gy, V30 Gy, V40 Gy) were used for reporting the dose volume parameters (Tables 4 and 5).
Table 4. Dosimetric parameters indicative of planning target volume (PTV) coverage

TB: tumour bed; SCF: supraclavicular fossa; IMN: internal mammary node.
Table 5. Dose and volume parameters for organs at risk

* Dose maximum (not mean dose).
C/L: contralateral, LAD: left anterior descending artery.
Statistical analysis
The patient, treatment and pathological characteristics have been reported as numbers and percentage. The dosimetric characteristics have been reported as mean value with range. Actuarial survival curves were generated by Kaplan–Meier method and compared using log – rank test for univariate analysis. Disease-free survival (DFS) was measured as the time between initial treatment (date of surgery) and first evidence of disease progression. Overall survival (OS) was calculated from the date of initial treatment to date of death from any cause or last follow-up. Disease recurrence in the ipsilateral breast/chest wall was defined as local recurrences and those in ipsilateral axillary, supraclavicular, or IMN were defined as regional recurrences. Multivariate analysis was done by Cox proportional–hazards model and it included factors significant (p-value < 0·05) on univariate analysis. The analysis was done in Statistical Package for Social Sciences (SPSS) version 22.
Results
Dosimetric results
Tables 4 and 5 summarise the dosimetric parameters achieved for the target volumes and OARs. The 95% PTV coverage for the primary, SCF, IMN and tumour bed was 93·47, 96·85, 90·72 and 98·39%, respectively. The inconsistent dose/volume parameters may be due to being a retrospective analysis. The mean dose to the total lung, heart and contra-lateral breast was 10·6 (7·8–13·7) Gy, 6·92 (2·34–14·89) Gy and 4·32 (2·04–10·67) Gy, respectively. The mean dose maximum to the LAD was 21·5 (2·91–52·60) Gy. The dosimetric results in relation to the type of surgery and inclusion of sternum in the target volumes have been provided in the Supplementary Tables 1–4.
Acute toxicities
Radiation Therapy Oncology Group (RTOG) grading criteria was used to assess toxicity. No patient developed grade III skin or oesophageal toxicity. Forty-seven patients had grade I and 18 patients had grade II skin toxicity, whereas 39 patients had grade I and 4 patients had grade II oesophageal toxicity.
Disease-related outcome
The median follow-up for the study cohort was 36 months (95% CI 34–42·8). Twenty-one patients had progression out of which 18 patients had distant metastasis alone, while 3 also had locoregional recurrence. The sites of progression were multiple with brain, liver metastasis being common sites. Ten patients died of disease progression.
LRC, DFS and OS
The median survival times for DFS and OS have not been reached. The 3-year loco-regional control, DFS, and OS were 93·5% (95% CI 86·1–100·0), 73·9% (95% CI 62·9–84·9) and 85·9 (95% CI 76·9–95·0), respectively (Figures 2, 3 and 4). On univariate analysis, age, menopausal status, laterality, hormone receptor status, Her2 status, presence of oligo-metastatic disease, response to NACT, type of surgery and pathological nodal status were analysed to see the impact on the DFS. Of these, absence of oligometastatic disease at presentation and significant response to chemotherapy were associated with improved outcome. The 3-year DFS for patients with oligometastatic disease was 34% (95% CI 7–60·7) versus 74% (61·1–86·9) for non-metastatic patients (p 0·003). With regard to OS, only response to chemotherapy predicted survival on univariate analysis.

Figure 2. Kaplan–Meier curves for loco-regional control.

Figure 3. Kaplan–Meier curves for disease free survival.
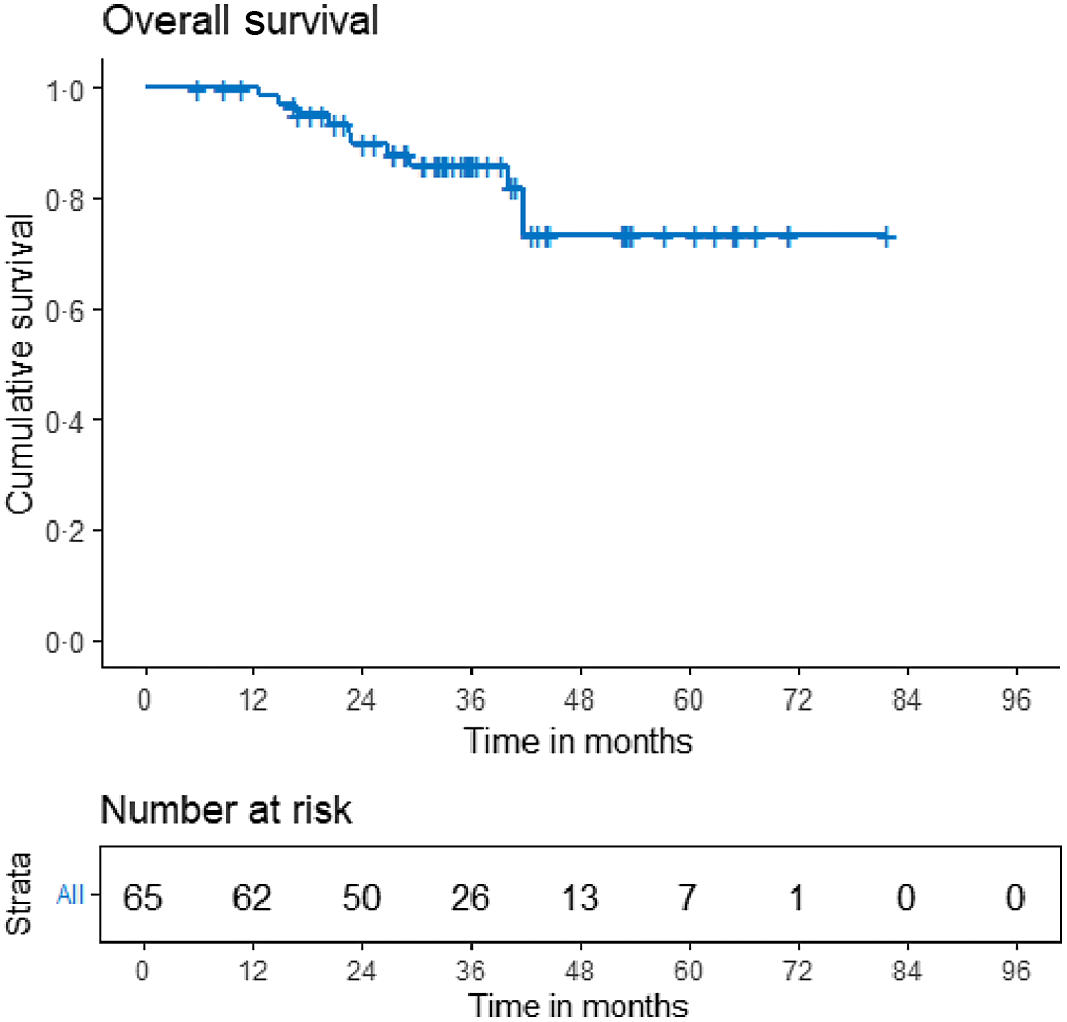
Figure 4. Kaplan–Meier curves for overall survival.
Discussion
It is seen that IMN metastases occur in 28–52% of patients having positive ALN and in 5–17% of patients without ALN. Reference Veronesi, Cascinelli and Greco18,Reference Cody and Urban19 The authors have not come across any series reporting on a homogenous cohort of patients who underwent IMN irradiation with single fractionation schedule and uniformly treated on HT. Albeit a small series, we report our initial clinical experience of IMN irradiation on HT.
IMN irradiation invariably leads to increased cardiac and pulmonary irradiation thereby increasing the risk of developing late effects. Reference Taylor, Wang, Macaulay, Jagsi, Duane and Darby20–Reference Choi, Kim and Shin22
In our study, mean dose received by the heart was 6·92 (2·34–14·89) Gy. In the 37 right-sided patients, Dmean of heart was 4·68 (2·34–7·70) Gy. Totally, 28 patients had left-sided cancers, Dmean was 9·89 (5·29–14·89) Gy while concomitant boost was delivered in 16 patients to a dose of 58–61 Gy of which 8 were left sided, Dmean for heart in these patients was 10·37 Gy.
Dosimetric comparison of 3–4 field technique IMRT with HT by Caudrelier et al. in stage III left-sided breast cancer reported cardiac sparing potential of HT in terms of significant reduction of V30 Gy but not with respect to Dmean. Reference Caudrelier, Morgan, Montgomery, Lacelle, Nyiri and Macpherson23 Initial dosimetric experience with HT reported by Goddu et al. reported a decrease in mean V35 Gy but also an increase in heart Dmean compared to 3D conformal radiotherapy (CRT). Reference Goddu, Chaudhari and Mamalui-Hunter24
The incidence of radiation pneumonitis has been associated with volume of the lung receiving low dose spill. A cut-off of 42% has been reported by Wang et al. Reference Wang, Liao and Wei25 Therefore, planner has to achieve reasonable dosimetric goals by maximum reduction of lung V20 Gy, V5 Gy, and mean lung dose. Practically, it is difficult to achieve the stringent cut-off of V5 Gy as observed in the current series as well as by Goddu et al. Reference Goddu, Chaudhari and Mamalui-Hunter24 In our study, mean V5 Gy of the I/L lung was 81·83% (50·30–99·34) and of C/L lung was 41·31% (11·80–71·14).
However, the follow-up period of the study is less to comment on whether the dose achieved for heart and lung has any effect on late cardiac and pulmonary toxicity.
While for acute toxicity, due to nil acute grade III events, dosimetric comparison has not been possible.
Matching of the field junctions is a challenging task while delivering IMN irradiation. Traditional junctional solutions (asymmetric jaws and photon-electron combination) make patient’s set-up complex and time-consuming. Reference Fournier-Bidoz, Kirova and Campana26 Moreover, dose homogeneity is very difficult to achieve resulting in either hot/cold spots within the target volumes. Most of these challenges are circumvented by HT that delivers radiation without any issue of field junctions. Moreover, daily image guidance provides the opportunity of correction of daily set up errors.
A dosimetric study including 13 patients (eight left sided) evaluated the feasibility of SIB comparing 3DCRT with HT. Reference Hijal, Fournier-Bidoz and Castro-Pena27 Dose prescribed was 50·68 Gy and 64·4 Gy in 28 fractions. With comparable coverage, conformity was better with HT with significantly less spillage outside the tumour bed (V107 = 12·47 versus 30·83%). It is important to control spillage as high doses to adjacent breast lead to increase in fibrosis and poor cosmetic outcome. Reference Vrieling, Collette and Fourquet28 In our study, 16 patients received SIB to a dose of 58–61 Gy/25F, in whom the mean of V107 = 8·88% (0·00–20·89).
The largest series by Kyubo Kim et al of patients with baseline supraclavicular and/or IMN, treated with conventional radiation to a median dose of 50·4 Gy, reported 5-year DFS and OS of 57·8 and 75·1%, respectively. Reference Kim, Jeong and Shin15 Similarly in a recent study by Kim et al. in patients with clinically positive IMN, treated by NACT followed by surgery and radiation, the 5-year DFS and OS was 68·6 and 81·8%, respectively. Reference Haeyoung, Su Ssan and Ik Jae29 In our study, the 5-year DFS and OS were 51·8 and 68%, respectively. The relatively inferior outcome is probably related to the differences in patient profile as the current study included oligo-metastatic patients as well as infrequent use of targeted therapy. The authors have reported multiple adverse prognostic factors predicting outcome which include initial clinical T stage, histologic grade 3, triple-negative (TNBC) subtype, response to NACT, lymphovascular invasion, involvement of both SCL and IMN, >= 4 ALN, Ki67 > 10%. In the current study, response to NACT was predicted for both DFS and OS, while oligometastatic presentation impacted only DFS.
Conclusion
This is a study for analysing efficacy of HT for radiating IMN, and it shows similar encouraging results compared to already published literature. It is tolerated well and results in minimal toxicity. However, the sample size is small, and follow-up is relatively short. The predominant pattern of failure was distant metastases that suggests need for intensification of systemic control especially in the non-responders. Patients having oligo-metastatic disease have a worse outcome.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1460396921000625
Acknowledgements
We acknowledge the coordinators of the breast working group for help in updating the follow-up details of the patients.
Financial Support
None.
Conflicts of Interest
None.












